ABSTRACT
Background
Nonmetastatic castration-resistant prostate cancer (nmCRPC) patients are often older and use concurrent medications that increase the potential for drug–drug interactions (pDDIs). This study assessed pDDI prevalence in real-world nmCRPC patients treated with apalutamide, darolutamide, or enzalutamide.
Research design and methods
Castrated prostate cancer patients without metastases prior to androgen receptor inhibitor initiation were identified retrospectively via Optum Clinformatics Data Mart claims data (8/2019–3/2021). The top 100 concomitant medications were assessed for pDDIs.
Results
Among 1,515 patients (mean age: 77 ± 8 years; mean Charlson Comorbidity Index: 3 ± 3), 340 initiated apalutamide, 112 darolutamide, and 1,063 enzalutamide. Common concomitant medication classes were cardiovascular (80%) and central nervous system (52%). Two-thirds of the patients received ≥5 concomitant medications; 30 (30/100 medications) pDDIs were identified for apalutamide and enzalutamide each and 2 (2/100 medications) for darolutamide. Most pDDIs had risk ratings of C or D, but four for apalutamide were rated X. Approximately 58% of the patients on apalutamide, 5% on darolutamide, and 54% on enzalutamide had ≥1 identified pDDI.
Conclusions
Results showed a higher frequency of pDDIs in patients receiving apalutamide and enzalutamide vs darolutamide. The impact of these could not be determined retrospectively. DDI risk should be carefully evaluated when discussing optimal therapy for patients with nmCRPC.
1. Introduction
Prostate cancer (PC) is the most commonly diagnosed cancer and the second-leading cause of cancer-related death among males in the United States (US), representing approximately 27% of all new cancer diagnoses and 11% of all cancer-related deaths in 2022 [Citation1]. The majority (74%) of patients with PC have hormone-sensitive, localized disease at the time of initial diagnosis, which is generally treated with surgery or radiation [Citation2,Citation3]. In cases of biochemical recurrence, if salvage surgery or radiation is ineffective or not an option, androgen deprivation therapy (ADT) is the standard of care. Despite ADT, 10% to 20% of the patients may develop castration resistance, typically within the first 5 years post-treatment (ie, castration-resistant prostate cancer [CRPC]) that may be associated with metastatic (mCRPC) or nonmetastatic (nmCRPC) disease [Citation3–6].
nmCRPC is defined by rising levels of serum prostate-specific antigen (PSA) and an absence of detectable metastases on conventional imaging, despite castrate levels of testosterone in patients receiving ADT or orchiectomy [Citation3,Citation4,Citation7,Citation8]. In 2020, there were an estimated 60,000 people living with nmCRPC in the US [Citation3,Citation9].
The Food and Drug Administration (FDA) approval of novel androgen receptor (AR) inhibitors (apalutamide, enzalutamide, and darolutamide) offers patients with nmCRPC a new treatment option [Citation10–12]. First-generation anti-androgen agents inhibit the androgen-dependent cellular cascade that stimulates prostate cancer growth through inhibition of testosterone and dihydrotestosterone binding to the AR; however, in CRPC, resistance develops through a variety of potential mechanisms including AR overexpression, AR gene mutations, development of AR variants, and altered steroidogenesis. Second-generation agents are able to potentially overcome resistance through increased specificity to the androgen receptor over other steroidal receptors and higher affinity for the androgen receptor; further, they are exclusively antagonistic to the AR and do not elicit the androgen withdrawal syndrome [Citation13]. Accordingly, these treatments represent a major improvement in the management of nmCRPC, demonstrating prolonged metastasis-free survival and overall survival. As a result of their approval, clinical management shifted from watching and waiting for the development of metastases and associated costs and complications to earlier intervention to delay the development of metastases and improve survival outcomes. This improvement in outcomes is particularly important as nmCRPC progression accounted for 86% of new mCRPC cases in 2020 [Citation3,Citation6] with 33% to 46% of patients with nmCRPC developing bone metastases within 2 years [Citation4,Citation14] and nmCRPC annual mortality rates ranging from 16% [Citation3] to up to 48% [Citation15].
While nmCRPC is generally an asymptomatic disease, the affected population tends to be older [Citation16,Citation17] and may exhibit age-related symptoms (e.g., sarcopenia and fatigue), have multiple comorbidities (e.g., depression, anemia, and hypertension) [Citation18], receive chronic concomitant medications (e.g., statins, antiplatelet agents, beta-blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, nonsteroidal anti-inflammatory drugs, and nonopioid analgesics) [Citation19,Citation20], and experience ADT-related adverse events (AEs) (e.g., cardiovascular complications, cognitive impairment, insulin sensitivity, and decreased bone health) [Citation19,Citation21–23]. Patients with multiple comorbidities may experience polypharmacy, which can be associated with diminished attention, concentration, and physical function, and medication nonadherence [Citation24]. In nmCRPC, the combination of older age and multiple comorbidities treated with concomitant medications increases the risk for drug–drug interactions (DDIs).
Recent studies have identified severe or major DDIs in an estimated 20% to 25% (and up to 50%) [Citation25,Citation26] of patients who received oral antineoplastics [Citation27]. Potential DDIs (pDDIs) may increase the risk of therapy discontinuation and AEs and thus negatively affect the efficacy and safety of nmCRPC therapies. Treatment-related AEs, which may be exacerbated by pDDIs, are burdensome, and physician and patient preference studies in patients with nmCRPC show value in reduction of treatment-related AEs (e.g., cognition, fatigue, and fractures) over improvements in overall survival ranging from 4 to 7 months [Citation28,Citation29]. Additionally, patients with prostate cancer who experience central nervous system (CNS)-related AEs while on AR inhibitor therapy incur higher annual total medical costs and utilize more healthcare resources (e.g., inpatient visits, emergency department visits, outpatient visits) vs those without any AEs [Citation30]. As such, selecting a regimen requires consideration of all of these factors: age, underlying comorbidities and concomitant medications, and ADT-induced AEs [Citation9].
Apalutamide and enzalutamide are extensively metabolized by hepatic cytochrome P450 (CYP) enzymes, which may result in pDDIs in patients taking concomitant medications [Citation10,Citation12]. Use of concomitant sensitive substrates of CYP3A4, CYP2C9, CYP2C19, uridine 5’-diphosphoglucuronosyltransferase, P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), or organic anion-transporting polypeptide 1B1 may render loss of effectiveness of medications common in the nmCRPC population, such as midazolam, warfarin, omeprazole, fexofenadine, and rosuvastatin [Citation10]. In fact, previous studies confirm CYP enzyme induction by enzalutamide and apalutamide is associated with the risk of loss of efficacy of common concomitant drugs in the nmCRPC population such as warfarin, thyroxine, and omeprazole [Citation31], which may have deleterious health effects (e.g., increased risk of stroke, hypertension, fatigue, impaired cognition).
In contrast, darolutamide is a structurally distinct AR inhibitor that exhibits no significant inhibition of CYP enzymes in vitro, suggesting it may have minimal clinically relevant effects on the administration of comedications metabolized by CYP enzymes [Citation31,Citation32]. However, to date, the real-world burden of pDDIs associated with darolutamide is unknown.
Because the nmCRPC population is complex and would benefit from nmCRPC-specific treatment agents that elicit low rates of pDDIs, it is crucial to evaluate the pDDIs of the novel AR inhibitors, especially given the increased use of long-term oral anticancer therapies in recent years [Citation33]. To address this gap in knowledge, we described the prevalence of pDDIs (via Lexicomp risk rating) associated with apalutamide, darolutamide, and enzalutamide in a cohort of nmCRPC patients treated with these novel AR inhibitors. Additionally, the secondary objectives of this study were to describe the real-world demographics (including comorbidities and concomitant medications) and clinical characteristics of darolutamide with other AR inhibitors in order to help aid clinicians in nmCRPC patient management.
2. Methods
2.1. Data source
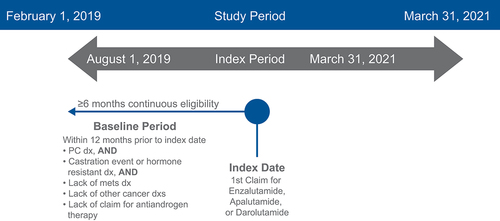
This study was a retrospective observational cohort study. shows the patient identification methodology. Patients were identified using US administrative claims data from the Optum Clinformatics Data Mart (CDM). The CDM is composed of data on commercial health plan and Medicare Advantage members and is geographically diverse, spanning all 50 states [Citation34]. Note that this study is exempt from institutional review board (IRB) approval as it utilized secondary, unidentifiable data. As such, no patient consent or waiver from an ethics committee was required for this research.
2.2. Patient identification
The full study period spanned from 1 February 2019 through 31 March 2021. The index period was between 1 August 2019 and 31 March 2021, once all 3 agents (apalutamide, enzalutamide, and darolutamide) were FDA approved for nmCRPC. Patients were included in the analysis if they were adult males with a diagnosis of prostate cancer and met all of the following three criteria occurring during the index period: (1) ≥1 medical claim for prostate cancer (International Classification of Diseases [ICD]-9-Clinical Modification [CM]: 185, 233.4; ICD-10-CM: C61, D07.5) in any diagnosis position on the medical claim during the study index period within 6 months prior to first pharmacy claim; and (2) ≥1 surgical claim for surgical castration (ie, bilateral orchiectomy) or medical castration (ie, hormone therapy) during the study index period within 6 months prior to the first pharmacy claim; or (3) ≥1 medical claim for CRPC (ICD-10-CM: Z19.2) in any diagnosis position on the medical claim within 6 months prior to the first pharmacy claim.
Additional inclusion criteria were no evidence of metastases prior to initiation with apalutamide, darolutamide, or enzalutamide and ≥1 pharmacy claim for an AR inhibitor during the index period (defined as the index date). Patients were also required to have continuous eligibility, defined as ≥6 months of continuous health plan eligibility for both medical and pharmacy benefits prior to the study index date. Patients were excluded if they had evidence of other nonprostate cancer/CRPC primary cancers, except skin cancers occurring any time during the 6-month period prior to the study index date (first pharmacy claim for treatment) or during the post-index period, including the index date; a medical claim for any metastasis diagnosis any time 6 months prior to the study index; or a medical or pharmacy claim for an antiandrogen therapy any time 6 months prior to the study index.
2.3. Variable definitions
Concomitant medications were defined as therapy with at least 1 day of overlap with apalutamide, darolutamide, or enzalutamide. The top 100 most prevalent concomitant medications at index date were identified, and pDDIs between each novel AR inhibitor (i.e., darolutamide, enzalutamide, and apalutamide) were assessed using the Lexicomp compendium. Each identified pDDI was flagged and further assessed for severity levels. Polypharmacy was defined as the evidence of ≥5 concurrent medications with the index AR inhibitors among medications filled or on hand during ±30 days of the index date.
2.4. Data analysis
Lexicomp provides information on what drug would possess pDDIs with the drug of interest and the severity levels of the interactions [Citation35]. Lexicomp categorizes the severity levels by letter grade of A (no known interaction), B (no action needed), C (monitor therapy), D (consider therapy modification), and X (avoid combination). Details on the definitions of severity levels in Lexicomp are included in the appendix (Supplemental Table S1). The Lexicomp compendium was utilized because it is the most widely used in clinical practice and is the most accurate among the common DDI screening databases; in an evaluation of five databases (Lexicomp, Micromedex, Medscape, Epocrates, and Drugs.com) to detect pDDIs of newly approved oral antineoplastics, Lexicomp received the highest score for accuracy among all databases [Citation27].
3. Results
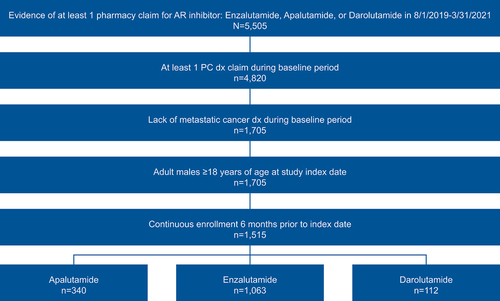
A total of 1,515 patients (apalutamide: n = 340; enzalutamide: n = 1,063; darolutamide: n = 112) were identified for analysis during the study index period between 1 August 2019 and 31 March 2021 ().
Mean age was 77 years old (standard deviation [SD]: 8), 49% of the patients were White (Black: 15%; Hispanic: 9%; Asian: 2%; unknown: 24%), and most had unknown/other commercial insurance (52%) followed by health maintenance organization insurance (24%) (). Comorbidities were common in the identified nmCRPC patient population, with a median Charlson Comorbidity Index (CCI) score of 2 (SD: 3). The most common comorbidities included hypertension (79%) and diabetes (38%) ().
Table 1. Baseline demographics.
Table 2. Charlson Comorbidity Index and select comorbidities.
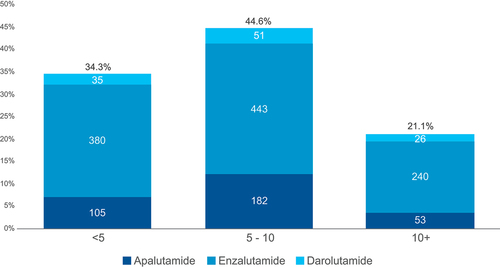
The majority of patients (66%) were prescribed ≥5 concomitant medications at baseline (45% had 5–10 concomitant medications and 21% had more than 10 concomitant medications), indicating polypharmacy burden within the identified nmCRPC population ().
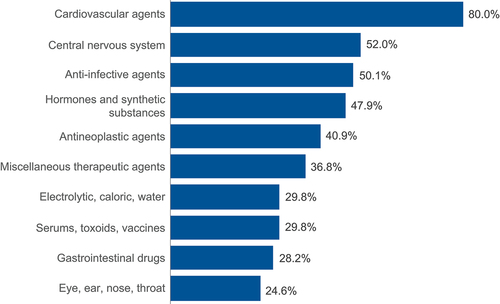
The most common concomitant medication classes were cardiovascular (80%), CNS (52%), and anti-infective (50%) (). Of the 100 most prevalent comedications (covering 99% of patients) utilized in the nmCRPC population, Lexicomp flagged 2 (2/100 medications) pDDIs for darolutamide, 30 (30/100 medications) for apalutamide, and 30 (30/100 medications) for enzalutamide (Supplemental Table S2). At least 1 pDDI (ie, patient was on ≥1 pDDI medication) was identified among over half of nmCRPC patients receiving apalutamide (58%; n = 197/340) and enzalutamide (54%; n = 577/1,063), while a pDDI was identified among only 5% (n = 6/112) of patients receiving darolutamide.
The majority of interactions (93%) had risk ratings of C (monitor therapy) (67%; apalutamide: n = 18; enzalutamide: n = 20; darolutamide: n = 0) or D (consider modification) (26%; apalutamide: n = 6; enzalutamide: n = 8; darolutamide: n = 1). However, four interactions for apalutamide were rated X (avoid combination) (). Implicated enzymes for pDDIs are listed in .
Figure 5. pDDIs out of the 100 most commonly prescribed concomitant medications by AR inhibitor.
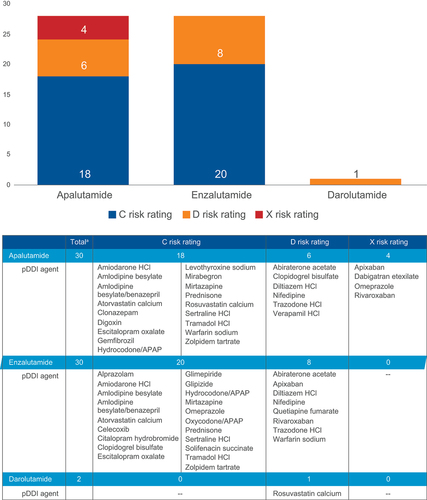
Table 3. Implicated enzymes for pDDIs.
4. Discussion
Novel AR inhibitors (i.e., darolutamide, apalutamide, and enzalutamide) are oral agents approved for nmCRPC, where duration of therapy can last years and therefore the potential for DDIs is an important consideration when managing nmCRPC patients receiving these agents. Our findings show that darolutamide has the benefit of fewer pDDIs, likely due to minimal CYP interactions, compared to apalutamide and enzalutamide. We also found a high proportion of comorbidities and concomitant medication use among this population. Given the multifaceted aspects of aging that intersect with nmCRPC, the risk-benefit profile of novel AR inhibitors is an essential clinical consideration; treatments that do not contribute to therapeutic burden due to DDIs or compound ADT-related AEs (e.g., cognitive function) should be considered and prioritized in therapy selection [Citation9,Citation36]. The findings of this study can help guide pharmacists and physicians to optimize therapy (e.g., AR inhibitors) for patients with nmCRPC by considering pDDIs related to their patients’ comorbidities and concomitant medications.
This study aligned with previously published data [Citation9,Citation16,Citation17,Citation37,Citation38] in terms of patient demographics, as patients with nmCRPC tended to be older (77 ± 8 years of age), have multiple comorbidities (mean CCI score: 3), and have high polypharmacy burden (≥5 concomitant medications: 66% of the patients). Darolutamide had numerically fewer pDDIs (5% of the patients had ≥1 pDDI) than both apalutamide and enzalutamide (58% and 54% of the patients had ≥1 pDDI, respectively). These findings highlight the value of proactively screening for pDDIs and the advantage of using darolutamide in patients with nmCRPC who have a high risk for pDDIs with other medicines to potentially optimize duration of therapy and maximize clinical benefit. Further, a recent systematic review and meta-analysis showed that patients treated with next-generation AR inhibitors had a 26% reduction in the risk of death compared with placebo; compared with other AR inhibitors, darolutamide had the lowest rate of grade 3 and 4 AEs and the lowest discontinuation rate due to any grade AEs [Citation39].
Polypharmacy (frequently defined as ≥5 medications daily) is common in older patients and is associated with AEs such as increased mortality, adverse drug reactions, and falls [Citation37,Citation38]. Furthermore, as patients tend to be on ADTs long term, the resulting hormonal depletion may be associated with induction or exacerbation of AEs particularly related to cardiovascular health, cognitive function, and bone health (including falls and fractures) [Citation9,Citation21]. The main finding of this study demonstrates low pDDIs between medications in the top 100 most commonly prescribed concomitant medications in the nmCRPC population and darolutamide (2 medications with pDDIs) compared with apalutamide and enzalutamide (30 medications with pDDIs each). Because only 2% of medications (2/100 most commonly prescribed medications) have pDDIs with darolutamide and 30% of medications (30/100 most commonly prescribed medications) have pDDIs with apalutamide and enzalutamide, lower numbers of patients were found to be at risk of pDDIs in the darolutamide population (5% receiving at least 1 of the 2 medications with pDDIs) compared to apalutamide (58% receiving at least 1 of the 30 medications with pDDIs) and enzalutamide (54% receiving at least 1 of the 30 medications with pDDIs) in this cohort. This supports the notion that darolutamide’s distinct molecular structure may contribute to its decreased pDDIs [Citation31,Citation32]. In the pivotal phase 3 study ARAMIS, the only potential interactions noted with darolutamide were BCRP substrates and combined P-gp and CYP3A4 inducers/inhibitors [Citation11,Citation32]. In vitro studies reported that the only potentially clinically relevant interaction of darolutamide was in combination with rosuvastatin (substrate for BCRP and OATP drug transporters) – an interaction that did not appear to result in increased AEs in the ARAMIS safety data analysis [Citation31,Citation32]. Our study found rosuvastatin was the only medication flagged by Lexicomp as a pDDI with darolutamide that had an associated actionable risk rating (category D: monitor therapy).
In contrast, both apalutamide and enzalutamide demonstrated a high rate of pDDIs compared with darolutamide, findings that are reinforced by product-prescribing information and previous DDI studies [Citation10,Citation12,Citation40,Citation41]. Apalutamide and enzalutamide induce enzymes CYP3A4, CYP2C9, and CYP2C19, which metabolize up to 50% of medicines [Citation10,Citation12,Citation40]. In a post hoc analysis of patients receiving apalutamide in the SPARTAN study, clinical characteristics that were associated with an increased risk of falls were evaluated. Results showed concomitant antidepressants or alpha-blockers were associated with falls, and multivariate analysis showed alpha-blockers were independently associated with falls, highlighting a potential result of a DDI [Citation41]. Apalutamide may also interact with P-gp, BCRP, OATP1B1, and uridine-diphosphate glucuronosyltransferase [Citation10]. Duran et al. assessed the potential enzyme and transporter inhibitor/inducer effects of apalutamide on single-dose pharmacokinetics of substrates for CYP2C9 (warfarin), CYP3A4 (midazolam), CYP2C19 (omeprazole), CYP2C8 (pioglitazone), BCRP/OATP1B1 (rosuvastatin), and P-gp (fexofenadine) in patients with CRPC [Citation22]. Due to clinically relevant interactions observed with apalutamide and CYP3A4, CYP2C19, and CYP2C9 substrates, author recommendations were to substitute, when possible, any concomitant medication predominantly metabolized via these CYP enzymes in patients who receive apalutamide [Citation22].
Enzalutamide may also interact with CYP2C8 inhibitors or inducers [Citation12]. Benoist et al. conducted a retrospective review of pharmacy records to evaluate the prevalence of pDDIs in a tertiary center cohort of mCRPC patients who received enzalutamide (N = 105) [Citation19]. Out of 205 different comedications, 56 (27%) comedications were identified as pDDIs with enzalutamide by at least 1 of 3 compendia databases (Lexicomp, Micromedex, Dutch database). Fentanyl, carbamazepine, nifedipine, and ticagrelor had the highest risk rating (Level X: avoid) in Lexicomp and were used by 31% of the patients. The majority of patients (85%) received at least 1 medication with a Major (Level D: treatment modification suggested) risk rating, and 44% of the patients received at least 1 concomitant medication with a Moderate (Level: C monitor therapy) risk rating [Citation19]. Furthermore, 63% (66/105) of patients received CNS depressants, with 45% using 2 or more CNS depressants. CNS depressants and proton pump inhibitors are often used in this patient population (63% and 56% of mCRPC patients received these medications, respectively) [Citation19]. Due to its side-effect profile, enzalutamide may contribute to further CNS-depressing effects and has been shown to decrease proton pump inhibitor plasma concentrations metabolized via CYP2C19 by approximately 70% [Citation19].
Consequently, in order to reduce the risk of pDDIs, clinicians should carefully consider the patient with nmCRPC, taking into account age, comorbidities, and concomitant medications when selecting nmCRPC therapy [Citation10,Citation12]. While delaying metastases onset, ultimately prolonging survival, is the main goal of nmCRPC therapy, pDDIs should be considered on an individualized basis in novel AR inhibitor treatment selection. They may negatively impact efficacy and safety of concomitant medications and increase the risk of AEs and therapy discontinuation of nmCRPC treatment, potentially leading to decreased survival and/or quality of life [Citation9,Citation19,Citation20] and increased healthcare resource utilization [Citation42]. Thus, darolutamide may be a beneficial therapy option for patients with nmCRPC and a high pharmacotherapy burden compared with apalutamide and enzalutamide.
There are limitations to this study. First, the Optum CDM is a subset of beneficiaries from a national health plan and may not be fully representative of the US nmCRPC population. However, the Optum CDM encompasses commercial health plan and Medicare Advantage members from all 50 states [Citation34]. Next, the use of claims data has inherent limitations, including potential errors from coding inaccuracies and possible misclassification of patient nmCRPC status. pDDI evaluations typically use a combination of DDI compendia and clinical review [Citation27]. A single compendium, in addition to claims data, was used in this study to identify pDDIs. Variability exists among DDI databases and, therefore, the use of other compendia may yield different results. While some published DDI evaluations utilized at least 2 DDI screening databases [Citation27,Citation43,Citation44], many reports used a single DDI database [Citation25,Citation27,Citation45,Citation46]. Utilizing newly approved oral antineoplastics, Bossaer et al. evaluated 5 databases (Lexicomp, Micromedex, Medscape, Epocrates, and Drugs.com) for specificity (229 DDI pairs) and sensitivity (64 false DDI pairs) to detect pDDIs. Considerable variability was observed among the datasets in both sensitivity (Drugs.com: 90.8%; Lexicomp: 88.6%; Epocrates: 85.6%; Medscape: 81.2%; Micromedex: 69.0%) and specificity (Lexicomp: 96.9%; Epocrates: 95.4%; Drugs.com: 90.6%; Micromedex: 71.9%; Medscape: 62.5%). However, Lexicomp scored consistently high in both sensitivity (88.6%) and specificity (96.9%) and achieved the highest (355) score among databases in terms of accuracy (max score = 400) (Epocrates: 344; Drugs.com: 352; Medscape: 280; Micromedex: 270) [Citation27], supporting its use in the current study as the single DDI compendium. Additionally, this study was unable to report the clinical relevance of the pDDI due to lack of clinical information available. Still, in clinical practice, there are no universally accepted standards for identifying clinically relevant pDDIs, which highlights the importance of proactive collaboration of clinicians, pharmacists, and other clinical decisions makers in identifying, monitoring, and reporting DDIs [Citation27]. Finally, it was assumed that patients would continue the same concomitant medications when prescribed apalutamide, enzalutamide, or darolutamide, which may not reflect clinical practice.
5. Conclusions
This claims-based analysis of real-world patients with nmCRPC treated with AR inhibitors identified that patients were older with an associated high comorbidity burden and a substantial polypharmacy burden. Additionally, a novel finding of this study was that considerably fewer pDDIs were observed among darolutamide-treated patients (2%) vs enzalutamide- or apalutamide-treated patients (30% each); this finding has been previously unreported in the literature. Accordingly, darolutamide may be a beneficial therapy option compared with apalutamide and enzalutamide for patients with nmCRPC treated with AR inhibitor therapy in order to decrease pDDIs. As the impact of pDDIs was beyond the scope of this study, future prospective studies are needed to assess the true relevance of DDIs in this patient population, and future real-world studies are also warranted in order to characterize the clinical and economic impact of pDDIs in patients with nmCRPC.
Declaration of interests
S Appukkuttan, G Ko, SX Kong, and J Jhaveri are employees of Bayer. C Fu was an employee of Bayer at the time of work. B Bannister was an employee of Cencora at the time of work. SJ Freedland is a paid consultant to Bayer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have substantially contributed to the conception and design of the article and interpreting the relevant literature. All authors have been substantially involved in writing the article or revised it for intellectual content. All authors agreed on the journal to which the article has been submitted and reviewed and agreed on all versions of the article before submission and during revision. S Appukkuttan, G Ko, C Fu, SX Kong, and J Jhaveri were involved in the study design, data collection, analysis, and interpretation of data. Breyanne Bannister was involved in the study analysis and data interpretation. SJ Freedland was involved in the study design and interpretation of data. All authors agree to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Supplemental Material
Download MS Word (63.6 KB)Acknowledgments
This research was previously presented at the Virtual ISPOR 2021 meeting (doi: 10.1016/j.jval.2021.04.250). We would like to acknowledge Charley Hallock and Bridgette Kanz Schroader, of Cencora for their aid in medical writing and Kylie Matthews, of Cencora for her aid in editing. Their permission was obtained to mention their names in this manuscript for publication.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737140.2024.2328778
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7–33.
- SEER. Cancer stat facts. 2021 [cited 2022 Sep 14]. Available from: https://seer.cancer.gov/statfacts/html/prost.html
- Scher HI, Solo K, Valant J, et al. Prevalence of prostate cancer clinical states and mortality in the United states: estimates using a dynamic progression model. PLoS One. 2015;10(10):e0139440. doi: 10.1371/journal.pone.0139440
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011 Nov;65(11):1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x
- Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008 Mar 1;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487
- Shore N, Oliver L, Shui I, et al. Systematic literature review of the epidemiology of advanced prostate cancer and associated homologous recombination repair gene alterations. J Urol. 2021 Apr;205(4):977–986.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with Darolutamide. N Engl J Med. 2020 Sep 10;383(11):1040–1049. doi: 10.1056/NEJMoa2001342
- Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016 Apr 20;34(12):1402–1418. doi: 10.1200/JCO.2015.64.2702
- Saad F, Bogemann M, Suzuki K, et al. Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors. Prostate Cancer Prostatic Dis. 2021 Jun;24(2):323–334.
- Erleada® (apalutamide) prescribing information. Horsham (PA): Janssen Products, LP; Sep 2021.
- Nubeqa® (darolutamide) prescribing information. Whippany (NJ): Bayer HealthCare Pharmaceuticals Inc; 2022.
- Xtandi® (enzalutamide) prescribing information. Northbrook (IL): Astellas Pharma US, Inc; 2022 Jan.
- Rice MA, Malhotra SV, Stoyanova T. Second-generation Antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019;9:801. doi: 10.3389/fonc.2019.00801
- Smith MR, Cook R, Lee KA, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011 May 15;117(10):2077–2085. doi: 10.1002/cncr.25762
- Freedland SJ, Sandin R, Sah J, et al. Treatment patterns and survival in metastatic castration-sensitive prostate cancer in the US veterans health administration. Cancer Med. 2021 Dec;10(23):8570–8580.
- Droz JP, Albrand G, Gillessen S, et al. Management of prostate cancer in elderly patients: recommendations of a task force of the international society of geriatric oncology. Eur Urol. 2017 Oct;72(4):521–531.
- American Cancer Society. Cancer facts & figures 2020. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancerfacts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771–787. doi: 10.1007/s12603-019-1273-z
- Benoist GE, van Oort IM, Smeenk S, et al. Drug-drug interaction potential in men treated with enzalutamide: mind the gap. Br J Clin Pharmacol. 2018 Jan;84(1):122–129.
- Li H, Hodgson E, Watson L, et al. Comorbidities and concomitant medication use in men with prostate cancer or high levels of PSA compared to matched controls: a GPRD analysis. J Cancer Epidemiol. 2012;2012:1–13. doi: 10.1155/2012/291704
- Ryan C, Wefel JS, Morgans AK. A review of prostate cancer treatment impact on the CNS and cognitive function. Prostate Cancer Prostatic Dis. 2020 Jun;23(2):207–219. doi: 10.1038/s41391-019-0195-5
- Duran I, Carles J, Bulat I, et al. Pharmacokinetic drug-drug interaction of apalutamide, part 1: clinical studies in healthy men and patients with castration-resistant prostate cancer. Clin Pharmacokinet. 2020 Sep;59(9):1135–1148.
- Tombal B. Non-metastatic CRPC and asymptomatic metastatic CRPC: which treatment for which patient? Ann Oncol. 2012 Sep;23(Suppl 10):x251–x258. doi: 10.1093/annonc/mds325
- Ritchie CS, Kvale E, Fisch MJ. Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract. 2011 Nov;7(6):371–374. doi: 10.1200/JOP.2011.000460
- Marcath LA, Coe TD, Hoylman EK, et al. Prevalence of drug-drug interactions in oncology patients enrolled on national clinical trials network oncology clinical trials. BMC Cancer. 2018 Nov 22;18(1):1155. doi: 10.1186/s12885-018-5076-0
- Keller KL, Franquiz MJ, Duffy AP, et al. Drug-drug interactions in patients receiving tyrosine kinase inhibitors. J Oncol Pharm Pract. 2018 Mar;24(2):110–115.
- Bossaer JB, Eskens D, Gardner A. Sensitivity and specificity of drug interaction databases to detect interactions with recently approved oral antineoplastics. J Oncol Pharm Pract. 2022 Jan;28(1):82–86. doi: 10.1177/1078155220984244
- Srinivas S, Mohamed AF, Appukkuttan S, et al. Patient and caregiver benefit-risk preferences for nonmetastatic castration-resistant prostate cancer treatment. Cancer Med. 2020 Sep;9(18):6586–6596.
- Srinivas S, Appukkuttan S, Mohamed AF, et al. Physician benefit-risk preferences for non-metastatic castration-resistant prostate cancer treatment (nmCRPC). Presented at: 2019 ASCO annual meeting; May 31-June 1, 2019; Chicago, Illinois. J Clin Oncol. 2019;37(15_suppl):e16610. doi: 10.1200/JCO.2019.37.15_suppl.e16610
- Shah A, Shah R, Kebede N, et al. Real-world incidence and burden of adverse events among non-metastatic prostate cancer patients treated with secondary hormonal therapies following androgen deprivation therapy. J Med Econ. 2020 Apr;23(4):330–346.
- Zurth C, Koskinen M, Fricke R, et al. Drug-Drug Interaction Potential of Darolutamide: In Vitro and Clinical Studies. Eur J Drug Metab Pharmacokinet. 2019 Dec;44(6):747–759.
- Shore N, Zurth C, Fricke R, et al. Evaluation of clinically relevant drug-drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol. 2019 Oct;14(5):527–539.
- Segal EM, Flood MR, Mancini RS, et al. Oral chemotherapy food and drug interactions: a comprehensive review of the literature. J Oncol Pract. 2014 Jul;10(4):e255–e268.
- Optum. Optum claims data. [cited 2022 Aug 11]. Available from: https://www.optum.com/business/life-sciences/real-world-data/claims-data.html
- Lexicomp Online. [cited 2022 May 12]. Available from: https://online.lexi.com
- Mateo J, Fizazi K, Gillessen S, et al. Managing nonmetastatic castration-resistant prostate cancer. Eur Urol. 2019 Feb;75(2):285–293.
- Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017 Oct 10;17(1):230. doi: 10.1186/s12877-017-0621-2
- Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008 Mar 15;336(7644):606–609. doi: 10.1136/bmj.39503.424653.80
- Ingrosso G, Bottero M, Becherini C, et al. A systematic review and meta-analysis on non-metastatic castration resistant prostate cancer: the radiation oncologist’s perspective. Semin Oncol. 2022 Oct;49(5):409–418.
- Del Re M, Fogli S, Derosa L, et al. The role of drug-drug interactions in prostate cancer treatment: Focus on abiraterone acetate/prednisone and enzalutamide. Cancer Treat Rev. 2017 Apr;55:71–82. doi: 10.1016/j.ctrv.2017.03.001
- Pollock Y, Smith M, Saad F, et al. Predictors of falls and fractures in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC) treated with apalutamide (APA) plus ongoing androgen deprivation therapy (ADT). J Clin Oncol. 2019;37(15_suppl):5025. doi: 10.1200/JCO.2019.37.15_suppl.5025
- Moura CS, Acurcio FA, Belo NO. Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12(3):266–272. doi: 10.18433/J35C7Z
- Ramos-Esquivel A, Viquez-Jaikel A, Fernandez C. Potential drug-drug and herb-drug interactions in patients with cancer: a prospective study of medication surveillance. J Oncol Pract. 2017 Jul;13(7):e613–e622. doi: 10.1200/JOP.2017.020859
- van Leeuwen RWF, Jansman FGA, van Leeuwen P, et al. Drug-drug interactions in patients treated for cancer: a prospective study on clinical interventions. Ann Oncol. 2015 May;26(5):992–997.
- Diaz-Carrasco MS, Almanchel-Rivadeneyra M, Tomas-Luiz A, et al. Observational study of drug-drug interactions in oncological inpatients. Farm Hosp. 2018 Jan 1;42(1):10–15. doi: 10.7399/fh.10857
- Ergun Y, Yildirim Ozdemir N, Toptas S, et al. Drug-drug interactions in patients using tyrosine kinase inhibitors: a multicenter retrospective study. J Buon. 2019 Jul;24(4):1719–1726.