ABSTRACT
Introduction
As the availability of new economic evaluations (EE) on adjuvant trastuzumab therapy for early-stage breast cancer (EBC) with HER2-positive since last search and other EEs missed warrant a more extensive review, this study aimed to systematically review EEs of adjuvant trastuzumab compared with chemotherapy alone for HER2-positive EBC.
Area covered
The search was performed in February 2019 using MEDLINE and Scopus. Reviewers independently selected studies based on eligibility criteria, extracted data, assessed quality of reporting, and appraised quality of data sources.
Expert opinion
22 studies were included which were from high-income (HICs) and upper-middle income countries (UMICs). Incremental cost-effectiveness ratios (ICERs) from HICs were within their cost-effectiveness thresholds and ranged from 6,018 to 78,929 USD per quality-adjusted life year (QALY) gained. ICERs from UMICs mostly exceeded their thresholds ranging from 3,526 to 174,901 USD per QALY gained. Evidence shows cost-effectiveness of trastuzumab for HER2-positive EBC in HICs. There were no methodological variations. The extent and adequacy of reporting were high. The quality of data sources was moderate to high. The quality of future EEs can be improved by enhancing the reporting quality, by using context-based data and real-world efficacy data, which would impact cost-effectiveness.
1. Introduction
Breast cancer continues to be the leading cancer among women with about 1.67 million cases and 521,907 deaths according to the 2012 GLOBOCAN cancer incidence, mortality, and prevalence report [Citation1]. The World Health Organization (WHO) reported that, in 2008, almost 50% of cases and 58% of deaths due to breast cancer had occurred in less developed countries [Citation2]. The report further noted a significant variation in survival rates, ranging from 80% or higher in North America, Sweden, and Japan, to around 60% in middle-income countries (MICs), and below 40% in low-income countries [Citation3]. Limited access to detection and treatment facilities in less developed countries contributed to lower survival rates [Citation4]. There is limited information on the economic impact of breast cancer, but it was estimated that its cost accounts to 10% to 20% of all cancer service costs, or about 0.15% of the Gross Domestic Product (GDP) of an average European nation [Citation5].
While breast cancer is commonly viewed as a single disease, it is comprised of several histological subtypes that are classified according to biological marker expression, which are all different in presentation, response to therapy, and prognosis [Citation6]. Among these subtypes are those detected with higher amount of ‘human epidermal growth factor receptor 2’ called HER2-positive breast cancer. HER2 is a tyrosine kinase receptor that facilitates signaling pathways of cell growth, division, motility, and repair [Citation6]. HER2-positive breast cancer possesses more aggressive biological and clinical behavior, and has less favorable survival outcomes [Citation7]. It is reported that such subtype is seen in 15% to 20% of all invasive breast cancers [Citation8]. The differentiation of subtypes has changed the course of treatment and led to the emergence of new therapies for breast cancer such as trastuzumab, which is the first monoclonal antibody-based therapy developed to specifically target HER2. Its antitumor activity against HER2-overexpression works through the downmodulation of HER2 expression by binding to the juxtamembrane domain of the receptor. Based on its demonstrated relative efficacy and acceptable safety through key pivotal trials [Citation9–16], trastuzumab in addition to standard chemotherapy is recommended both by US National Comprehensive Cancer Network (NCCN) Guideline 2017 [Citation17] and 2015 European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for the diagnosis, treatment, and follow-up of primary breast cancer [Citation18] for the management of early-stage breast cancer (EBC) with HER2-positive in adjuvant settings.
Notwithstanding its years of efficacy to improve disease-free and overall survival of breast cancer patients, the use of adjuvant trastuzumab therapy incurs substantial economic impact. A price survey among different countries reported by the WHO in 2012 showed varied costs of trastuzumab produced by Roche, which is the patent holder, ranging from 3,035.95 USD per gram in Pakistan to 10,000 USD per gram in Brazil and Oman [Citation19]. The report argued that trastuzumab had been costly even in India where price cuts were applied and lower priced versions were available. Adjuvant trastuzumab therapy also incurs additional cost of chemotherapy administration due to additional cycles of the regimen and due to monitoring and treating possible cardiotoxic effects associated with its use [Citation9–16].
Because of existing resource constraints in many health systems, assessing the cost-effectiveness of trastuzumab became important for policy-makers to inform financing decisions. There have been several economic evaluations (EEs) on trastuzumab conducted across many countries. While there are two published systematic reviews of cost-effectiveness analysis studies of trastuzumab by Chan et al., 2009 [Citation20] and Petrou 2019 [Citation21], the availability of newly published EEs since last search of previous reviews and other EEs they missed, and the lack of more comprehensive appraisal and analysis using relevant assessment tools warrant the need for an updated and more extensive systematic review. The goals of this study were to conduct a systematic review of published EEs of adjuvant trastuzumab for HER2-positive EBC and to comprehensively describe and evaluate them based on their methodology, transparency, and adequacy of reporting, and quality of input data sources using the latest guidelines and tools. We further aimed to analyze and compare the evaluation results based on their country's income status, which may provide relevant guidance to other countries of comparable economic status.
2. Patients and methods
2.1. Data sources and searches
Economic evaluation studies of adjuvant trastuzumab therapy for HER2-positive EBC patients were identified through MEDLINE (via PubMed) and Scopus. We used search terms: ((her2 AND positive) AND early AND ‘breast neoplasms’) AND (trastuzumab AND ‘chemotherapy, adjuvant’) AND (‘cost-benefit analysis’ OR ‘cost-utility analysis) in Medline, while ((her2 AND positive) AND early AND (‘breast neoplasms’ OR ‘breast cancer’) AND (trastuzumab AND (‘adjuvant’ OR ‘post-operative’) AND (‘cost-benefit analysis’ OR ‘cost-utility analysis’ OR ‘cost-effectiveness analysis’) for Scopus. Searches were run in February 2019. We did not limit the time period and language for the search.
2.2. Selection of studies
Two reviewers (AJG and MAG) independently assessed articles obtained from the databases. Studies were eligible and included if they were original EEs of any type that assessed cost-effectiveness, measured as incremental cost-effectiveness ratio (ICER), of one-year use of adjuvant trastuzumab therapy in addition to chemotherapy versus chemotherapy alone for EBC patients who are HER2-positive. Studies which assessed its cost-effectiveness with other anti-HER2 drugs or in neo-adjuvant settings were excluded. Full-text of eligible studies were obtained and reviewed independently. Any disagreements were resolved with the third reviewer (UC).
2.3. Data extraction and quality assessment
AJG and MAG independently extracted information on the research question, methods, and other general study characteristics using standard data extraction forms. The reviewers compared and validated data extraction tables for accuracy and completeness. The included studies were appraised in three domains: methodological variations, adequacy, and transparency of reporting and, quality of data input parameters. First, a standard extraction tool was used to provide a general overview of the study characteristics, in terms of study setting, first author affiliation, and funding source, and, to assess methodological variations by describing the types of EE, type of modeling used, incorporation of cardiotoxicity in the modeling as a significant side effect associated with the use of trastuzumab, study perspective, time horizon, cycle length, discounting, and uncertainty analysis. Second, the adequacy and transparency of reporting of the studies were evaluated using the 24-item Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [Citation22]. Third, the quality of input data sources was rated using a ranking algorithm developed by Copper et al., 2005 [Citation23] (see Supplementary ), which reflected that the quality of sources for input parameters substantially affects the credibility of EEs as much as the rigor of methodology does. The sources of the following input parameters were evaluated: baseline clinical data, clinical effect size, costs, and utilities. Rank 1 was given for parameters which were derived from the most appropriate source, while rank 9 was given for parameters with unclearly stated sources. AJG and MAG independently appraised and extracted the studies using the above tools. Any discrepancy in the assessment was resolved with the third reviewer (UC).
Table 1. General Study Information and Methodology of EEs on Adjuvant Trastuzumab regimen for HER2-positive EBC patients
2.4. Data synthesis and analysis
We compared value for money of trastuzumab for HER2-positive EBC across studies. As these EEs were undertaken in different time frames and settings, all ICERs were converted into a common currency – International dollars (I$) at 2017. Values were calculated using the national GDP deflator and implied purchasing power parity conversion rates from the International Monetary Fund (https://www.imf.org/external/datamapper/PPPEX@WEO/OEMDC/ADVEC/WEOWORLD) [Citation24]. Necessary currency conversion rates and inflation adjustments applied using Consumer Price Indexes (CPIs) were derived from OECD website (https://data.oecd.org/conversion/exchange-rates.htm) [Citation25] and World Bank website (https://data.worldbank.org/indicator/FP.CPI.TOTL) [Citation26], respectively. The grouping of studies relative to the income status of country setting was referred from the World Bank classification based on Gross National Income (GNI) [Citation27]. Studies which did not indicate the year of cost analysis were assumed to have the same base year as the year of publication.
3. Results
3.1. Review profile
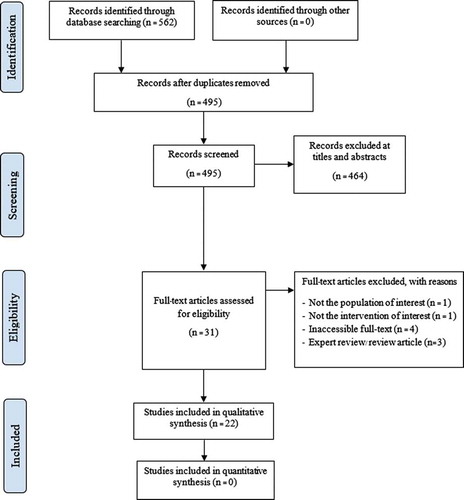
The search yielded 562 records. After removing duplicates, 464 records were screened for relevance based on set criteria, which then resulted in 31 eligible studies. Of these articles, nine studies were excluded – four were inaccessible for full-text, three were experts’ reviews, one was not the population of interest, and one was not the intervention of interest. There were three additional full-text papers identified through cited reference searching. Finally, we included 22 publications in this review. The flow diagram is shown in .
3.2. General study information and methodological variations
We identified 22 EEs comparing adjuvant trastuzumab therapy with chemotherapy alone for HER2-positive EBC, published from year 2006 to 2018 (). As shown in , studies were mostly from high-income countries (HICs) [Citation28–43], while some [Citation44–49] were from upper-middle-income countries (UMICs). About half [Citation15,Citation28–37,Citation44] were conducted and published as early as 2006 to 2009, which was within the early years of trastuzumab’s market entry for HER2-positive early stage indication in 2006. More than half [Citation28–40,Citation44] have been published even before 2013 when the WHO reviewed its inclusion in the WHO Essential Medicines List [Citation50]. In terms of methodology, majority of the studies [Citation30–32,Citation36–49] adopted cost-utility analysis, while some [Citation28,Citation29,Citation33–35] used cost-effectiveness analysis. All except two studies [Citation5,Citation10] used decision analytic Markov modeling technique. Chemotherapy regimens were varied, but the most common was trastuzumab with anthracycline-taxane combination of standard chemotherapy [Citation28,Citation30–32,Citation38,Citation41,Citation44–46]. More than half of the studies [Citation30,Citation32,Citation33,Citation37–46,Citation48] incorporated cardiotoxicity effect of trastuzumab in the analysis. Health care payer or insurance perspective was the most commonly applied [Citation28,Citation35–37,Citation39–41,Citation44,Citation45]. Half of the studies modeled for lifetime horizon [Citation30,Citation34,Citation37,Citation39,Citation42–45,Citation47–49], while others varied from 10 to 50 years. One-year cycle length was commonly applied [Citation29,Citation38,Citation41–47]. As regards to discounting, both costs [Citation29–38,Citation41,Citation42,Citation44,Citation46,Citation49] and outcomes [Citation30–33,Citation35,Citation36,Citation38,Citation46,Citation49] were mostly discounted at 3.0%. Finally, most studies handled uncertainty by conducting both one-way and probabilistic sensitivity analyses [Citation30,Citation31,Citation34–36,Citation39–41,Citation43–46,Citation48,Citation49].
Table 2. Summary of general study characteristics and different methodologies used in the included EEs (n = 22)
3.3. Adequacy and transparency of reporting
The assessment of adequacy and transparency of reporting guided by the CHEERS checklist resulted in scores ranging from 61% by Neyt et al., 2006 [Citation28] to 96% by Hall et al., 2011 [Citation39] and Shiroiwa et al., 2008 [Citation35]. The scores among HIC studies were from 61% [Citation28] to 96% [Citation35,Citation39], while the scores among UMIC studies were from 83% [Citation44,Citation49] to 92% [Citation45–47]. Less than half of the studies [Citation29,Citation30,Citation32,Citation35,Citation39,Citation42,Citation45–48] attained high scores of 90% or higher. Of the 24 reporting domains in the checklist, only 10 items were reported by all studies. These were: abstract; background and rationale; target population and subgroups; setting and location; estimation of costs and resources; discount rates; analytical methods; incremental costs and outcomes; characterization of uncertainty; and, study findings. On the contrary, measurement, and valuation of preference-based outcomes, currency price date and conversion rate, assumptions, characterization of heterogeneity, and conflict of interest statements were noted to be the most commonly missing or unstated reporting items. Moreover, while input parameters were tabulated, not all parameter values and distributions were presented. In the discussion section, not all papers adequately explained their study limitations. The CHEERS scoring per reporting domain is shown in .
Table 3. Summary results of CHEERS scoring per reporting domain (n = 22)
3.4. Quality assessment of input data sources
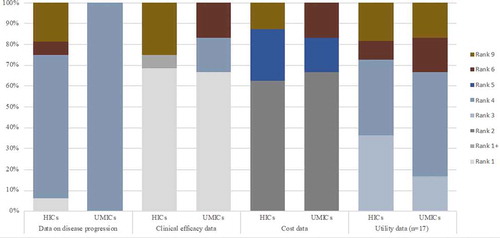
The baseline clinical data which transition probabilities were derived from were generally sourced from published reports of randomized clinical trials (RCTs), while two studies [Citation42,Citation43] used real-world country data. Correspondingly, the source of trastuzumab efficacy data was generally the same RCT source of the baseline clinical data. One study [Citation32] referred to published meta-analysis results for the relative treatment effect. Majority of the studies [Citation28,Citation30,Citation34,Citation37–45,Citation47–49] derived costing parameters from local data sources, while the remaining studies referred to published data sources from other jurisdictions [Citation31,Citation32,Citation36,Citation40,Citation46] or were not clearly stated [Citation33,Citation35]. For studies which employed cost-utility analysis, the quality of utility parameter sources was varied. Some were referred from studies which employed direct utility assessment, while others were from studies with unstated method of elicitation from unclearly reported sources. The ranking was not much different between HIC and UMIC studies – a varied ranking quality across all parameter domains. The references and corresponding ranking of the parameters domains of each study are shown in (see Supplementary ).
Figure 2. Quality assessment of evidence used in economic evaluations of trastuzumab. Y-axis represents percentage of economic evaluations having a rank based on the Quality Assessment of Sources of Input Data using tool from Cooper et al. X-axis accounts for the high-income countries (HICs) and upper-middle-income countries (UMICs) assessed based on different input parameters
3.5. Cost-effectiveness analysis results
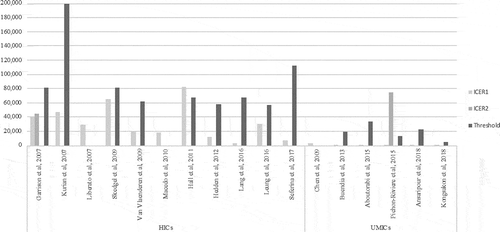
The results were measured in terms of cost per quality-adjusted life year (QALY) gained for cost-utility analyses, and were converted to international dollars per outcome for comparison (). The resulting ICERs from HICs ranged from 6,018 to 78,929 USD per QALY or 3,492 to 82,575 international dollars per QALY gained for cost-utility analyses. The values were within their corresponding country cost-effectiveness thresholds, except for Skedgel et al., 2009 [Citation36] because of the absence of a cost-effectiveness threshold in the study’s setting. Studies conducted by Liberato et al., 2007 (32) and Macedo et al., 2010 (39) also did not report their corresponding thresholds. Still, adjuvant trastuzumab was consistently concluded for its value for money among HER2-positive EBC in HICs.
Table 4. Summary of cost-effectiveness analysis results of EEs of adjuvant trastuzumab therapy for HER2-positive EBC
Among UMICs, the ICER range was relatively wider at 3,526 to 174,901 USD per QALY or 1.76 to 74,905 international dollars per QALY. Notably, the ICERs of Kongsakon et al., 2018 [Citation49] at 3,526 USD per QALY of Chen et al., 2009 [Citation44] at 9,976 USD per QALY were significantly lower compared with the ICERs of all other UMIC studies. Correspondingly, all UMIC studies except Kongsakon et al, 2018 [Citation49] and Chen et al., 2009 [Citation44] concluded that adjuvant trastuzumab therapy was not cost-effective in their settings ().
Figure 3. Incremental cost-effectiveness ratios (I$, 2017) of trastuzumab and corresponding thresholds reported from health economic evaluation. Y-axis represents the incremental cost-effectiveness ratios (ICERs) reported from different economic evaluations. X-axis accounts for EEs assessed from high-income countries (HICs) and upper-middle-income countries (UMICs)
4. Discussion
In this review, we identified a sensible number of EEs (22 studies) on adjuvant trastuzumab therapy for HER2-positive EBC considering that it has only been approved in the market for such indication for the last decade. Since the last search in 2018 by Petrou’s study, which identified 20 studies [Citation21], 18 studies from our review were found to overlap with the previous review. There were many studies conducted from a healthcare payer perspective that covered only direct medical costs and guided policy decision-making on its coverage. Notably, the patients’ out-of-pocket (OOP) expenses varied significantly among countries, such as Cambodia (74%), Indonesia (47%), China (32%), Japan (14%), and Thailand (12%) [Citation51]. Thus, OOP expenses should also be considered since all healthcare costs may not be covered by the healthcare payers. According to our review, four economic evaluations [Citation30,Citation31,Citation38,Citation49] were performed based on a societal perspective, which covered direct medical, non-medical, and indirect costs. However, none reported the patients’ OOP expenses from direct medical costs. Furthermore, only two studies revealed that patients’ OOP expenses from direct non-medical costs were transportation [Citation30,Citation48] and food [Citation48] costs. About half of the studies were conducted during the first few years of its market introduction and were undertaken by HICs, which evidently have the larger fiscal space and higher capacity to afford such medication for national coverage. In contrary, the limited number of published EEs among MICs, which were mostly published recently, likely suggests the low priority among these countries to consider high-cost therapies even with proven relative treatment effect. This may also be attributed to the current technical and context-specific challenges that researchers working in lower-middle income countries (LMICs) face, such as limited expertise to conduct EEs or lack of reliable data.
Overall, the methodology of studies was appropriate and of good quality. This may due to the fact that they were undertaken by HICs where expertise on health economics is well-established, and with their first authors mostly affiliated with the academe where conduct of research is generally considered to be of high quality. Further, in the context of the evolution of health economics research, these studies were conducted in recent times where developments in EE methods have been importantly explored, and where guidelines for more robust and better quality evaluations are clearer.
As trastuzumab is relatively new in the market and in the clinical practice, it is evident that the studies identified applied modeling technique to evaluate the cost-effectiveness considering the paucity of longer follow-up data. As expected, the models were highly varied in terms of the number of health states but the key health states were comparable, with many of them incorporating cardiotoxicity as a significant side effect of trastuzumab use. There were no detected major inconsistencies across their methods.
Although the range of scoring for the transparency of reporting was wide, most studies attained high scores since they were recently conducted when reporting tools have been released and recommended. Notably, the extent of reporting based on the CHEERS scoring guide was moderately higher in studies from UMICs (89%) compared with studies from HICs (85%). This may be explained by the fact that the most recent CHEERS checklist used in our review was only released in 2013, and most HIC studies were published prior to that year. Almost all UMIC studies, on the other hand, were published in 2013 and beyond.
As regards the appraisal of input data sources, the ranking scores were quite varied. Among all the parameter domains, only the data source/s clinical effect size attained mostly high ranking score across all studies and scored for the highest possible rank in that parameter domain. Each of the source parameter domains had a small number of studies (ranging from one to four studies) with the lowest ranking. It was evident though that there were more studies from HICs with lowest ranking (i.e., three studies in epidemiological data sources, four in clinical efficacy source, and two in cost data source) as compared with those studies from UMICs (i.e., one study in utility data source). This may be accounted for the fact that studies from UMICs were more recently published, hence had better quality of reporting. Although relatively transferable, the dependence of most studies on RCT data for clinical data may suggest the lack of established cancer registries even among HICs. The need for real-world data may be more imperative in developing countries where a possible significant difference in the observed efficacy under controlled conditions versus the actual effectiveness in the clinical practice cannot be ignored. We also noted that most RCT sources were of short follow-up period (i.e., three years) with about a decade of its entry in the market and the release of latest follow-up data on trastuzumab efficacy [Citation9], utilizing longer follow-up clinical data is imperative, considering that duration of efficacy was cited by most studies to be an influential parameter in cost-effectiveness results. Moreover, the reliance of some studies on published costing data outside their jurisdiction for costing parameters, given its low transferability, may result in unreliable results. Furthermore, it is noted that there may be a difference in the costing data between the actual costs and the costs obtained from published studies since, in reality, approximately 14–17% of HER2- positive EBC patients are not provided with adjuvant trastuzumab [Citation52], specifically for those with advanced age and who have co-morbidities, and about 15% discontinue the adjuvant treatment due to its cardiotoxicity [Citation53]. Nonetheless, the overall input data sources of majority of the studies were of acceptable quality.
As with value for money, adjuvant trastuzumab for HER2-positive EBC was found to be cost-effective in HICs with ICERs ranging from 6,018 to 78,929 USD per QALY gained. Trastuzumab was found to be cost-effective in China [Citation44] and in Thailand [Citation49], contrary to the results of all other studies in UMICs where it was concluded as not cost-effective with ICERs at 18,088 to 174,901 USD per QALY. Several factors may have affected their significantly lower ICERs (i.e., 9,976 USD per QALY gained in China and 3,526 USD per QALY gained in Thailand) and favorable cost-effectiveness results. First, Chen et al., 2009 used a lower hazard ratio, thereby modeling for a more favorable trastuzumab efficacy. It also modeled for five-year efficacy duration with decreasing efficacy in a stepwise function for the trastuzumab cohort simulation, while other studies mostly applied a 5-year duration of efficacy only with zero applied for benefit onwards. On the other hand, Kongsakon et al., 2018 did not incorporate cardiac events in their analysis, resulting in an underestimated ICER.
The overall quality of future EEs on trastuzumab can be enhanced by improving the reporting quality through explicitly stating and discussing commonly missed reporting information that we have identified in this review – complete table of input parameters, their values, distribution, and sources; the underpinning model assumptions; the currency price date and conversion rate; comprehensive discussion section with limitations of the study; the funding source; and the conflict of interest statements. Future EEs on trastuzumab are further recommended to consider the use of local data parameters for a more contextualized and appropriate results that can guide decision-making. We also note the significance of using real-world clinical data of longer follow-up period that can better reflect the true effectiveness, and therefore cost-effectiveness of trastuzumab.
Our main findings are consistent with previously published reviews showing that majority of the studies showed favorable results mainly because majority were from HICs with higher willingness-to-pay or cost-effectiveness thresholds. Similarly, Chan et al., 2009 [Citation20] rated a high rating for the quality of the studies based on a checklist; although our study applied CHEERS checklist which is a more comprehensive standard reporting list that what they used. The main limitation of our review is the non-inclusion of unpublished papers which may possibly capture EEs on adjuvant trastuzumab therapy among LMICs. Identifying and making them accessible to fellow LMICs, especially of those with lower capacity to conduct such evaluations, may guide them on their decision-making on trastuzumab coverage.
Nevertheless, it is noteworthy to highlight that six trastuzumab biosimilars have been recently approved by the European Union and are presently available in the market [Citation54]. In effect, this has led to a significant price reduction by 20% to 30% [Citation55]. Since trastuzumab is considered to be an effective drug used for the treatment of HER2-positive EBC and has already been included in the WHO Essential Medicines List since 2013 [Citation50], the debate on its cost-effectiveness should be closed. Furthermore, the standard adjuvant treatment for HER2-positive EBC patients is currently transitioning after the approval of new drugs, such as pertuzumab, trastuzumab emtansine, and neratinib for oral use [Citation56]. Consequently, future research on their cost-effectiveness as adjuvant therapy for HER2-positive EBC patients should be further investigated.
5. Conclusion
Our review, based on available EEs on adjuvant trastuzumab, suggests that the therapy, in comparison with chemotherapy alone, for HER2-positive EBC, may be cost-effective in HICs. We have yet to see more evidence on its value for money in developing countries, especially among LMICs where no economic evaluation currently exists. While the quality of methods and the adequacy and transparency of reporting of EEs on trastuzumab were generally high, the quality of input data sources is challenged with the paucity of high-quality data. Future EEs on trastuzumab are recommended to consider reliable context-based data parameters, as well longer and real-world clinical data that can capture the true effectiveness of adjuvant trastuzumab therapy which significantly affects its value for money. Nevertheless, trastuzumab biosimilars are currently approved and available in the market with a reduced price by about 20% to 30% [Citation55] and such information can absolutely change the results of our review.
Authors’ contribution
AJG and MAJG performed systematic reviews, quality assessment, and interpretation as well as drafted and revised the paper. AT and TR participated in the analysis and interpretation of data and the drafting of the paper. UC is involved in the conception and design, analysis, and interpretation of the data, the drafting of the paper, and revising it critically for intellectual content. All authors granted the final approval of the version to be published and agreed to be accountable for all aspects of the work.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (15.2 KB)Acknowledgments
The authors would like to thank Mahidol University Health Technology Assessment (MUHTA) Graduate Program at Mahidol University for supporting the research facility.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Cancer WHO-IAfRo. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 [web page]. World Health Organization; 2012 [cited 2017]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- World Health Organization. GLOBOCAN 2008: cancer incidence and mortality worldwide. World Health Organization, Geneva; 2010.
- Coleman MP, Quaresma M, Berrino F, et al. CONCORD working group. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730–756.
- World Health Organization. Breast cancer: prevention and control. World Health Organization, Geneva; 2017.
- Taylor D. The reality of economics for oncologists. Breast. 2017 Jun;33:183–190.
- Burstein H. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005 Oct 20;353(16):1652–1654.
- Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11(2):263–275.
- Wolff AC, Hammond M, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/College of American pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013.
- Cameron D, Piccart-Gebhart M, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205.
- Perez EA, Romond E, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N983. J Clin Oncol. 2014;23(31):7811–7819.
- Romond EH, J J, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol. 2012;30(31):3792–3799.
- Advani PP, Ballman K, Dockter TJ, et al. Long term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34(6):581–587.
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283.
- Joensuu H, Bono P, Kataja V, et al. Fluorouacil, epirubicin and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer trial. J Clin Oncol. 2009;27(34):5685–5692.
- Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27(36):6129–6134.
- Joensuu H, Kellokumpu-Lehtinen P, Huovinen R, et al. Outcome of patients with HER2-positive breast cancer treated with or without adjuvant trastuzumab in the Finland Capecitabine Trial (FinXX). Acta Oncol. 2014;53(2):186–194.
- National Comprehensive Cancer Network. Guidelines clinical practice guidelines in oncology- breast cancer version 2. National Comprehensive Cancer Network, Plymouth Meeting; 2017.
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–30.
- World Health Organization. Proposal for the inclusion of trastuzumab in the who model list of essential medicines for the treatment of her2-positive breast cancer. World Health Organization, Geneva; 2012.
- Chan AL, Leung H, Lu CL, et al. Cost-effectiveness of trastuzumab as adjuvant therapy for early breast cancer: a systematic review. Ann Pharmacother. 2009 Feb;43(2):296–303.
- Petrou P. Looking for Her (2+): A systematic review of the economic evaluations of Trastuzumab in early stage HER 2 positive breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2019 Apr; 19(2):115–125.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)—Explanation and elaboration: A report of the ISPOR health economic evaluations publication guidelines good reporting practices task force. Value Health. 2013;16:231–250.
- Cooper N, Coyle D, Abrams K, et al. Use of evidence in decision models: an appraisal of health technology assessments in the UK since 1997. J Health Serv Res Policy. 2005 Oct;10(4):245–250.
- Fund IM. Implied PPP conversion rate - National currency per international dollar international monetary fund 2018 [cited 2018 Sept 15]. Available from: https://www.imf.org/external/datamapper/PPPEX@WEO/OEMDC/ADVEC/WEOWORLD
- Development OfEC-oa. Exchange rates - total, national currency units/US dollar, 2000 – 2017: organisation for economic co-operation and development 2000 – 2017 [ cited 2018 Sep 14]. Available from: https://data.oecd.org/conversion/exchange-rates.htm
- Group TWB. Consumer price index (1960-2017) - international monetary fund, international financial statistics and data files: the world bank group; 2018 [cited 2018 Jul]. Available from: https://data.worldbank.org/indicator/FP.CPI.TOTL
- Bank TW. World bank country and lending groups: the world bank; 2018 [cited 2018 Sept 17]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- Neyt M, Albrecht, J, Cocquyt V. An economic evaluation of herceptin in adjuvant setting: the breast cancer international research group 006 trial. Ann Oncol. 2006;17(3):381–390.
- Dedes K, Szucs TD, Imesch P, et al. Cost-effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a model-based analysis of the HERA and FinHer trial. Ann Oncol. 2007;18(9):1493–1499.
- Garrison LP Jr, Lubeck D, Lalla D, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007 Aug 1;110(3):489–498.
- Kurian AW, Thompson R, Gaw AF, et al. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007 Feb 20;25(6):634–641.
- Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol. 2007;25(6):625–633.
- Norum J, Olsen J, Wist EA, et al. Trastuzumab in adjuvant breast cancer therapy. A model based cost-effectiveness analysis. Acta Oncol. 2007;46(2):153–164.
- Neyt M, Huybrechts M, Hulstaert F, et al. Trastuzumab in early stage breast cancer: a cost-effectiveness analysis for Belgium. Health Policy. 2008;87(2):146–159.
- Shiroiwa T, Fukuda T, Shimozuma K, et al. The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat. 2008 Jun;109(3):559–566.
- Skedgel C, Rayson D, Younis T. The cost-utility of sequential adjuvant trastuzumab in women with Her2/neu-positive breast cancer: an analysis based on updated results from the HERA trial. Value Health. 2009 Jul-Aug;12(5):641–648. .
- Van Vlaenderen I, Canon J, Cocquyt V, et al. Trastuzumab treatment of early stage breast cancer is cost-effective from the perspective of the Belgian health care authorities. Acta Clin Belg. 2009 Mar-Apr;64(2):100–112.
- Macedo A, Monteiro I, Andrade S, et al. [Cost-effectiveness of trastuzumab in the treatment of early stages breast cancer patients, in Portugal]. Acta Med Port. 2010 May-Jun;23(3):475–482.
- Hall PS, Hulme C, McCabe C, et al. Updated cost-effectiveness analysis of trastuzumab for early breast cancer: a UK perspective considering duration of benefit, long-term toxicity and pattern of recurrence. Pharmacoeconomics. 2011 May;29(5):415–432.
- Hedden L, O’Reilly RS, Lohrisch C, et al. Assessing the real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu positive breast cancer. Oncologist. 2012;17(2):164–171.
- Lang HC, Chen H, Chiou TJ, et al. The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu-positive early breast cancer in Taiwan. J Med Econ. 2016 Oct;19(10):923–927.
- Leung W, Kvizhinadze G, Nair N, et al. Adjuvant Trastuzumab in HER2-positive early breast cancer by age and hormone receptor status: a cost-utility analysis. PLoS Med. 2016 Aug 9;;13(8):e1002067.
- Seferina SC, Ramaekers B, de Boer M, et al. Cost and cost-effectiveness of adjuvant trastuzumab in the real world setting: A study of the Southeast Netherlands breast cancer consortium. Oncotarget. 2017;8(45):79223–79233.
- Chen W, Jiang Z, Shao Z, et al. An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health. 2009 Nov-Dec;12(Suppl 3):S82–4.
- Buendía JA, Vallejos C, Pichón-Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomedica. 2013 Jul-Sep;33(3):411–417.
- Aboutorabi AHM, Ghaderi H, Salehi M, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant treatment for early breast cancer. Glob J Health Sci. 2014 Aug 14;;7(1):98–106.
- Pichon-Riviere A, Garay O, Augustovski F, et al. Implications of global pricing policies on access to innovative drugs: the case of trastuzumab in seven Latin American countries. Int J Technol Assess Health Care. 2015 Jan;31(1–2):2–11.
- Ansaripour A, Uyl-de Groot CA, Redekop WK. Adjuvant trastuzumab therapy for early HER2-positive breast cancer in Iran: a cost-effectiveness and scenario analysis for an optimal treatment strategy. Pharmacoeconomics. 2018;36(1):91–103.
- Kongsakon R, Lochid-Amnuay S, Kapol N, et al. From research to policy implementation: trastuzumab in early-stage breast cancer treatment in Thailand. Value Health Reg Issues. 2018;18:47–53.
- World Health Organization. Essential medicines selection: trastuzumab. World Health Organization, Geneva. (cited 2020 Aug 24). 2013.
- World Health Organization. Out-of-pocket expenditure as a percentage of total expenditure on health 2014 [cited 2020 Aug 24]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/out-of-pocket-expenditure-as-a-percentage-of-total-expenditure-on-health
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015 March 14;385(9972):977–1010.
- Mustacchi G, Puglisi F, Molino AM, et al. Observational study on adjuvant trastuzumab in HER2-positive early breast cancer patients. Future Oncol. 2015;11(10):1493–1500.
- Barbier L, Declerck P, Simoens S, et al. The arrival of biosimilar monoclonal antibodies in oncology: clinical studies for trastuzumab biosimilars. Br J Cancer. 2019;121(3):199–210.
- Deloitte. Winning with biosimilars: opportunities in global markets 2015 [ cited 2020 August 24]. Available from: https://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-biosimilars-whitepaper-final.pdf
- Debiasi M, Polanczyk C, Ziegelmann P, et al. Efficacy of anti-HER2 agents in combination with adjuvant or neoadjuvant chemotherapy for early and locally advanced HER2-positive breast cancer patients: a network meta-analysis. Front Oncol. 2018;8:156.