ABSTRACT
Introduction
Biosimilars have improved access to biologic medicines; however, historical thinking may jeopardize the viability of future markets.
Areas covered
An expert panel of eight diverse European stakeholders provided insights about rethinking biosimilars and cost-savings, reducing patient access inequalities, increasing inter-market equity, and improving education. The insights reported here (Part 2) follow a study that provides perspectives on leveraging the holistic benefits of biosimilars for market sustainability based on independent survey results and telephone interviews of stakeholders from diverse biosimilar markets (Part 1). Directional recommendations are provided for payers.
Expert Opinion
The panel’s market maturity framework for biosimilars has three stages: ‘Invest,’ ‘Expand’ and ‘Harvest.’ Across market stages, re-thinking the benefits of biosimilars beyond cost-savings, considering earlier or expanded access/new indications, product innovations, and re-investment of biosimilar-generated cost-savings should be communicated to stakeholders to promote further engagement. During ‘Expand’ and ‘Harvest’ stages, development of efficient, forward-looking procurement systems and mechanisms that drive uptake and stabilize competition between manufacturers are key. Future biosimilars will target various therapy areas beyond those targeted by existing biosimilars. To ensure a healthy, accessible future market, stakeholders must align their objectives, communicate, collaborate, and coordinate via education, incentivization, and procurement, to maximize the totality of benefits.
1. Introduction
The use of biologic medicines, biotherapeutics, or ‘biologics’ is well established in Europe for the treatment of severe and life-threatening diseases across several therapy areas, including oncology, rheumatology, gastroenterology, dermatology, supportive care, diabetes, and fertility. Due to their targeted actions, biologics have the potential to improve the management of chronic and/or life-threatening diseases with relatively fewer off-target side effects than previous generations of small molecule therapeutics with low or variable specificity [Citation1]. Although biologics have irrevocably changed treatment landscapes in some therapeutic areas, access to these treatments has been variable. This means the benefits of biologics, both in terms of patient outcomes and affordability, have not reached all patients. It was thought that after expiration of exclusivity rights, follow-on products (as sometimes referred to in the United States and Canada) – here called biosimilars – might improve accessibility and affordability as a result of competition in the market. A biosimilar is a biological medicine highly similar to another already approved biological medicine (the ‘reference medicine’) [Citation2]. Biosimilars are approved according to the same standards of pharmaceutical quality, safety and efficacy that apply to all biological medicines [Citation2]. Because they are generally available at a lower cost (given, among other things, the lower costs of research and development) making them more affordable, they are increasingly attractive treatment options by which to treat larger patient populations. Biosimilars can potentially be used across a wide range of diseases, in more treatment settings, for wider target populations (if restricted previously) and earlier in the treatment lifecycle into the foreseeable future.
Biosimilars have been marketed in Europe since 2006, following approval of biosimilar recombinant human growth hormone by the European Medicines Agency (EMA). In 2013, when the first major monoclonal antibody (mAb) loss of patent protection (market exclusivity) event occurred in some European countries, biosimilar infliximab was introduced in Europe. This provided an opportunity for healthcare systems to treat patients with its lower-cost biosimilar [Citation3]. Thereafter, biosimilar versions of many biologic therapies have been developed. Multiple studies have established the clinical equivalence of biosimilars to reference products in terms of their safety, efficacy, and immunogenicity profiles [Citation3–5].
The acceptance of biosimilars continues to grow, and in the absence of ongoing significant debate we appear to be at the beginning of a new era in which both the market dynamics and possible outcomes show unprecedented promise for biosimilars. Previously (in Part 1), we focused on the past, highlighting the benefits and learnings about biosimilars garnered from European countries since their launch in the marketplace [Citation6]. In this report, we offer perspectives that focus on aspects relevant for payers in the medicines market. We explore the future of biosimilars, having the benefit of hindsight from past reported experiences across a variety of therapeutic areas and European markets.
This perspective paper delves into ways in which the holistic benefits of biosimilars can be expanded, harnessed, and maintained into the future. We consider the evolving competitive landscape, new market entrants, long-term impacts of COVID-19, and the likely goals of key stakeholders across markets.
2. Body of the paper
2.1. General considerations
This report is Part 2 of a two-part study that provides expert perspectives on the previously published Part 1 of the study [Citation6]. The study design and conduct of the survey in Part 1 were previously described in detail [Citation6]. Briefly, survey respondents were clinicians, pharmacists, and payers from across six European markets (Czech Republic, France, Germany, Italy, Sweden, and the UK) [Citation6]. In this perspective report, an expert panel of eight diverse European stakeholders considered the results of the survey previously reported in Part 1 of the study [Citation6]. With a focus on the future, the expert panel provided their perspectives and insights on the following topics that they reached through consensus discussions:
Rethinking biosimilars and cost-savings
The role biosimilars play in maintaining innovations and improving quality of care, thereby further improving patient outcomes
Reducing inequality in patient access, and increasing equity between markets, and
Improving education based on market needs.
A ‘market maturity framework’ devised by the expert panel is the tool used to provide directional recommendations or considerations for key stakeholders across European and other markets globally (). In this context the same country can be in different stages of the framework for different biologic molecules. Where applicable, published articles about biosimilars are used in support of the perspectives presented.
Figure 1. The three stages of the market maturity framework.a
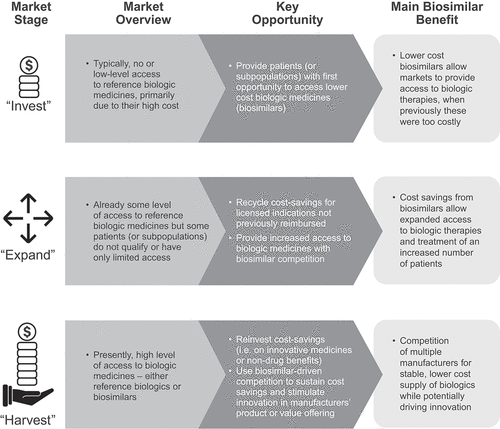
The expert panel comprised clinicians, pharmacists, policy advisors, and health economists from diverse countries, markets, and disciplines, who shared a multitude of perspectives with respect to biosimilar uptake and healthcare system structure. Jorge Mestre-Ferrandiz, Economics and Policy Consultant; Josef S. Smolen, Rheumatologist; Silvio Danese, Gastroenterologist; Matti S. Aapro, Oncologist; Arnold G. Vulto, Hospital Pharmacist/Policy Advisor; Paul Cornes, Policy Advisor, Pharma Economist, and Oncology Clinician; Margaret Kyle, Economist/Health Economist; and Marcin Czech, Policy Advisor and Health Economics and Outcomes Research Expert contextualized the survey results in Part 1 [Citation6] and provide prospective insights here in Part 2.
2.2. Rethinking biosimilars and cost-savings: the need for a shift in perspective
The historical perspective explored in Part 1 of this two-part review reported highlights of the cost-savings related to biosimilar competition and various ancillary downstream benefits of recycling cost-savings [Citation6]. However, past observations of biosimilar-generated cost-savings – often characterized by one-time, non-recurrent or incremental cost-savings – are likely to look considerably different to those in the future. Therefore, underpinning future decision-making based largely on a legacy mind-set could hinder important uptake drivers for the ongoing advancement of biosimilars. Three contemporary influential factors suggest the need for a shift in perspective during prospective decision-making by key stakeholders. First, the magnitude of potential future cost-savings is variable across countries, health systems (e.g. in- or outpatients markets) and disease areas. Second, the next wave of anticipated biosimilars will also target a variety of therapy areas beyond those targeted by existing biosimilars. Third, there is a medium- to long-term impact of the COVID-19 pandemic on healthcare budgets and changes to practice behaviors. Lastly, an additional consideration that should not be novel to stakeholders, but which they should be encouraged to remain mindful of, is that cost-savings are not the only benefit biosimilars can offer.
2.3. Magnitude of potential future cost-savings
Within a biosimilar market, the key stages of the market maturity framework – Stage 1: ‘Invest,’ Stage 2: ‘Expand,’ and Stage 3: ‘Harvest;’ as discussed in Part 1 – will influence potential future cost-savings [Citation6]. The ‘Invest’ stage refers to markets in which access to a biologic is restricted and the cost-savings generated are minimal because of current low-level utilization of the biologic. Under these circumstances, the entry of biosimilars into existing markets with reference products can result in two scenarios. Firstly, the lack of incentive for the relevant stakeholders (e.g. policy makers and budget holders) to drive biosimilar uptake via the implementation of new policies, procurement systems, and other mechanisms that can divert the focus to the holistic value of biosimilars. This can entail expanding or increasing patient access with a negative impact on the budget in the face of additional administration complexity and associated costs. Secondly, if access to a reference biologic is negligible and the entry of biosimilars cannot generate cost-savings, the lower acquisition cost of biosimilars (or price decrease of reference products) may make for an effective investment when the focus is to grant access to improve patient outcomes.
The ‘Expand’ stage of the maturity framework refers to markets that have received a level of access to a reference biologic before the entry of biosimilars, which subsequently lowers acquisition costs for the biosimilars and/or reference products. The magnitude of future cost-savings is tied to the effectiveness of the instituted policies and mechanisms (each directed to one or multiple agents involved), as well as biosimilar competition. In particular, policy changes can include enforced price reductions for biosimilars, speed of an administrative process, the creation of competitive pressure via the implementation of procurement methods, such as national level tenders, prescription quotas, benefit sharing systems, and proactive plans for the utilization of generated cost-savings. Although cost-savings are conceivably the main driver, there may be a neutral impact on the budget and stakeholders should ideally be amenable to the benefits attainable beyond cost savings alone.
The ‘Harvest’ stage of market maturity is reached when most patients who may benefit from a biologic medicine already have access to it because they are generally reimbursed by their healthcare systems. As such, further price evolution is limited, and lower incremental cost-savings must be expected in conjunction with the protection and reinvestment of savings within the healthcare system. If savings occur initially and rarely thereafter, they will be key in addition to savvy procurement mechanisms designed to ensure reliable, long-term, and stable competition. The ‘Harvest’ stage optimizes the opportunity for payers and decision makers to plan to improve patient outcomes by leveraging biosimilar medicines as drivers of value package offerings and product innovation, the latter of which can lend support to the provision of next-generation innovative therapies [Citation7]. This stage of market maturity would support a healthy and sustainable biosimilar industry over the medium and long term.
2.4. The next wave of biosimilars
Across European markets, it has been estimated that by 2029 the total value of biologic medicines faced with loss of exclusivity will more than triple the 2020 value (based on sales volume) and reach approximately €9 billion, as the loss of market exclusivity for many biologics takes effect () [Citation8]. In addition to some of the high-value biologics (e.g. infliximab, adalimumab, and rituximab) there are those developed to target relatively small populations with rare diseases, which do not generate the same cost-savings (e.g. denosumab, eculizumab) [Citation9]. Under these circumstances, procurement via regional or national tendering across the board, focusing solely on reducing costs, and replete with all its associated administrative expenses, will not be conducive to optimizing cost-savings, and consequently the magnitude of cost savings is likely to be modest [Citation10]. Although it is predicted that up to ~120 distinct biosimilars will enter the marketplace in the next decade beginning around 2024, expiring exclusivities do not automatically mean that biosimilars will be developed [Citation11,Citation12]. Also, biosimilars for the treatment of rare diseases may not yield the same cost-savings as for the first wave of widely used biologic products [Citation10]. Therefore, payers and policy-/decision-makers will need to develop innovative, and efficient procurement methods to drive price reductions beyond an initial large, short-term influx of savings generated predominantly from a small number of high-value products. Markets use various tendering procedures to procure biologics that are no longer patent-protected and biosimilars. In a best-case scenario, tenders should be designed based on market maturity to maximize timely competition using strategic approaches that safeguard long-term sustainability, including contract award criteria that are transparently defined and not driven solely by price, or limited by a single supplier in the market [Citation13,Citation14].
Figure 2. Estimated value of biologic products faced with loss of exclusivity in Europe (2010‒2029). (Reproduced from, the impact of biosimilar competition in Europe, Troein, P., Newton, M., Scott, K. © IQVIA Inc. 2020; by permission of IQVIA Inc.) [Citation8].
![Figure 2. Estimated value of biologic products faced with loss of exclusivity in Europe (2010‒2029). (Reproduced from, the impact of biosimilar competition in Europe, Troein, P., Newton, M., Scott, K. © IQVIA Inc. 2020; by permission of IQVIA Inc.) [Citation8].](/cms/asset/f92edf7e-3bb7-4dec-a86c-48443e6cbd9a/ierp_a_2310684_f0002_b.gif)
2.5. Healthcare in a post-covid-19 economy
Unpredictable factors, such as the COVID-19 pandemic and its associated costs, can erode pharmaceutical budgets, requiring policy makers to remain vigilant about the resiliency of healthcare systems. For example, it may be necessary to redirect certain spending to accommodate shifts aimed at the digitization of healthcare and the provision of services outside of programs in the hospital setting to improve the maneuverability of healthcare providers and hospitals under such circumstances. In the face of some stark choices, decision makers would do well to remain mindful of the holistic benefits that reference biologics and biosimilars offer beyond cost-savings. This could provide the impetus to explore further opportunities for low-cost treatment options and to generate cost-savings through biosimilar medicines.
2.6. Further improving patient outcomes
To support future decision making as it relates to biosimilars, the panel of experts explored how upcoming competition may evolve to improve patient outcomes based on each market’s characteristics, position in the maturity framework, and the specific goals of stakeholders and decision makers.
The key opportunities to be weighed by policy-/decision-makers in ‘Invest’ markets, where access to reference biologics is either low or non-existent due to the high cost of these medicines, are two-fold: (i) to provide patients with expanded/increased access to biologic medicines despite minimal cost savings; this investment in better patient wellbeing (reduced morbidity) is one of the holistic benefits biosimilars can deliver beyond cost reductions, given the minimal budget impact linked to additional administration and/or the expense required for policy changes and (ii) to expand the limited access to biologic medicines (reference or biosimilar) in the absence of any cost savings as an investment in patient outcomes (better health and lower future costs for disease worsening). When markets with some access to biologics can attain lower acquisition costs via biosimilar competition, patient access will be increased or expanded with budget neutrality. This would hinge on cost-savings related to biosimilars being recycled within the same healthcare system, ideally within the same therapeutic area/department where they were generated, to maximally incentivize clinicians and their colleagues, rather than funneling cost-savings into other budgets or the broader system. In markets where patients have not had access to specific reference biologics, payers can decide to accept the budget impact and provide lower-cost biologics as an investment opportunity, so improved patient outcomes in a sizable patient population may be realized with a modest budget impact.
At the ‘Expand’ market stage, there is access to biologics and once biosimilar competition has already delivered significant cost-savings that have been appropriately recycled within the healthcare system to further improve patient outcomes, there are some additional strategies that can be adopted when future objectives are the priority and payers are aware of opportunities to improve the effectiveness of treatment. Extending access to subpopulations (for example, those who may benefit from earlier access to a treatment not previously reimbursed until symptoms become more severe) can confer a patient benefit [Citation15]. In more complex healthcare systems, payers may need to be intentional in their approach to reinvest cost-savings into the same therapeutic area in which they were generated to expand access. With collaboration and communication between all the key stakeholders to ensure alignment with respect to goals, incentives and behaviors, the opportunity for success among clinicians, managers, and patients can be enhanced.
For biosimilar markets in the ‘Harvest’ stage of market maturity, most patients who benefit from biologic medicines already have access to them. The greatest opportunities to improve patient outcomes at this stage are by harvesting savings to reinvest (i.e. in staff, new equipment or the provision of more services) that indirectly benefit patients as the overall standard of care is elevated, or driving innovation through reinvesting cost-savings to enable access to innovative medicines. In England, the National Health Service (NHS), the UK regulatory agency, and the National Institute for Health and Care Excellence (NICE) work in partnership to provide patients, for whom reimbursement decisions could be delayed [Citation16], with rapid, early access to novel cancer therapies using a special Cancer Drugs Fund [Citation17] at an annual cost of £340 M [Citation18]. Although the financing of this fund has not been directly attributed to savings from biosimilar competition following medicine patent expirations, it illustrates how cost-saving could be reinvested to support funding within other sectors of national healthcare systems in a bid to improve patient outcomes. NHS England also supports an Innovative Medicines Fund to provide faster access for patients to non-cancer drugs [Citation19]. Recently, a similar mechanism has been implemented in Poland targeting rare diseases and oncology, therapeutic areas in which biologic medicines are used widely. The Medical Fund is dedicated to cover the financing of high clinical value and highly innovative medicinal products [Citation20].
2.7. Maintaining innovation
Biologic competition in the past led to innovations in products and value offerings by manufacturers; although, they have often gone unnoticed as a key benefit that biosimilars offer to patients and healthcare systems. For instance, the etanercept biosimilar SB4 was associated with fewer injection site reactions and lower immunogenicity compared with the reference product with similar efficacy in a comparative study conducted in patients with moderate-to-severe RA [Citation21]. This difference has been ascribed to innovation in terms of the formulation since SB4 does not contain L-arginine and the needle shield is latex free [Citation22]. These benefits were most applicable to decision makers in more mature markets (i.e. Expand and Harvest). As the potential for further price reductions of the available biosimilars will lessen in the future, exploration of other methods to safeguard the value of biosimilars in the marketplace are necessary. With healthy competitive dynamics and improved stability, it is envisioned that biosimilar manufacturers will be attracted to making long-term investments capable of supporting innovations, although due to manufacturer heterogeneity, a one-size-fits-all approach will not suffice. Procurement mechanisms that allow for multi-winner national tendering or several subnational tenders with weighted tender criteria (as in France), not solely linked to price, show promise in terms of encouraging more patient support, or care management programs with the potential to stimulate innovative responses from manufacturers or criteria that expands the range of treatable patients to include pediatrics, for example [Citation13]. The impetus for product innovations and the provision of value-added services may become even more attractive to payers and decision makers because of the COVID-19 pandemic. Consequently, there is increased interest and potential value in reconfiguring the delivery of services to facilitate care outside of hospital settings (e.g. subcutaneous formulations able to be administered in homecare settings and providing home care) as well as innovating infusion products so they take less time to administer.
It will be important for physicians to be aware of and to prescribe biosimilars in order for markets to achieve cost-savings from biosimilar competition that support and stimulate innovative future clinical research for novel indications or approaches, an example being first-line treatment of pediatric inflammatory bowel disease (IBD, both Crohn’s disease and ulcerative colitis) with biosimilar infliximab [Citation15]. Furthermore, reduced acquisition costs may allow for innovative treatment approaches to be explored, such as combination therapy or earlier treatment, which may not have been possible before biosimilars due to the higher costs of reference biologics.
2.8. Reducing inequality and increasing equity between markets
Historical evidence presented in Part 1 of this two-part review describes significant variability in the magnitude of price decreases for biologic medicines and the uptake of biosimilar medicines across European markets [Citation6]. Based upon lessons from the past, there are several actions that payers and policy-/decision-makers could take to reduce the level of inequality and increase parity among European countries. Firstly, they can ensure a common understanding of the biological and clinical equivalence of biosimilars to reference biologics across European markets. This is important because in some European countries, skepticism around biosimilar clinical equivalence persists [Citation23], despite a wealth of literature that supports switching [Citation3–5], including a joint statement from the EMA and Heads of Medicines Agency endorsing the interchangeability of biosimilar medicines approved in the European Union (EU) and their reference medicine or with an equivalent biosimilar [Citation24]. Approximately half of the countries’ regulatory agencies do not have information supportive of biosimilars on their websites [Citation25], impacting (negatively) on their adoption. Stakeholders in more mature stages of market development may get additional traction by seeking to support and promote their experience with biosimilars to markets where this discussion is unresolved, and when reinforcing the importance of effective communication, collaboration, and coordination. Several physician associations have published position papers on biosimilars including the European Society for Medical Oncology (ESMO) [Citation26], and the European Crohn’s and Colitis Organisation (ECCO) [Citation27]. Similarly, provision of informative literature in various languages has been attempted by some patient associations, with excellent examples including Digestive Cancers Europe [Citation28] and the International Alliance of Patients’ Organizations (IAPO) [Citation29]. Stakeholders could also aim to increase awareness of the wide-ranging benefits that biosimilars have to offer and support the development of policy, procurement mechanisms, and uptake incentives designed to maximize the number of benefits captured. This includes demonstrating how competition between biosimilar and reference biologic manufacturers can be harnessed to minimize the budget impact of investment in previously difficult-to-access treatments. Finally, stakeholders in mature biosimilar markets should be cognizant of the wider impact of their decision making on patient access in other countries and strive to support peers in such markets where decision making is influenced by supranational decision making. This may include ensuring that Health Technology Assessments (HTAs), reimbursement decisions, and clinical guidelines are updated in a timely fashion upon the entrance of biosimilar competition, as well as supporting the development of HTA processes, where required.
2.9. Improving education based on market needs
According to stakeholders, the most frequently reported driver of change to improve access to biologics is awareness of biologic drugs [Citation6]. Education is a key uptake driver across markets, regardless of stage in the maturity framework. Educating medical and life science undergraduates and postgraduates about the cost-effective prescribing of biologics, including biosimilars, should not be overlooked [Citation30]. Payers need to invest in tailored educational efforts of healthcare professionals (HCPs) to meet specific market needs, as biosimilars for new indications will require the education of a different group of stakeholders (HCPs, payers and prescribers). An example of this in action is the Australian biosimilar hub [Citation31] that provides a wealth of information for HCPs, patients and their carers. A similar, ‘one-stop’ website representing biosimilar markets in European countries in their own language would be beneficial.
In markets where biosimilars have not yet reached wide levels of acceptance, there may be a need for further education on the clinical equivalence and safety of biosimilars. Again, position statements by health authorities [Citation24], physician organizations [Citation26,Citation27] and patient advocacy groups [Citation29] play an important supportive role. While these concepts are widely proven, regulatory bodies in at least half of the countries across Europe do not mention biosimilar equivalence in their resources, or refer to any additional educational materials supporting their use [Citation25]. As the biosimilars market continues to evolve, in areas such as oncology [Citation32], ophthalmology [Citation33], neurology [Citation34] and rare diseases [Citation35], stakeholders need to anticipate next steps within their market and encourage education to maximize benefits gained from biosimilar competition.
Education also remains vital in markets where biosimilars are already widely accepted and used. With movement within markets, such as new competitors entering and old products exiting, future discussions will likely include moving to multiple switching, including cross switching between biosimilars. In non-English speaking countries, not all physicians, payers, or patients will be able to participate in international conferences or read the latest publications, and this could lead to disparities in the acceptance of biosimilars and awareness of their benefits. For education to be effective, it is important for policy-/decision-makers and payers to communicate well, and to address this issue in ongoing educational campaigns and provide easy-to-access information in local languages. Ideally, information should be easily available in all countries in their own language. In all European markets, ongoing education on the wide and varied benefits of biosimilars is needed to ensure that the full potential of biosimilars continues to be realized. Although the EMA has provided excellent resources [Citation2] (with some available in the 23 official languages of the EU), countries should also produce their own educational materials that are directly relevant to the local patients and health system – and ensuring the material is adapted to the needs of the stakeholder being targeted. As the biosimilars landscape continues to evolve, it will be important for stakeholders to anticipate the future and invest in any educational campaigns that will support the ongoing generation of those benefits. This could start with the undergraduate education that all healthcare providers receive, given that doctors lack training related to new aspects of cost-effective prescribing and the biosimilar development paradigm [Citation30].
3. Discussion
3.1. Final recommendations for key stakeholders
The findings of this research have far-reaching implications for the various stakeholders across markets. Importantly, there is no ‘one-size-fits all’ framework that can be used to maximize capturing biosimilar generated benefits. Policy and investment decisions must be made based on consideration of specific – and preferably aligned – aspirations of the key stakeholders in the country and the ‘maturity’ of their respective biosimilar (sub)market, be that ‘Invest,’ ‘Expand’ or ‘Harvest’ stages. Within a jurisdiction, different levels of maturity may exist between markets. Stages of maturity are not entirely mutually exclusive: there may be differences in the stage of maturity across disease indications and patient groups, and some recommendations will be applicable across several stages of the maturity framework within a country.
Active coordinated collaboration between all stakeholder groups (patients, physicians, pharmacists, payers and pharmaceutical manufacturers) is needed to achieve a sustainable biosimilar market in the future [Citation36,Citation37]. This is a pertinent point, particularly among some payers who could underestimate the implications of the actions and policies they implement on the future of the biosimilar industry if they focus exclusively or myopically on cost-savings over the short-term, which will do little to contribute to keeping biosimilars as a viable alternative in the coming years. It is concerning given evidence that suggests the erosion of sustainable biosimilar markets can occur despite driving increased access to biologics. Where the biosimilars market is most established, payer-driven switching and single-winner tenders have been identified as a significant risk to long-term sustainability of the market [Citation12,Citation36,Citation37]. In the US, biosimilars are considered not to have achieved their full potential. Aspects such as delayed market entry of FDA-approved products, low utilization of biosimilars on the market, lack of price transparency of originators, the withdrawal of approved biosimilars, and originator manufacturers’ strategic responses on prices, have hampered competition and undermined trust at the prescriber and patient level [Citation38]. Moreover, not all originator biologics with expired exclusivities attract biosimilar competition, suggesting additional cost or other considerations have a bearing on biosimilar manufacturer strategic decision making in such instances [Citation12]. Not only can this harm competition, but it can undermine the trust of prescribers and patients. Ideally, policies predicated on a holistic approach, in more mature markets, will be more conducive to supporting market sustainability. The following recommendations, which may help to mitigate these factors, focus on the role of the payer.
3.2. Key points for payers
3.2.1. Invest stage markets
Along with the importance of implementing procurement policies that generate price reductions, it is also necessary to create a stable competitive market environment into the future. Payers with foresight will likely reframe their thinking about biosimilars so that important cost-savings are coupled with non-cost saving benefits, such as enabling increased access, including earlier use, or additional services (e.g. therapeutic drug monitoring, switching support and education initiatives) [Citation39]. An approach that maximizes the totality of benefits from biologic competition, for instance through increased access, may in turn ensure greater improvements in terms of patient outcomes, such as decreased serious (and costly) morbidity in the short term and better health in the future. This point is exemplified by the treatment of chemotherapy-related febrile neutropenia, when pegfilgrastim has shown superior benefits over filgrastim that stem from the longer dosing interval, in terms of achieving target dose intensity and reducing the incidence of febrile neutropenia [Citation40]. In this paradigm, biosimilar pegfilgrastim offers both adherence benefits versus filgrastim and economic benefits versus reference pegfilgrastim.
Payers that implement incentive mechanisms will better facilitate the capture of biosimilar-generated benefits beyond cost-savings. Benefit-sharing programs are examples of initiatives that generate tangible benefits that can be realized across stakeholders, including payers, HCPs and patients (e.g. when the savings made by hospitals are directed to resourcing nursing) [Citation41,Citation42]. Complementary initiatives include position statements from medical associations or scientific associations to promote confidence in implementation of switching, guidelines (and indicators of adherence), implementation of prescription target agreements, transparency with gainsharing to motivate switching, use of electronic prescribing systems to promote price awareness, and increased recognition of the recent EMA statement supporting the interchangeability of biosimilars with reference medicines [Citation24,Citation30].
3.2.2. Expand stage markets
An examination of the current procurement systems and mechanisms used to drive uptake and to determine whether they are appropriate for generating stable future competition between manufacturers should be within the remit of payers. This is particularly pertinent for the ‘next wave’ of biosimilars for rare diseases for which cost-savings are anticipated to be lower than first-wave biologic products with higher sales. Payers could also explore the financial flows of biosimilar-generated cost-savings within the healthcare system and optimize flow to maximize the potential benefits to patients or medical department facilities/staffing. Of critical importance is to publicize this information transparently and widely to improve visibility/tangibility for patients and prescribers [Citation30]. Furthermore, a concerted effort to maximize access to biologic treatments in all patient groups who could benefit from them would be useful. It may be prudent to revisit reimbursement/HTA decisions for biologic molecules after biosimilars become available [Citation42,Citation43] and publicly communicate any changes to reimbursement decisions due to biosimilars to ensure policy makers in other countries are aware of such changes [Citation42,Citation44–46].
3.2.3. Harvest stage markets
Overall, the recommendations in the ‘Expand’ stage of market maturity are all broadly applicable to markets in the ‘Harvest’ stage of maturity too, with additional recommendations for payers. Firstly, prepare a procurement system that will be effective at generating both stable competition and cost-savings over the next major period of biologics loss of exclusivity (2025‒2030); that is, look to invest in procurement contracts beyond year-end that will allow the system to adapt in response to market dynamics and potentially increase the likelihood of long-term gains over 5 or more years. This system should also include biologics for rare diseases.
Secondly, payers who communicate the benefits of biosimilar-generated cost-savings to broader stakeholders (e.g. governmental budget holders) are more likely to ensure that pharmaceutical budgets retain growth elements. This may include the provision of incentives to ensure savings remain within the healthcare system, preferably allocated to pharmacotherapy as opposed to lost somewhere else in the overall system. This is a frequent complaint among physicians and patients, and it can compromise the willingness to prescribe biosimilars. But it will be important to ensure the savings are shared appropriately across all stakeholder groups in a transparent manner. It may also involve increasing the weighting of non-price winning criteria in tenders to simulate innovative manufacturer value offerings and product innovation. Indeed, such non-price criteria may be product-, service-, or patient-driven and represent a pertinent additional viewpoint on which to base decisions around selection of off-patent biologicals and biosimilars in clinical practice [Citation14]. For instance, the treatment of ulcerative colitis with infliximab, is an example of when biosimilar competition may have contributed to a change in a national prescribing guideline (NICE) [Citation47], which in turn led to expedited expanded access to include adult patients with moderate as well as severe disease [Citation48]. Furthermore, superiority of first-line biosimilar infliximab (CT-P13: Inflectra®, Remsima®) versus conventional therapy was recently reported for treatment of pediatric IBD [Citation15]. A significant advance was also made for approximately 25,000 British patients with rheumatoid arthritis when NICE published guidance recommending that those with moderate disease not responding to conventional therapies should have the option of biosimilar tumor necrosis factor inhibitor treatment made available to them, in addition to patients with severe disease [Citation49].
Finally, it is important to communicate to both the medical community and the public what happened with the savings that were ascertained, thereby proving that biosimilars are of benefit to society in general. Incentive programs that aim to generate savings that can ultimately be shared among stakeholders was noted as one of several key actions identified to maximize the potential of benefit-sharing programs across Europe [Citation41]. Indeed, such savings can be substantial: a study investigating utilization of biosimilars following a ‘best-value biological’ medicine initiative for adalimumab and etanercept in Ireland reported €3.6 million raised as result of a gain-share incentive for reinvestment in patient care [Citation50]. In this regard, policy makers also have a duty to engage with payers in ‘Invest’ and ‘Harvest’ stage markets to share evidence of biosimilar-generated benefits (e.g. prevent costly disease worsening, improved quality of life) in addition to cost savings, for example, increased or enhanced patient access.
3.3. Study limitations
Our research has limitations. This report provides a consensus of our perspectives on recommendations for the future of the biosimilars market; however, we did not utilize Delphi methodology or other formal consensus methodology. Currently, there are no substantial prospectively collected market data to support some of the recommendations made. The prospective views reported here have been collected from several experts emanating from their respective fields of focus. Therefore, these findings should be perceived as presenting an expert opinion, with the purpose of providing perspectives on the future biosimilars market environment.
4. Conclusions
The future of the biosimilar market should not be taken for granted and payers have an important responsibility for its sustainability. The rising costs of drugs represent a public health problem, especially in oncology [Citation51], and the increased use of biosimilars could help control drug spending [Citation52,Citation53]. Already, we see signs of erosion that threaten the future of biosimilar markets. The heterogeneity we see in markets should be interpreted as a sign that some are underdeveloped – with poor patient access to biologics or biosimilars – while in other jurisdictions we see strong price-driven markets that may not be sustainable. Payers have a duty to act on behalf of patients and to think responsibly, beyond the current book-year, to optimize the wellbeing of their patients, given the budget constraints they face. Improving the access and use of biosimilars can be an excellent tool for that purpose.
5. Expert opinion
Since 2006 the EMA has approved 106 biosimilars, of which there are now (November 2023) 84 available on the market as some products were withdrawn due to market reasons. Over the years, the discussion around acceptance of biosimilars has evolved from concerns about quality, efficacy, safety, and immunogenicity to, finally, concerns about interchangeability; however, all these (perceived) issues have been resolved. Kurki et al. [Citation54] published an extensive review on the safety and immunogenicity of all licensed biosimilar monoclonal antibodies and fusion proteins in the European Union. This analysis spanning over 1 million patient-treatment years of safety data demonstrated there were no clinically relevant differences between biosimilars and their respective reference products, suggesting that both the industry and regulators have set a high standard for drug development.
When looking at patient access to these equally efficacious and safe versions of reference products, we observe a less successful story. Within Europe there is considerable inequity regarding how patients benefit from the usually lower-cost biosimilar version of biological medicines. Countries that had a high per capita use of biological medicines showed a faster acceptance of biosimilars, as the health system was aware of the potential for cost savings. For countries with a low usage of biological medicines, even the lower cost of biosimilars could mean an increase in drug spending. However, this can be seen as an investment in patient health, by preventing higher costs later in the course of a disease. The conclusion is that there is no one solution for improving patient access to biological medicines and that a more tailormade approach is needed. For this reason, we have developed a model to address these differences in market structure, differentiating between 1) countries that must invest in a better health system by improving patient access to biological therapies 2) countries that can expand biosimilar competition to further improve patient outcomes and 3) countries with mature biosimilar markets, where health systems can harvest biosimilar benefits in various ways.
Consequently, there is a need to rethink and shift perspectives on biosimilars and their from one-size-fits all to a more individualized approach. Loss of exclusivity affects both high- and low-market value biologics, including those for rare diseases where competition generates small savings. Future decision-making based on a legacy mind-set (usually top-down) could hinder the uptake drivers for the ongoing advancement of biosimilars. The current market is dominated by competing interests of stakeholders and short-term policies of health authorities and payers. It can be envisioned that price reductions will lessen in the future. Driven by competition, manufacturers will likely invest in product innovations to safeguard biosimilars’ value. Payers will need innovative procurement methods to reduce prices beyond initial large, short-term savings from a few high-value products. Unpredictable costs, such as those resulting from the COVID-19 pandemic, can erode drug budgets, so policymakers must remain vigilant to the resiliency of healthcare systems. Furthermore, payers and policymakers must take various actions to reduce the level of inequality and increase parity.
The future of the biosimilar market is not guaranteed, with some signs of erosion already evident. We have to accept that given the high cost of biosimilar development there must be room for developers to recoup their investment. Here payers have a responsibility toward achieving a healthy competitive market and the sustainability of affordable drug treatment. The heterogeneity in biosimilar access across markets is a sign that some are underdeveloped while others are unsustainably price driven. Payers have a duty to think responsibly, beyond the current book-year, to optimize patients’ wellbeing, given the budget constraints they face. Improving access to and use of biosimilars may be an excellent tool for this purpose.
Article highlights
Biologics can improve the management of chronic and/or life-threatening diseases; however, access to biologics is variable, which means that not all patients have benefited from the improved outcomes and affordability of biologics.
Biosimilars are generally more affordable than biologics, providing healthcare systems with lower-cost alternatives to biologics with which to treat patients. However, despite the cost-savings, the future of the biosimilars market is not guaranteed and payers bear a large responsibility for its sustainability.
There is no ‘one-size-fits all’ framework to maximize capturing biosimilar generated benefits. Policy and investment decisions must be made based on specific aspirations of the country’s key stakeholders (alignment of their respective interests) and the ‘maturity’ of their biosimilar market and usage of the specific biologic.
Along with the importance of implementing procurement policies that generate price reductions, a stable competitive market environment for the future is needed.
Payers should examine the current procurement systems and mechanisms used to drive uptake and determine whether they are appropriate for generating stable future competition between manufacturers, particularly for the ‘next wave’ of biosimilars for rare diseases.
For the next major period of loss of exclusivity for biologic products (2025‒2030), there is a need for procurement systems that will generate stable competition and cost-savings; that is, investment in procurement contracts beyond year-end so the system can respond to market dynamics, potentially increasing the likelihood of long-term gains.
Compliance with ethical standards
Ethical approval was not applicable to this work as it was based on previously reported results from Part 1 [6]. Research in Part 1 was considered exempt from institutional research board review because it involved survey procedures and was conducted according to the following codes of conduct: BHBIA/MRS/ESOMAR/Data Protection Act. Informed consent was not applicable to this paper as it is a prospective report that makes recommendations for the future based on the biosimilar market maturity framework that was introduced in Part 1.
Declaration of interest
J Mestre-Ferrandiz declares an honorarium received from Pfizer for participation in the expert panel in support of the preparatory work upon which this manuscript is based.
M Czech declares a consulting/advisory role in AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Biogen, EliLilly, GSK, Janssen, MSD, Novartis, Pfizer, Sanofi, Takeda and UCB.
J S. Smolen has received grants for his institution from AbbVie, AstraZeneca, Janssen, Lilly, Novartis, and Roche; and honoraria for consulting or speaking engagements from AbbVie, Amgen, Astro, BMS, Celgene, Celltrion, Chugai, Gilead, ILTOO, Janssen, Lilly, MSD, Novartis-Sandoz, Pfizer, Roche, Samsung, Sanofi, and UCB.
P Cornes declares honoraria or support for attending meetings from Accord Healthcare, Amgen, Astro Pharma, European Association for Hospital Pharmacists, European Commission, Medicines for Europe/European Generics Association, Medscape, Mylan, Napp, Pfizer/Hospira, Sandoz and Teva.
M S Aapro declares a consulting/advisory role in Amgen, BMS, Daiichi Sankyo, Fresenius Kabi, G1 Therapeutics, Genomic Health, Helsinn Healthcare, Merck, Merck KGaA, Novartis, Pfizer, Pierre Fabre, Roche, Sandoz, Tesaro, and Vifor Pharma; speakers’ bureau at Accord Research, Amgen, Biocon, Dr Reed, Genomic Health, Helsinn Healthcare, Mundipharma, Novartis, Pfizer, Pierre Fabre, Roche, Sandoz, Taiho Pharmaceutical, Tesaro, and Vifor Pharma; and research funding from Helsinn Healthcare, Novartis, Pierre Fabre, and Sandoz.
S Danese declares consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, Applied Molecular Transport, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, EliLilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, Morphic, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB Inc., Vial, and Vifor Pharma; and lecture fees from Abbvie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, and Takeda.
S Deitch was a full-time employee of Charles River Associates when the work was conducted and was contracted by Pfizer in connection with the development of the manuscript.
H Tyldsley and W Foster are full-time employees of Charles River Associates and were contracted by Pfizer in connection with the development of the manuscript.
P Shah and M Latymer are full-time employees of and hold stock and stock options in Pfizer.
A G Vulto founded the KU Leuven Fund on Market Analysis of Biologics and Biosimilars following Loss of Exclusivity (MABEL Fund). He is involved in consulting, advisory work and speaking engagements for Accord Healthcare, Amgen, Biogen, Effik, Fresenius/Kabi, Medicines for Europe, Mundipharma, Novartis, Pfizer/Hospira, and Sandoz.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
All authors contributed to content development, writing, and critical review of the paper. M Latymer is the overall guarantor. All authors have read and agreed to the published version of the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Interactive summary
Download PDF (1.5 MB)Acknowledgments
Margaret Kyle, Professor of Economics, Centre d’économie industrielle (Cerna), Mines ParisTech, and a subject-matter expert, participated on the expert panel and endorsed the final survey report. Medical writing support was provided by Sue Reinwald, PhD, and Elyse Smith, PhD, of Engage Scientific Solutions and funded by Pfizer.
Data availability statement
Not applicable.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2024.2310684
Additional information
Funding
References
- Mócsai A, Kovács L, Gergely P. What is the future of targeted therapy in rheumatology: biologics or small molecules? BMC Med. 2014 Mar 13;12(43). doi: 10.1186/1741-7015-12-43
- European Medicines Agency. Biosimilar medicines: Overview. 2023 [cited 2023 Feb 2]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview
- Gimeno-Gracia M, Gargallo-Puyuelo CJ, Gomollón F. Bioequivalence studies with anti-TNF biosimilars. Expert Opin Biol Ther. 2019 Oct;19(10):1031–1043. doi: 10.1080/14712598.2019.1561851
- Chingcuanco F, Segal JB, Kim SC, et al. Bioequivalence of biosimilar tumor necrosis factor-α inhibitors compared with their reference biologics: a systematic review. Ann Intern Med. 2016 Oct 18;165(8):565–574. doi: 10.7326/M16-0428
- Singh I, Patel A, Patel R, et al. Pharmacokinetic and pharmacodynamic bioequivalence study of a pegfilgrastim biosimilar INTP5 in healthy subjects. Cancer Chemother Pharmacol. 2018 Aug;82(2):329–337.
- Mestre-Ferrandiz J, Czech M, Smolen JS, et al. Capturing the holistic value of biosimilars in Europe – part 1: a historical perspective. Expert Rev Pharmacoecon Outcomes Res. 2023;24(2):237–250. doi: 10.1080/14737167.2023.2297926.
- Cornes P. The innovation opportunity. 2021. [updated 2021 Jan; cited 2023 Jan 28]. Available from: https://www.pharmatimes.com/magazine/2021/januaryfebruary_2021/the_innovation_opportunity
- Troein P, Newton M, Scott K. The impact of biosimilar competition in Europe. 2020 [ updated 2020 Dec; cited 2023 Jun 27]. updated 2020 Dec: https://health.ec.europa.eu/system/files/2021-01/biosimilar_competition_en_0.pdf
- Generics and Biosimilars Initiative. Patent expiry dates for biologicals: 2018 update. 2019 [updated 2019 Feb 7; cited 2023 Feb 7]. Available from: http://gabi-journal.net/patent-expiry-dates-for-biologicals-2018-update.html
- Mestre-Ferrandiz J, Palaska C, Kelly T, et al. An analysis of orphan medicine expenditure in Europe: is it sustainable? Orphanet J Rare Diseases. 2019 Dec 11;14(1):287. doi: 10.1186/s13023-019-1246-7
- Niazi SK. The coming of age of biosimilars: a personal perspective. Biologics. 2022;2(2):107–27. doi:10.3390/biologics2020009
- IQVIA. Assessing the biosimilar void: achieving sustainable levels of biosimilar competition in Europe. 2023 [updated 2023 Oct; cited 2023 Dec 8]. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/assessing-the-biosimilar-void
- Barbier L, Simoens S, Soontjens C, et al. Off-patent biologicals and biosimilars tendering in Europe—a proposal towards more sustainable practices. Pharmaceuticals (Basel). 2021 May 24;14(6):499. doi: 10.3390/ph14060499
- Barbier L, Vandenplas Y, Boone N, et al. How to select a best-value biological medicine? A practical model to support hospital pharmacists. Am J Health Syst Pharm. 2022 Nov 7;79(22):2001–2011. doi: 10.1093/ajhp/zxac235.
- Jongsma MME, Aardoom MA, Cozijnsen MA, et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe crohn’s disease: an open-label multicentre randomised controlled trial. Gut. 2022 Jan;71(1):34–42.
- Wood EM, Hughes DA. The new and non-transparent cancer drugs fund. PharmacoEconomics. 2020 Jan;38(1):1–4. doi: 10.1007/s40273-019-00871-9
- NHS England. Cancer drugs fund list 2021. [ updated 2023 Jun 22; cited 2023 Jun 27]. Available from: https://www.england.nhs.uk/publication/national-cancer-drugs-fund-list/
- Hawkes N. New cancer drugs fund keeps within £340m a year budget. BMJ. 2018 Jan 29;360:k461. doi: 10.1136/bmj.k461
- NHS England. Innovative medicines fund. 2023 [cited 2023 Jun 27]. Available from: https://www.england.nhs.uk/medicines-2/innovative-medicines-fund/
- Krzyzanowski J. Poland: new bill on the medical fund amending certain reimbursement regulations. 2020. [ updated 2020 Jul 21; cited 2023 Jun 27]. updated: https://insightplus.bakermckenzie.com/bm/investigations-compliance-ethics/poland-new-bill-on-the-medical-fund-amending-certain-reimbursement-regulations
- Emery P, Vencovsky J, Sylwestrzak A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017 Jan;76(1):51–57.
- Girolomoni G, Feldman SR, Emery P, et al. Comparison of injection-site reactions between the etanercept biosimilar SB4 and the reference etanercept in patients with rheumatoid arthritis from a phase III study. Br J Dermatol. 2018 Mar;178(3):e215–e216.
- Shah-Manek B, Baskett A, Baynton E, et al. Perceptions of biosimilars across physician specialities in Europe. Value Health. 2018;21:S265. doi: 10.1016/j.jval.2018.04.1771
- European Medicines Agency. Biosimilar medicines can be interchanged. 2022 [updated 2022 Sep 19; cited 2023 Jun 27]. Available from: https://www.ema.europa.eu/en/news/biosimilar-medicines-can-be-interchanged.
- Barbier L, Vulto AG. Interchangeability of biosimilars: overcoming the final hurdles. Drugs. 2021 Nov;81(16):1897–903. doi: 10.1007/s40265-021-01629-4
- Schiestl M, Krendyukov A. The ESMO position paper on biosimilars in oncology: enhancing the provision of accurate education and information. ESMO Open. 2017;2(3):e000245. doi: 10.1136/esmoopen-2017-000245.
- Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis. 2017 Jan;11(1):26–34. doi: 10.1093/ecco-jcc/jjw198.
- Digestive Cancers Europe. Biosimilars education. 2021 [cited 2023 Jul 28]. Available from: https://biosimilars.education.digestivecancers.eu/
- International Alliance of Patients’ Organizations. The global voice for patient-centered healthcare. 2023 [cited 2023 Dec 8]. Available from: https://www.iapo.org.uk/biosimilars-toolkit
- Moorkens E, Vulto AG, Huys I. Biosimilars in Belgium: a proposal for a more competitive market. Acta Clin Belg. 2021 Dec;76(6):441–452. doi: 10.1080/17843286.2020.1761690
- GBMA Education. Biosimilar hub. 2022 [cited 2023 Jun 27]. Available from: https://biosimilarhub.com.au/
- Bachu RD, Abou-Dahech M, Balaji S, et al. Oncology biosimilars: new developments and future directions. Cancer Rep (Hoboken). 2022 Nov;5(11):e1720.
- Joszt L. Ophthalmologists see promise and peril, but lack familiarity, with biosimilars. American Journal of Managed Care. 2022 [updated 2022 Jul 19; cited 2023 Jun 27]. Available from: https://www.ajmc.com/view/ophthalmologists-see-promise-and-peril-but-lack-familiarity-with-biosimilars
- Aitken M, Tewary V, Abouzid A. Unlocking biosimilar potential: learnings from physicians across therapy areas. 2023 [updated 2023, Apr; cited 2023 Apr 28]. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/unlocking-biosimilar-potential/iqi-unlocking-biosimilar-potential-04-23-forweb.pdf
- Drelichman G, Castaneda-Hernandez G, Cem Ar M, et al. The road to biosimilars in rare diseases – ongoing lessons from gaucher disease. Am J Hematol. 2020 Mar;95(3):233–237.
- Vulto AG, Vanderpuye-Orgle J, van der Graaff M, et al. Sustainability of biosimilars in Europe: a delphi panel consensus with systematic literature review. Pharmaceuticals (Basel). 2020 Nov 17;13(11):400. doi: 10.3390/ph13110400
- Aitken M, Rodríguez I, Diamantara J, et al. Advancing biosimilar sustainability in Europe: a multi-stakeholder assessment. 2018 [updated 2018 Sep 4; cited 2023 Jun 27]. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/advancing-biosimilar-sustainability-in-europe.pdf?_=1671113499133
- Zhai MZ, Sarpatwari A, Kesselheim AS. Why are biosimilars not living up to their promise in the US? AMA J Ethics. 2019 Aug 1;21(8):E668–78.
- Dutta B, Huys I, Vulto AG, et al. Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2020 Apr;34(2):159–170. doi: 10.1007/s40259-019-00395-w.
- Cornes P, Gascon P, Vulto AG, et al. Biosimilar pegfilgrastim: improving access and optimising practice to supportive care that enables cure. BioDrugs. 2020 Jun;34(3):255–263.
- Barcina Lacosta T, Vulto AG, Turcu-Stiolica A, et al. Qualitative analysis of the design and implementation of benefit-sharing programs for biologics across Europe. BioDrugs. 2022 Mar 01;36(2):217–229.
- National Institute for Health and Care Excellence. Health technologies adoption programme. Introducing biosimilar versions of infliximab: Infectra and Remsima. 2015 [updated 2015 Jul 31;cited 2023 Jun 27]. Available from: https://www.biosimilars-nederland.nl/wp-content/uploads/2015/08/NICE-Adoption-Resource-Biosimilar-Infliximab-2015_07_31.pdf
- Moorkens E, Lacosta TB, Dawoud D, et al. A systematic literature review of gaps and challenges in value assessment of biosimilars: an ISPOR special interest group report. Value Health. 2023 Aug;26(8):1137–1144.
- Ascef BO, Lopes ACF, de Soarez PC. Health technology assessment of biosimilars worldwide: a scoping review. Health Res Policy Syst. 2020 Aug 26;18(1):95.
- Balijepalli C, Desai K, Yan K, et al. PNS278 a review of health technology assessment guidelines on the approval of biosimilars. Value Health. 2019;22:S810. doi: 10.1016/j.jval.2019.09.2178
- Mestre-Ferrandiz J, Towse A. What is the role of HTA for biosimilars? 2014 [updated 2014 Jan 4; cited 2023 Jun 27]. Available from: https://www.ohe.org/publications/what-role-hta-biosimilars/
- National Institute for Health and Care Excellence. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy. 2015 [ updated 2015 Feb 25; cited 2023 May 30]. updated: https://www.nice.org.uk/guidance/ta329/chapter/1-Guidance
- European Medicines Agency. Remsima. Summary of product charateristics. 2013 [ updated 2023 May 30; cited 2023 May 30]. updated: https://www.ema.europa.eu/en/documents/product-information/remsima-epar-product-information_en.pdf
- National Institute for Health and Care Excellence. NICE recommends several treatment options to help thousands with moderate rheumatoid arthritis. 2021 [ updated 2021 Jun 10; cited 2023 Jun 27]. updated: https://www.nice.org.uk/news/article/nice-recommends-several-treatment-options-to-help-thousands-with-moderate-rheumatoid-arthritis
- Duggan B, Smith A, Barry M. Uptake of biosimilars for TNF-α inhibitors adalimumab and etanercept following the best-value biological medicine initiative in Ireland. Int J Clin Pharm. 2021 Oct;43(5):1251–1256. doi: 10.1007/s11096-021-01243-0
- Bonetti A, Giuliani J. Implications of drugs with rebate in Europe. Lancet Reg Health Eur. 2021 Apr;3:100060. doi: 10.1016/j.lanepe.2021.100060
- Giuliani J, Bonetti A. The economic impact of biosimilars in oncology and hematology: the case of trastuzumab and rituximab. Anticancer Res. 2019 Jul;39(7):3971–3973. doi: 10.21873/anticanres.13552
- Giuliani J, Fiorica F, Albanese V, et al. Financial toxicity and cancer treatments: help from biosimilars – the explanatory case of bevacizumab. Eur J Cancer. 2021 Jan;143:40–42.
- Kurki P, Barry S, Bourges I, et al. Safety, immunogenicity and interchangeability of biosimilar monoclonal antibodies and fusion proteins: a regulatory perspective. Drugs. 2021 Nov;81(16):1881–1896.

