ABSTRACT
Objective
We describe the impact of acute myeloid leukemia (AML) diagnosis on workplace absenteeism and disability days among patients and their caregivers.
Methods
This retrospective study included adults with newly diagnosed AML (2009–2019) and adult caregivers of patients with newly diagnosed AML, identified from the US Merative™ MarketScan® Commercial Database. The Merative MarketScan Health and Productivity Management Database provided linked patient-level records of workplace absence and short-term (STD) and long-term disability (LTD) data. Endpoints included workplace absence, STD and LTD for patients and caregivers during 12 months pre-AML (baseline) and ≤3 years’ follow-up, and corresponding cost of work loss.
Results
Patient workplace absence decreased in the months post-AML diagnosis, but the number of STD and LTD leave days claimed increased significantly by sixfold and fourfold, respectively. The proportion of patients making STD leave claims increased within 4–5 months of diagnosis, while the proportion making LTD leave claims increased significantly starting from month 5. Caregiver workplace absence peaked in the first 2 months post-diagnosis and remained elevated versus baseline throughout the study.
Conclusion
AML diagnosis leads to workplace absenteeism and increased economic burden for patients with AML and their caregivers.
1. Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia in adults, with an age-adjusted incidence rate of 4.1 cases per 100,000 in the United States during 2016–2020, with approximately 20,380 newly diagnosed in 2023 [Citation1]. According to 2020 figures, >73,000 people in the United States were estimated to be living with an AML diagnosis [Citation1]. While the median age at AML diagnosis is 69 years, 40% of individuals are of working age (younger than 65) when they receive their diagnosis [Citation1]; this portion of the cancer patient population is identified as having excess financial burden compared with older patients with cancer [Citation2], and one in whom the likelihood of being in debt is significantly higher than in older individuals with cancer [Citation3], making the need to continue working, return to work, or both even more important.
For patients younger than 75 years with AML, especially those who are medically fit and with good functional status, treatment includes intensive chemotherapy with or without hematopoietic stem cell transplantation (HSCT) [Citation4], which require hospitalization [Citation5]. While intensive treatments are a key driver of the direct cost of therapy for patients with AML [Citation6,Citation7], indirect costs, including work absences and work loss (or lost productivity), are believed to constitute a substantial proportion of the economic burden of AML that may be underappreciated [Citation7]. Indeed, different cancer types are known to be associated with different risks of adverse work outcomes, which could be explained by differences in treatments and their long-term consequences [Citation8]. The diagnosis of, and the often extended treatment duration for, hematologic malignancies like AML can result in slow or insufficient recovery of the physical and mental capabilities of patients, which impacts their ability to return to work [Citation9]. A cohort study of lung and colorectal cancer survivors later identified the clinical stage of the disease and a lower level of education as factors negatively influencing return to work [Citation10]. In a study exploring the burden experienced by family caregivers of patients with AML at three centers in the United States, there was substantial work loss as a result of treatment for AML, with approximately 50% of patients failing to return to work and 80% of caregivers reporting a significant detrimental impact on household income 2 years after HSCT [Citation11]. Work-related and indirect costs for caregivers of patients with AML have not been well characterized, despite acknowledgment that family caregivers are often the primary source of support for patients with cancer, including the provision of financial support [Citation12,Citation13]. Of the published studies available, a higher financial burden was found to have a significant impact on psychological morbidities [Citation13], demonstrating the humanistic burden of AML on family caregivers.
There is limited recent evidence describing the impact of an AML diagnosis and treatment on the working lives of patients and their caregivers, which could help health-care teams to identify those requiring access to rehabilitation programs and additional support to enable them to return to work. The aim of the current study was to describe the impact of AML diagnosis on workplace absenteeism and disability days among patients and their caregivers in a US commercial health insurance setting and compare outcomes before and after the AML diagnosis.
2. Methods
2.1. Study design
This was an observational, retrospective, self-controlled case series study of adult patients newly diagnosed with AML between 1 January 2009 and 1 December 2019, as well as adult caregivers of patients newly diagnosed with AML, as identified from the US Merative™ MarketScan® Commercial Database. This database contains inpatient, outpatient, and outpatient prescription drug experiences of employees and their dependents who are covered under fee-for-service and managed care health plans. However, any medications received as part of a clinical trial were not included unless a claim, covered by one of the health plans included in the MarketScan Database, had been submitted to their insurance. A dependent could be a spouse or other dependent. Medical claims, linked to outpatient prescription drug claims and person-level enrollment data, were available for 25–50 million enrollees per year during the study timeframe. A linked database, the Merative MarketScan Health and Productivity Management (HPM) Database provided patient-level records of workplace absence, short-term disability (STD) and long-term disability (LTD) data in relation to medical and prescription drug claims for a subset of the employees in the Commercial Database. The HPM Database included 3.5–5.5 million employees per year during the study timeframe and included a subset of the commercially insured US population concentrated in large companies, such as those in the manufacturing, financial services and shipping/transportation sectors. All data contained in the databases were self-reported by employers. Data assessment was conducted between 31 January 2022 and 2 August 2022.
A patient cohort and a caregiver cohort were identified in the databases for inclusion in the study. Patients were followed from the index date, defined as the earliest date of a medical claim with AML diagnosis, for at least 30 days of continuous enrollment up to 3 years or until the end of continuous enrollment, end of database eligibility, or end of the study, whichever occurred first. A person’s continuous enrollment or eligibility for entry in the database ended when they had a change in employer, the employer stopped contributing data, or when they died. A separate cohort of caregivers was identified as adult family members covered under the same health plan as an adult patient with AML; index date for caregivers was defined as the earliest date of AML diagnosis for the linked patient.
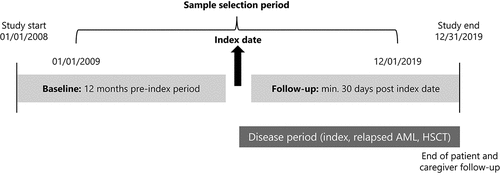
This was a pre–post index, self-controlled study. The study design is illustrated in . The baseline period comprised a 12-month period prior to the index date during which patient and caregiver clinical characteristics and work absence outcomes were recorded, for comparison with follow-up outcomes. The follow-up period included three AML disease periods: index; relapsed AML; and HSCT. The index date for patients was the earliest date of a medical claim with an AML diagnosis; for caregivers, it was the date of the family member’s first AML diagnosis. The index period was defined as the time from the index date to the earliest of relapse (identified via diagnosis code), first HCST (identified via HSCT code), or the end of follow-up. The relapsed AML period was defined as the time from a diagnosis of relapsed AML (patients previously in remission had return of myeloid leukemia cells) until inpatient admission for HSCT or end of follow-up, whichever occurred first. The HSCT period was defined as the time from an HSCT-related admission and continued until diagnosis of relapsed AML or end of follow-up, whichever occurred first. During follow-up, endpoints were described overall and separately for each of the three disease phases (index, relapsed AML, or HSCT).
2.2. Inclusion and exclusion criteria
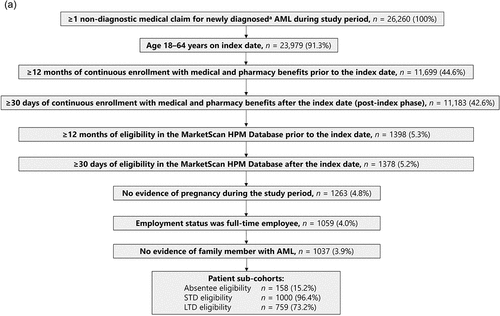
Patients aged 18–64 years on the index date were included in the study if they had a new diagnosis of AML (International Classification of Diseases 9th revision [ICD-9] code 205.00, or ICD 10th revision [ICD-10] codes C92.60, C92.A0, C92.00, C92.40, and C92.50) between 1 January 2009 and 1 December 2019 and had not been diagnosed with AML prior to the index date. Patients had to be full-time employees and must have had at least 12 months of continuous enrollment in the database prior to index date and at least 30 days of continuous enrollment after the index date. They must also have had at least 12 months of absentee, STD, or LTD eligibility in the HPM Database prior to the index date and at least 30 days of data after the index date.
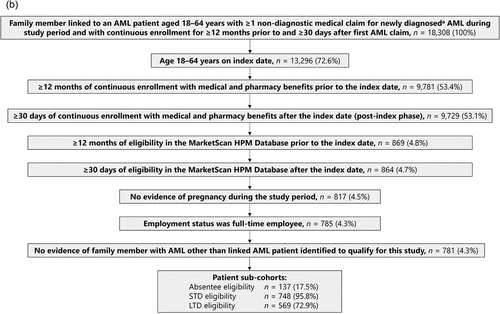
AML caregivers were included in the study if they were aged 18–64 years on the index date and were a family member of an adult AML patient between 1 January 2009 and 1 December 2019. Caregivers had to be full-time employees and must have had at least 12 months of continuous enrollment in the database prior to index date and at least 30 days of continuous enrollment after the index date. They must also have had at least 12 months of absentee, STD, or LTD eligibility in the HPM Database prior to the index date and at least 30 days of data after the index date.
Patients and caregivers whose employment status was part time, early retiree, or retiree, or who had evidence of pregnancy during the baseline or follow-up periods were excluded. Families with more than one AML patient covered under the same health plan were excluded from both the patient and caregiver cohorts.
2.3. Outcomes
Patient-level variables were included to describe the study population. These included demographic characteristics (age, sex, US geographic region, type of residence, insurance plan type, family insurance coverage, and employer industry), comorbid clinical characteristics, and AML-related characteristics (e.g., hospital admission, length of stay, and treatments received).
A comparison was made of endpoints before and after the first AML diagnosis for patients, and for caregivers before and after a family member’s AML diagnosis. The primary endpoint was work loss due to absence, STD, and LTD for patients with AML and for caregivers, during the 12-month baseline period and overall during the follow-up period (up to 3 years’ duration). Work loss was recorded as mean number of days per patient per month (PPPM) and percentage of patients reporting each outcome. STD absence was defined in the databases as wage replacement insurance for individuals with a temporary disability because of non-occupational injury or illness lasting ≤6 months. LTD absence was defined in the databases as wage replacement insurance for individuals with permanent disability (partial or total) lasting >6 months. Patient-level data in relation to work absence, STD, and LTD were based on available data in each category and may not have included the same individual patients across categories. We considered performing a combined analysis of patients with data for all three categories; however, the number of qualifying patients was too low (n = 110) for meaningful outcomes and the analysis was not conducted.
Secondary endpoints included a summary of lost wages associated with each work loss type (work absence, STD, LTD) PPPM during the 12-month baseline period and overall during the follow-up period. Lost wages were defined as the estimated monetary value associated with work loss due to each work loss type and were estimated by multiplying the number of work days lost by the mean daily wage specific to each patient’s age, sex, and geographic region of residence. Mean daily wages were obtained from the Q4 2019 Labor Statistics/Bureau of the Census Current Population Survey (CPS) Annual Social and Economic Supplement (ASEC).
Exploratory endpoints included a description of absenteeism and disability-lost days, and lost wages for patients with AML for each AML disease period (index, relapsed AML, and HSCT). Within each disease period, endpoints were described PPPM during each period and during each analytical month of follow-up.
2.4. Ethics
All database records were de-identified and fully compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 for ensuring the rights of participants in non-interventional studies. Since this study used de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board approval to conduct the study was not sought. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies [Citation14].
2.5. Statistical analyses
Continuous variables are presented as means with standard deviations and/or medians (where appropriate), while dichotomous or categorical variables are presented as the number of observations and percentages. All analyses were conducted separately for patients and caregivers with eligibility for each absence type. Intra-individual analysis was used to control for underlying differences in baseline characteristics of patients and caregivers.
In the analysis of the primary endpoints, proportions before and after AML diagnosis (overall follow-up period) were compared using McNemar’s chi-square test, and days of work loss PPPM were compared using a paired t-test to account for the paired nature of these data. In the analysis of the secondary endpoints of mean lost wages PPPM before and after AML diagnosis (overall follow-up period), a paired t-test was used to account for the paired nature of these data. A fixed effect multivariable linear regression model was implemented, with normal distribution and an identity link function and fixed effects design was used in the exploratory analysis to estimate incremental absentee and STD days during each disease phase, separately, for patients and caregivers. To account for the paired nature of these data, proportions were compared using McNemar’s chi-square test, and days of work loss PPPM and mean lost wages PPPM were compared using a paired t-test. For all comparisons, a critical value of 0.05 was specified a priori as the threshold for statistical significance.
The statistical analysis software program used for this study was WPS version 4.2 (World Programming, UK).
3. Results
3.1. Study cohorts
Of 26,260 patients with ≥1 non-diagnostic medical claim for newly diagnosed AML identified in the HPM Database during the study period, 1,037 patients met the inclusion criteria (). A total of 18,308 individuals were identified as caregiver family members of patients with an insurance claim for newly diagnosed AML meeting the study inclusion criteria, and of these, 781 caregivers met the inclusion criteria and comprised the caregiver cohort ().
3.2. Patients
3.2.1. Demographics
The mean (standard deviation [SD]) age of patients with AML included in the study was 49.8 (9.8) years and most (70.5%) were male (). Just over half (56.2%) of patients contributed at least 6 months of data following their AML diagnosis ().
Table 1. Baseline demographics and characteristics for (a) patients with AML and (b) caregivers of patients with AML.
3.2.2. Comorbid conditions
The mean (SD) number of comorbidities recorded per patient at baseline was 2.63 (2.1) (). Other than any malignancy (including lymphoma and leukemia, except for malignant neoplasm of the skin; 46.8%), anemia (38.3%), hypertension (36.2%), fatigue (22.3%), and diabetes (21.6%) were the most common comorbidities ().
Table 2. Baseline comorbid conditions recorded for patients with AML.
3.2.3. AML-related clinical characteristics
Of the 1,037 patients with newly diagnosed AML in the cohort, 384 (37.0%) entered a remission phase during the follow-up period, with mean 3.9 months to disease remission (). During follow-up, 152 (14.7%) patients were recorded as having relapsed AML, with relapse occurring mean 6.4 months after index date (). The proportion of patients with a record of admission to hospital increased from 25.6% during the baseline period to 71.8% during follow-up, with the corresponding mean length of stay increasing from 8.0 days to 16.5 days, respectively (). Only 3.3% of patients were recorded as receiving FLT3 inhibitor treatment ().
Table 3. AML-related health-care resource use and clinical characteristicsa recorded for patients with newly diagnosed AML.
3.2.4. Workplace absence and associated wage loss
Work absence and indirect costs incurred during baseline and follow-up in the patient cohort showed that the proportion recorded as having a workplace absence decreased by 10% from baseline to the follow-up period, with no significant difference in PPPM days of absence between the two periods (). However, the proportion of patients recorded as having STD and LTD leave claims, and the mean number of days of leave taken PPPM, were significantly higher at follow-up compared with baseline: STD claims doubled from 24.6% to 50.0% (p < 0.001) and LTD claims increased fivefold, from 4.0% to 20.0% (p < 0.001) (). There were corresponding significant increases in wage losses, with STD-related wage loss PPPM increasing from $203 in the baseline period to $1194 during follow-up (p < 0.001), and LTD-related wage loss PPPM increasing from $57 in the baseline period to $330 during follow-up (p < 0.001) (). Work loss due to STD recorded for patients was the highest up until 4–5 months after AML diagnosis before declining sharply; this decline coincided with an increase in LTD-related workplace loss ().
Figure 3. Mean monthly AML workplace loss by work loss type for (a) patients with AML and (b) caregivers. AML, acute myeloid leukemia; HSCT, hematopoietic stem cell transplant.
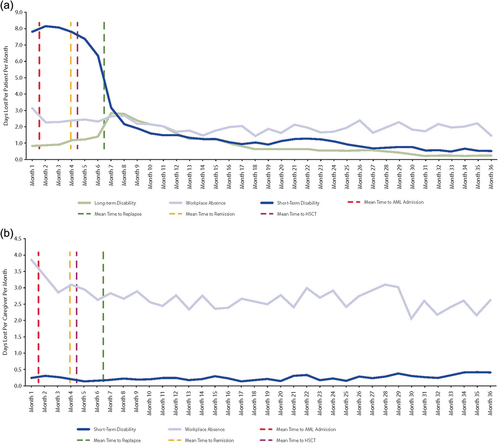
Table 4. Work loss and associated indirect costs incurred during baseline and full follow-up period for (a) AML patients and (b) caregivers of AML patients.
Exploratory analysis results describing work absence and associated indirect wage loss incurred for patients with AML during baseline and each AML disease period (index, relapsed AML, and HSCT) are shown in Supplemental Table S1a. There was a significant decrease of nearly 16% in the proportion of patients with a record of workplace absence during the index period compared to baseline (p = 0.003), and an almost doubling in the proportion of patients with STD claims over the same period, from 24.6% to 47.2% (p < 0.001) (Supplemental Table S1a). From baseline to index AML, the number of days lost PPPM due to STD leave increased significantly by more than sixfold (0.85 to 5.88; p < 0.001), with a corresponding increase in STD-related wage loss ($203 to $1425; p < 0.001) (Supplemental Table S1a). There was also a threefold increase in the proportion of patients with LTD claims (p < 0.001), and an almost fourfold increase in the number of days PPPM lost due to LTD (p < 0.001), with a corresponding increase in LTD-related wage loss ($57 to $220; p < 0.001) (Supplemental Table S1a).
For patients eligible for analysis in the HSCT period, there was a 38% relative decrease in workplace absence compared with the baseline period (p < 0.001) (Supplemental Table S1a). Over the same period, STD claims almost doubled (p < 0.001), and days PPPM lost due to STD leave increased almost tenfold (p < 0.001), as did STD-related wage losses (p < 0.001) (Supplemental Table S1a). Likewise, LTD claims increased almost threefold (p = 0.001), number of days PPPM lost due to LTD leave increased more than sevenfold (p < 0.001) and LTD-related wage losses increased almost eightfold (p < 0.001) over the same period (Supplemental Table S1a).
Compared with baseline, the proportion of patients with workplace absence decreased in the relapsed AML period, while the number of days PPPM lost due to workplace absence and associated wage losses during the relapsed AML period did not differ significantly (Supplemental Table S1a). However, over the same period, STD claims nearly doubled (p < 0.001), and both the number of days PPPM lost due to STD and STD-related wage loss increased more than eightfold (p < 0.001) (Supplemental Table S1a). Similarly, LTD claims increased almost ninefold (p < 0.001), the number of days PPPM lost due to LTD leave increased more than fortyfold (p < 0.001), and LTD-related wage losses increased almost fiftyfold (p < 0.001) (Supplemental Table S1a).
3.3. Caregivers
3.3.1. Demographics
Caregiver family members of newly diagnosed AML patients had a mean (SD) age of 50.9 (7.9) years and most were male (69.8%) (). While the detail of the relationship between patients and caregivers was not recorded in the claims data, 97.2% of the caregivers had a spouse covered under the same health insurance and 76.8% had a dependent covered under the same health insurance ().
3.3.2. Workplace absence and associated wage loss
Similar proportions of caregivers were recorded as having workplace absence in the baseline (83.2%) and follow-up (83.9%) periods, and there was no significant difference in workplace absence PPPM between the two time periods (). The proportion of caregivers recorded as having STDs more than doubled from baseline to follow-up (from 5.4% to 11.8%; p < 0.001), while there were non-significant increases in the number of days PPPM lost due to STD (from 0.15 to 0.25 days) and associated wage losses (from $38 to $60) over this time period (). The proportion of caregivers with an LTD claim was small and remained similar between baseline and follow-up (0.4% and 0.7%, respectively), as did the number of days PPPM lost due to LTD and associated indirect costs (). For caregivers, days lost due to workplace absence were highest in the first 2 months following initial AML diagnosis, but there was a generally elevated level of workplace absence and some work loss due to STD recorded throughout the follow-up period ().
Supplemental Table S1b presents the exploratory analysis of work absence and associated indirect wage loss incurred for caregivers of patients with AML during baseline and each AML disease period. Compared with baseline, the number of days lost PPPM due to workplace absence increased significantly during the index (from 2.4 to 3.3 days, respectively; p = 0.023) and HSCT periods (from 2.0 to 3.7 days, respectively; p = 0.041), but the increase did not reach statistical significance for the comparatively lower number of caregivers with data for relapsed AML (from 2.5 to 4.1 days; p = 0.060) (Supplemental Table S1b). There were accompanying significant increases from baseline in indirect costs PPPM associated with workplace absence for caregivers during the index (from $619 to $857, respectively; p = 0.025) and HSCT periods (from $498 to $906, respectively; p = 0.048).
4. Discussion
Our study showed that in a cohort of employed patients with AML included in a US insurance claims database, workplace absence decreased in the months following the first AML diagnosis. However, there were significant increases from baseline to the post-AML diagnosis follow-up period in the proportion of patients making STD and LTD leave claims, and the mean number of STD- and LTD-related leave days PPPM that patients claimed from their full-time employment. Those patients who underwent HSCT or who had AML relapse had the greatest increase in STD and LTD leave. The increased incidence of STD and LTD leave claims corresponded to a significant increase in wage losses. The later decline in STD claims by patients from months 4–5 onwards, even after relapse, may reflect the fact that patients have used their STD allowance. Concurrently, a spike in LTD claims occurred after relapse. Patients thus appear to progress from STD to LTD and may eventually fall out of the workplace, which could have a substantial societal impact, especially when considering that the median age of the cohort was 52 years. These findings correspond with those of other studies in which patients with various cancer types reported taking time off work during cancer treatment [Citation15], or having repeated or long-term sick-leave absences after returning to work following treatment [Citation9,Citation16]. While our study did not explore the reasons for workplace absence and disability leave days, it is known that intensive chemotherapy to treat AML is associated with long-term fatigue and reduced physical performance measures [Citation17], which could influence a person’s ability to work. Another study of patients with AML identified the physical and mental impact of the disease and its treatment, disease- and treatment-related adverse effects, and concern about work relationships as barriers preventing return to work after HSCT [Citation5]. Indeed, HSCT appears to have a highly detrimental impact on patients with AML, with 41% of >1000 participants in a study from Japan resigning from work during the course of treatment [Citation18]. In addition to the direct impact of AML and its treatment on patients’ health, there appear to be a number of indirect workplace and financial effects that impact the lives of patients and their caregivers.
For caregivers of AML patients, there were similar proportions who recorded workplace absence before and in the overall follow-up period after diagnosis of AML. However, compared with baseline, caregivers had a high level of workplace absence in the first months after the initial AML diagnosis, which was followed by a general elevated level of workplace absence and some work loss due to STD leave throughout the follow-up period. This finding highlights a potentially problematic gap in support for the caregivers of someone with a longer-term illness: the absence of a long-term workplace leave option could lead to caregivers leaving the workforce entirely. A systematic literature review of the impact of cancer treatment on caregiver work productivity revealed a reduction of approximately 24% in productivity owing to work absence because of patients receiving intensive treatment, time spent on hospital appointments, and the adverse effects of treatment [Citation19]. According to a study from China, 46% of the familial caregivers of patients with cancer reduced their working hours to accommodate the primary treatment phase; 17% also stopped working altogether at this time, and one-quarter of caregivers reduced their working hours after treatment was complete [Citation20]. Upon closer inspection of work loss during the different AML disease periods in our study, caregivers had significant increases in number of days lost PPPM during the index and HSCT periods compared to baseline. Compared with baseline, there were significant respective increases of 38.4% and 81.9% in indirect costs PPPM associated with caregiver workplace absence during the index and HSCT periods. An Australian study used an economic model to calculate the projected societal impact of AML in a population aged 15–65 years, in terms of productivity-adjusted life years (PALYs). The authors estimated that between 2020 and 2029, there would be 7,337 PALYs lost to AML, amounting to the equivalent of US$971 million lost in gross domestic product during this period [Citation21].
We note that among our cohort of caregivers, the majority (~70%) were male, which was surprising given the higher incidence of AML in males than females [Citation1], and therefore the expectation that a majority of caregivers would be female. However, the caregiver and AML samples were independent of each other and were not paired, so the percentage of males in each sample is more likely to reflect the percentage of males in the database population. Although the reason for the relatively high percentage of male caregivers is uncertain, it is known that wives of patients with cancer are significantly less likely to be employed 2–6 years after diagnosis, compared with wives of individuals without cancer, whereas the impact on husbands was not significant [Citation22]. It is possible that a similar effect was seen among the caregivers of newly diagnosed AML patients in our study; if so, the departure of female caregivers from employment could have skewed the available data, and our findings may not reflect the true impact of AML on the work loss and wages of caregivers.
Comorbid conditions at baseline were recorded for patients with AML in this study; however, it would be interesting to explore the impact of an AML diagnosis and treatment on incident comorbidities over time among patients and caregivers. For instance, it would be anticipated that caring for a patient with AML could impact negatively on psychosocial factors, such as anxiety and depression [Citation23], and potentially lead to linked clinical factors, such as cardiovascular disease [Citation24]. A future study could investigate these indirect factors, in conjunction with employment and financial outcomes, as well as their impact on health-care costs, to determine the broader societal burden of AML.
Our study has several strengths, including the use of intra-individual analysis to control for underlying differences in the baseline characteristics of patients and caregivers. In addition, the analysis of absenteeism was not restricted to specific types of treatment, such as HSCT, meaning that we were able to achieve more complete examination of the impact of AML on employment. As the overall MarketScan Commercial Database links patients with AML to family members through shared medical insurance, the ability to connect various types of data for a large, national sample of individuals was another strength of our study. Furthermore, this large database enables the identification of likely caregivers for AML patients, which can otherwise be difficult to pinpoint outside of such survey data.
We also acknowledge some limitations of the study, including those inherent to retrospective analyses as a whole. Firstly, the small sample size in this study was further limited to only those with commercial health insurance coverage and those meeting eligibility in the MarketScan HPM Database. As a result, work loss outcomes in eligible patients or caregivers in the HPM Database with available workplace absence data may not be generalizable to work loss trends across all AML patients and caregivers. AML patients also typically rely on STD during the initial 6–7 months post-diagnosis; the need for extended leave following an AML diagnosis may have discouraged these patients from taking regular workplace absences. As such, this factor could be responsible for the reduced sample size deemed eligible for inclusion in the HPM Database for up to 12 months prior to index date. Moreover, the study population was limited to individuals from a single country with commercial health insurance coverage via employment in large companies. Consequently, the results may not be generalizable to patients with AML in other countries, those with health insurance not sponsored by an employer, those whose caregivers are covered under a different health plan, or those without health insurance coverage. Apart from STD/LTD, patients were also removed from the cohort if they ceased the employment that was providing commercial health insurance; as such, since employment is linked to eligibility for inclusion in the MarketScan HPM Database, durations of both pre- and post-index date follow-up periods were also limited. Additionally, the MarketScan HPM Database included only a limited subset of members (full-time employees or caregivers) from the overall MarketScan Database. Hence, the limited representation of patients or caregivers with available workplace absence data in the HPM Database is potentially another factor contributing to the non-generalizability of work loss outcomes to all AML patients and caregivers.
Since patients were identified via administrative claims data rather than through medical records, there was the potential for misclassification of various study aspects, including AML, covariates, and study outcomes. The data were self-reported from a collection of large employers and thus were susceptible to self-reporting bias and may not be generalizable to patients with AML or caregivers with different forms of employment and employment benefits. As remission in this study was identified from the presence of diagnosis codes on medical claims (at baseline or during any follow-up periods), the rate of remission may also be lower than expected due to underreporting of diagnosis codes in the database. Precise medical data was not collected, as building lines of therapy to identify remission/relapse patterns among patients or caregivers was beyond the scope of this study, though this can be explored in future research. Data on whether employees were salaried or paid hourly were also not available in the database. The number of patients with AML who had recorded data for all three categories of work absenteeism was also too low for meaningful analysis. Similarly, the number of LTD claims recorded for caregivers was also negligible, as under US regulations disability leave can only be claimed for illness or injury to employees themselves, not to care for others; interpretation of LTD claims findings in the caregiver cohort should therefore be treated with caution. The study did not control for non-disease-related factors that could have contributed to workplace absenteeism and disability leave days, such as age, sex, and socioeconomic status; however, the pre–post design of this study, which assessed changes in the same patient or caregiver, was intended to minimize the impact of this limitation. Indirect costs were estimated using mean daily wages, which did not account for industry type.
There was the potential to underreport the true burden of disease for caregivers, since some employees, such as white-collar workers, may still be able to work remotely while their family member is being cared for in hospital, thereby underestimating the burden of AML. Also, we could not rule out that individuals younger than 18 years, the minimum age for inclusion in this study, could still provide care for a parent with AML. Patients and caregivers in the HPM Database were not necessarily related, and the study could not confirm whether adults identified under the same health insurance plan as a patient with AML were taking the role of caregivers. As the selection of caregivers was limited to those covered under the same health plan as the patient, this study may have omitted a substantial proportion of family members or other caregivers who were covered under a separate health insurance plan. Additionally, as mentioned above, the disproportionate effect of a cancer diagnosis on the wives of patients may mean that our dataset did not capture the full impact of AML on carers. Finally, more than one individual could provide care for the same patient with AML and may, along with the patient, use unpaid leave or a flexible work schedule to care for patients, which would not be reflected in the HPM data. Consequently, as the database only recorded paid leave, work absence would likely be underreported. However, regardless of these limitations, a substantial difference in caregiver absence was recorded after the diagnosis of AML in a family member, although we may not have had the ability to capture the full impact.
5. Conclusion
This study provides insights into the impact of an AML diagnosis, AML relapse, and HSCT on work loss and subsequent wage implications among patients with AML and caregivers of AML patients in a US commercial health insurance setting. As such, our findings build on the available evidence on the societal impact of AML in relation to work and wage loss and could help inform interventions to support and assist families at risk of poor work outcomes. While workplace absence decreased for patients in the months following initial AML diagnosis, the number of STD and LTD leave days claimed increased significantly by six- and fourfold, respectively. The proportion of patients filing STD leave claims increased to months 4–5 after diagnosis, while the proportion making LTD leave claims increased significantly starting from 5 months after AML diagnosis. For caregivers, workplace absence peaked in the first 2 months after an AML diagnosis and remained elevated throughout the study period. Patients undergoing HSCT appeared to have a significant detrimental impact on their caregivers in terms of workplace absence and indirect costs. This is the first detailed evaluation of the impact of AML on both patients and caregivers, particularly as caregivers have very little coverage in the literature. While this study was non-interventional, observational, and based on large US commercial health insurance databases, the findings are informative but are restricted to a similar setting. However, these findings highlight the impact of the burden on caregivers of patients with AML who undergo HSCT or have disease relapses. Further research is still needed to explore how work loss burden varies by AML treatment type and/or treatment sequencing, to further assess the impact of AML on workplace absenteeism among patients and their caregivers.
Previous presentation
These data were submitted in part as an abstract to the 2023 American Society of Clinical Oncology annual meeting, June 2–6, Chicago, IL, USA, and was published online (J Clin Oncol 41, 2023 [Suppl 16; Abstr e19002]).
Declaration of interest
B J Pandya, C Young, B Xie, A Block, K Bernacki, and M Touya are employees of Astellas.
E R Packnett and T Lillehaugen are employees of Merative.
T W LeBanc has received grants/contracts from AstraZeneca, CareVive, GSK, Janssen, Bristol Myers Squibb, and Jazz Pharmaceuticals; royalties/licenses from UpToDate; consulting fees from AbbVie, Astellas, Agios/Servier, Bristol Myers Squibb/Celgene, Flatiron, GSK, Genentech, Pfizer, BlueNote Therapeutics, Novartis, and AstraZeneca; honoraria from AbbVie, Agios/Servier, Bristol Myers Squibb/Celgene for speaker programs; and support for attending meetings from Agios/Servier, AbbVie, and Bristol Myers Squibb/Celgene.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
Conception/study design: B J Pandya, C Young, E R Packnett, B Xie, T Lillehaugen, A Block, K Bernacki, T W LeBlanc
Data acquisition: E R Packnett, T Lillehaugen
Analysis of data: C Young, E R Packnett, B Xie, T Lillehaugen
Interpretation of study data: B J Pandya, C Young, E R Packnett, B Xie, T Lillehaugen, A Block, K Bernacki, M Touya, T W LeBlanc
All authors were involved in drafting the work or revising it critically for important intellectual content, approve the final version to be published, and agree to be accountable for all aspects of the work.
Data sharing statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (59.2 KB)Acknowledgments
Programming services were provided by Caroline Henriques, MPH, of Merative. Statistical analysis support was provided by David Smith, PhD, of Merative. These services were funded by Astellas Pharma Inc. Medical writing support was provided by Rhian Harper Owen, PhD, for Lumanity and funded by Astellas Pharma Inc. T W LeBlanc is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2024.2311305
Additional information
Funding
References
- U.S. Department of Health and Human Services. SEER cancer stat facts: AML [Internet]. [cited 2023 Dec 7]. Available from: https://seer.cancer.gov/statfacts/html/amyl.html.
- Smith GL, Lopez-Olivo MA, Advani PG, et al. Financial burdens of cancer treatment: a systematic review of risk factors and outcomes. J Natl Compr Canc Netw. 2019;17(10):1184–1192. doi: 10.6004/jnccn.2019.7305
- Bentley C, Teckle P, McQuarrie L, et al. Impact of cancer on income, wealth and economic outcomes of adult cancer survivors: a scoping review. BMJ Open. 2022;12(9):e064714. doi: 10.1136/bmjopen-2022-064714
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721–749. doi: 10.6004/jnccn.2019.0028
- Granroth G, Khera N, Arana Yi C. Progress and challenges in survivorship after acute myeloid leukemia in adults. Curr Hematol Malig Rep. 2022;17(6):243–253. doi: 10.1007/s11899-022-00680-6
- Zeidan AM, Mahmoud D, Kucmin-Bemelmans IT, et al. Economic burden associated with acute myeloid leukemia treatment. Expert Rev Hematol [Internet]. 2016;9:79–89. doi: 10.1586/17474086.2016.1112735
- Redaelli A, Botteman MF, Stephens JM, et al. Economic burden of acute myeloid leukemia: a literature review. Cancer Treat Rev. 2004;30(3):237–247. doi: 10.1016/j.ctrv.2003.11.002
- de Boer AGEM, de Wind A, Coenen P, et al. Cancer survivors and adverse work outcomes: associated factors and supportive interventions. Br Med Bull. 2023;145(1):60–71. doi: 10.1093/bmb/ldac028
- Persoon S, Buffart LM, Chinapaw MJM, et al. Return to work experiences of patients treated with stem cell transplantation for a hematologic malignancy. Support Care Cancer. 2019;27(8):2987–2997. doi: 10.1007/s00520-018-4596-0
- Earle CC, Chretien Y, Morris C, et al. Employment among survivors of lung cancer and colorectal cancer. J Clin Oncol. 2010;28(10):1700–1705. doi: 10.1200/JCO.2009.24.7411
- Denzen EM, Thao V, Hahn T, et al. Financial impact of allogeneic hematopoietic cell transplantation on patients and families over 2 years: results from a multicenter pilot study. Bone Marrow Transplant. 2016;51(9):1233–1240. doi: 10.1038/bmt.2016.103
- Pahlavanzade S, Khosravi N, Moeini M. The effect of a family need-based program on burden of caregivers of leukemia patients in Isfahan in 2013-2014. Iran J Nurs Midwifery Res. 2014;19(6):629–634.
- Grover S, Rina K, Malhotra P, et al. Caregiver burden in the patients of acute myeloblastic leukemia. Indian J Hematol Blood Transfus An Off J Indian Soc Hematol Blood Transfus. 2019;35(3):437–445. doi: 10.1007/s12288-018-1048-4
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010
- Paltrinieri S, Vicentini M, Mazzini E, et al. Factors influencing return to work of cancer survivors: a population-based study in Italy. Support Care Cancer. 2020;28(2):701–712. doi: 10.1007/s00520-019-04868-0
- Eriksson L, Wennman-Larsen A, Bergkvist K, et al. Important factors associated with sick leave after allogeneic haematopoietic stem cell transplantation—a 1-year prospective study. J Cancer Surviv. 2021;15(6):933–941. doi: 10.1007/s11764-020-00986-5
- Timilshina N, Breunis H, Tomlinson GA, et al. Long-term recovery of quality of life and physical function over three years in adult survivors of acute myeloid leukemia after intensive chemotherapy. Leukemia. 2019;33(1):15–25. doi: 10.1038/s41375-018-0162-5
- Kurosawa S, Yamaguchi T, Mori A, et al. Incidence and predictors of recurrent sick leave in survivors who returned to work after allogeneic hematopoietic cell transplantation. J Cancer Surviv. 2023;17(3):781–794. doi: 10.1007/s11764-022-01250-8
- Kamal KM, Covvey JR, Dashputre A, et al. A systematic review of the effect of cancer treatment on work productivity of patients and caregivers. J Manag Care Spec Pharm [Internet]. 2017;23:136–162. doi: 10.18553/jmcp.2017.23.2.136
- Liu S, Su M, Yao N, et al. Employment changes among Chinese family caregivers of long-term cancer survivors. BMC Public Health. 2020;20(1):1787. doi: 10.1186/s12889-020-09922-9
- Parker C, Liew D, Ademi Z, et al. Estimating the productivity impact of acute myeloid leukemia in Australia between 2020 and 2029, using a novel work utility measure: the productivity-adjusted life year (PALY). JCO Oncol Pract [Internet]. 2021;17:e1803–e1810. doi: 10.1200/OP.20.00904
- Hollenbeak CS, Short PF, Moran J. The implications of cancer survivorship for spousal employment. J Cancer Surviv. 2011;5(3):226–234. doi: 10.1007/s11764-011-0175-9
- Yucel E, Zhang S, Panjabi S. Health-related and economic burden among family caregivers of patients with acute myeloid leukemia or hematological malignancies. Adv Ther. 2021;38(10):5002–5024. doi: 10.1007/s12325-021-01872-x
- Pössel P, Mitchell AM, Harbison B, et al. Association of cancer caregiver stress and negative attribution style with depressive symptoms and cortisol: a cross-sectional study. Support Care Cancer [Internet]. 2022;30(6):4945–4952. doi: 10.1007/s00520-022-06866-1