ABSTRACT
Background
The treatment of chronic hepatitis C virus (HCV) infection using directly acting antivirals was recently adopted in the treatment guidelines of Zimbabwe. The objectives of this study were to design a simplified model of HCV care and estimate the cost of screening and treatment of hepatitis C infection at a tertiary hospital in Zimbabwe.
Methods
We developed a model of care for HCV using WHO 2018 guidelines for the treatment of HCV infection and expert opinion. We then performed a micro-costing to estimate the costs of implementing the model of care from the healthcare sector perspective. Deterministic and probabilistic sensitivity analyses were performed to explore the impact of uncertainty in input parameters on the estimated total cost of care.
Results
The total cost of screening and treatment was estimated to be US$2448 (SD=$290) per patient over a 12-week treatment duration using sofosbuvir/velpatasvir. The cost of directly acting antivirals contributed 57.5% to the total cost of care. The second largest cost driver was the cost of diagnosis, US$819, contributing 34.6% to the total cost of care.
Conclusion
Screening and treatment of HCV-infected individuals using directly acting antivirals at a tertiary hospital in Zimbabwe may require substantial financial resources.
1. Introduction
The World Health Organisation (WHO) launched a strategy to reduce hepatitis C virus (HCV) infections and HCV-linked mortality [Citation1]. Increasing the number of people with chronic HCV on treatment to 80% by 2030 is one of the targets in the WHO strategy [Citation1]. The WHO issued an update to the guidelines for the care and management of people living with chronic HCV infection in 2022 [Citation2]. The guidelines recommended that all children, adolescents, and adults infected with chronic HCV infection be treated using pan-genotypic directly acting antivirals (DAAs). The development and adoption of pan-genotypic DAAs eliminates the need for genotyping before initiating therapy. The need for genotyping was a major access barrier to HCV treatment in low to middle-income countries (LMICs) due to its high cost and unavailability [Citation3,Citation4].
Despite a high burden of HCV infection in LMICs, very few HCV-infected people know their status and are on treatment [Citation5–7]. One of the reasons for the low numbers of people on treatment is high medical costs associated with the screening and treatment of HCV using DAAs [Citation6]. The development and adoption of simplified HCV screening and treatment models has the potential to reduce the costs. Implementing simplified models of care characterized by reduced number of healthcare visits and fewer safety and viral load monitoring tests is now feasible due to the improved efficacy and safety of DAAs. Simplified models of care have been successfully implemented in LMICs yielding high patient retention and sustained virologic response rates (SVR) [Citation8–10]. A study in Pakistan reported SVR rates of 94.7% for sofosbuvir/velpatasvir(sof/velp) and sofosbuvir/daclatsvir (sof/dcv) respectively [Citation11]. Another study in real-world settings in South Africa reported SVR rates of 100% and 97% for sof/velp and sof/dcv respectively [Citation12]. In addition to the favorable clinical outcomes, there is growing evidence suggesting these simplified models of care are potentially cost effective and/or cost-saving [Citation13,Citation14].
The ministry of health in Zimbabwe adopted the WHO strategy and formulated the national strategy for the elimination of HCV in 2019 [Citation15]. Scaling up HCV screening and treatment are important for achieving the goals of this strategic plan. Adopting simplified models of HCV care minimizes the duration between screening and initiation of treatment [Citation16,Citation17]. Screening and treatment of HCV may require substantial investment [Citation18,Citation19]. Estimates of the costs required to screen and treat HCV-infected people are required for the planning and implementation of the national elimination strategy. This study aims to develop a simplified HCV screening and treatment model of care and estimate the cost of implementing the model at a tertiary hospital in Zimbabwe.
2. Methods
2.1. Development of the model of care
An ambulatory model of care for HCV infected individuals was developed based on the WHO 20182 guidelines and literature. The WHO guidelines provide recommendations on the diagnosis, treatment assessment and monitoring of chronic HCV infection. The recommendations were used to develop a draft schematic representation of the model of care. In addition to the WHO recommendations, we used literature to refine and simplify the model. The simplification of the model was largely informed by Medicines Sans Frontiers (MSF) HCV treatment program implemented in Cambodia [Citation8]. The program presented full, simplified and rural models of HCV care and the clinical and operational outcomes. Some aspects of the simplified model in MSF Cambodia program were adopted and incorporated in our draft model of care. The draft was then presented to experts who provided inputs/feedback using face to face consultations. These experts included two medical officers, two pharmacists, two laboratory scientists, a nurse and one counselor. For example, pharmacists were asked for their views on the frequency of DAAs dispensing (full 12-week supply versus monthly). We incorporated expert opinions into the final model of care and used the model as the basis for the costing analysis.
2.2. Costing procedure
We used standard methods to measure resource use, valuation of resources, and cost analysis. The methods are documented in the literature [Citation20–23].
2.3. Study setting
Data on resource use and unit costs were collected from Parirenyatwa Hospital Centre of Excellence for the period September to December 2019. The Centre of Excellence is an outpatient HIV clinic providing care to over 5000 adolescents and adults living with HIV. In 2016, the AIDS Healthcare Foundation (AHF) signed a memorandum of understanding with the Ministry of Health and Childcare in which they committed to providing funding for pharmacy, counseling, laboratory and clinical services for HIV/AIDS treatment at selected health centers including Parirenyatwa Hospital. Under the memorandum of understanding, AHF committed to paying salaries and benefits for senior staff that are directly linked to patient care in the aforementioned departments. Examples of staff who had their salaries and benefits paid by AHF include medical doctors, pharmacists, nurses, laboratory scientists, and counselors. In 2017, the Centre of Excellence initiated HCV screening among HIV-infected patients receiving care at the institution.
2.4. Identification and measurement of resources
We assumed a patient going through all the steps of the model of care at the Parirenyatwa Centre of Excellence and used the center as a site for measuring the costs. A micro-costing approach from a healthcare provider’s perspective was used to estimate the costs of HCV screening and DAA-based treatment. The costs of HCV screening and treatment were determined for the activities in the model of care. The major activities of the model of care were HCV screening, pre-treatment assessments, DAA treatment initiation and follow-up, and sustained virologic response (SVR) testing and disclosure. The activities were divided between the nursing, laboratory, counseling, medical, pharmacy, information technology, and administration departments. The staff members in these departments were interviewed to determine the exact staff performing specific activities, resources, and time required to perform each activity. For example, the pharmacist was interviewed to identify resources and time required to dispense DAAs and provide drug information. Details of the interview used to identify and measure the resources are recorded in Table S1 in the supplementary file 1. We also collected overhead resources used to support patient care provision, including buildings, furniture, vehicles, and equipment, and apportioned them to direct patient activities using the inpatient daily allocation method. We measured the spaces utilized for care in the pharmacy, clinic, counseling, laboratory, administration, and offices during interviews and used the information to allocate overhead costs.
2.5. Valuation of resources
Staff times were valued using salaries and benefits for each staff category obtained from the human resources departments of the hospital and AHF. Unit costs for the DAAs used in the treatment care model were obtained from private pharmaceutical wholesalers and average market prices were used as the unit costs for the analysis. Unit costs for laboratory tests performed at Parirenyatwa were estimated from the cost of materials used, equipment and staff time. Unit costs for outsourced laboratory tests were obtained from the private laboratories’ charge sheet provided by the Centre of Excellence’s laboratory department. Unit costs for consumables such as gloves were obtained from the respective heads of departments. Total hospital wide utility charges (water, electricity and municipal rates) were obtained from the hospital’s annual financial report. The total space occupied by the buildings of the Centre of Excellence as a fraction of the total space occupied by the hospital was used to apportion the utilities cost. The total number of patient visits per year in the HIV management program at the Centre of Excellence was obtained from the information technology department. Based on expert opinion, we assumed that the screening and treatment of HCV would result in a 35% increase in the hospital visits at the HIV clinic. The total cost of utilities was then divided by the total number of hospital visits (both HIV and HCV) to get an estimate of the cost per visit.
To estimate equipment costs, we calculated the annualized equipment replacement value. The acquisition costs for equipment were obtained from interviews performed with the heads of departments. The standard life years for equipment were obtained from World Health Organisation-Choosing Interventions that are Cost-effective website [Citation24]. The annualized values were calculated using the annuity function in Microsoft excel at an interest rate of 4% provided by the Reserve Bank of Zimbabwe [Citation25]. The total cost of equipment use was divided by the estimated number of patient visits (HIV and HCV visits) to estimate the cost of equipment per visit. The equipment cost per visit obtained was then allocated to every hospital visit in the model of care. The cost for the space utilized was determined using rental cost per square meter obtained from the estate agents in the area where the hospital is located.
2.6. Cost analysis
Resource use and costs were estimated for every activity performed under each hospital visit in the model of care. Fixed costs including general consumables costs, utilities costs, support staff costs (for staff not directly involved in patient care for example drivers), equipment costs (for example vehicles) and rental costs assigned to every visit. The total cost was computed by adding all the costs accrued assuming a patient completed all the visits in the model of care. We used the SVR rates for sofosbuvir/velpatasvir (sof/vel) and sofosbuvir/daclatasvir(sof/dcv) specified in the WHO 2018 [Citation2] guidelines for treatment of chronic HCV infection to estimate the total cost per SVR. The total cost per SVR was categorized by DAA regimen (sol/vel and sof/dcv). Zimbabwe uses a multicurrency system dominated by the Zimbabwean dollar (ZWD) and United States Dollar (USD). Data on some of the costs were obtained in ZWD currency for example government salaries and were converted to USD using the Reserve Bank of Zimbabwe’s monthly average rate [Citation25]. All costs are reported in 2019 USD.
2.7. Estimating cost of case finding
To estimate the cost of diagnosis, we calculated the cost of HCV rapid diagnostic test (RDT) and the cost of HCV nucleic acid test (NAT). We assumed HCV antibody prevalence of 3% from the literature [Citation26]. For the chronic HCV prevalence, we revised downwards the 76.6% observed in a cohort of people who inject drugs in Kenya to 60% because we assumed slightly less risk [Citation27]. The cost of diagnosis, which includes the cost of people who received the tests and were negative was calculated by factoring in the assumed antibody and chronic HCV prevalence. The formula used to calculate the cost of case finding is shown in supplementary file, section b.
2.8. Sensitivity analysis
To assess the impact of variation in input data to the total cost, we performed deterministic and probabilistic sensitivity analyses using Microsoft Excel. On the deterministic sensitivity analysis, we assumed a ± 20% change in staff salaries, laboratory, cost of RDT and DAAs costs and explored the impact on the total costs per patient per SVR. For SVR rates, we adopted ranges provided in the WHO 2018 guidelines (86%-100%)2. In the probabilistic sensitivity analysis, we performed 1000 simulations where random values of the input variables were sampled from a specified probability distribution. For costs, we used the gamma distribution and triangular distribution for the SVR rate.
2.9. Ethical considerations
Ethical approval to conduct the study was granted by the Joint Research Ethics Committee for the University of Zimbabwe College of Health Sciences and Parirenyatwa Hospitals (JREC/347/2018). All participants in the interviews signed informed consent before participating in the study. The study followed the principles of the Helsinki Declaration for research involving human subjects.
3. Results
3.1. The model of care
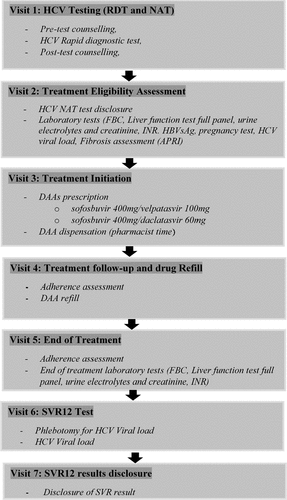
The simplified model of care comprised screening for HCV and DAA treatment services offered by trained nurses, counselors, pharmacists, and nonspecialist medical officers at an HIV treatment center in a tertiary hospital. The screening and treatment services are offered to individuals 18 years and older. Screening for HCV involves serological antibody testing using a rapid diagnostic test (RDT) and confirmation of chronic infection using a qualitative nucleic acid test (NAT). Individuals who test positive for HCV NAT are evaluated for treatment. Pregnancy testing and family planning counselings are offered to women of childbearing age. Baseline assessment in patients considered eligible for treatment includes tests such as HIV, HBV, HCV viral load, FBC, liver function tests (ALT, AST, ALP, total and direct bilirubin), and calculated glomerular filtration rate (eGRF). Non-invasive liver fibrosis assessment based on blood or serum markers using the Aspartate aminotransferase to platelet ratio index (APRI) is used to assess the presence of cirrhosis. Parameters for the computation of the index will be obtained from the laboratory results. Eligible individuals will be initiated on treatment treated sofosbuvir 400 mg/velpatasvir 100 mg (sof/vel) or sofosbuvir 400 mg/daclatasvir 60 mg(sof/dcv) for 12 weeks. Treatment response is assessed 12 weeks after completion of treatment using quantitative HCV NAT. A cure is defined as undetectable plasma RNA 12 weeks after treatment completion. is a schematic representation of the model of care.
Figure 1. Schematic for the simplified model of care for HCV screening and treatment RDT-rapid diagnostic test, NAT-nucleic acid test, HCV-hepatitis C virus, FBC-full blood count, INR-international normalized ratio, HBVsAg- hepatitis B virus surface antigen, APRI – aspartate transaminase-to–platelet ratio index, SVR- sustained virologic response.
3.2. Costs of the model of care
The unit costs for the major activities, laboratory tests and medicines of model of care using government salary scales are as shown in and . Counseling sessions including pre- and post-testing, adherence and alcohol reduction counseling were estimated to cost $0.76 per patient per session. Fibrosis assessment was estimated to cost $9.19. The unit costs per pill was $16.78 and $15.78 for sof/vel and sof/dcv respectively. The total cost per screening was estimated to be $67 and the cost per case detected was $819. The total cost per patient treated was estimated to be $2448(SD=$290) and $2364(SD = 263) per patient for sof/vel and sof/dcv respectively. The major contributor to the total cost, contributing 57.5% was the DAAs cost. The total costs per patient treated per SVR were $2550 and $2569 for sof/vel and sof/dcv respectively. The total costs for the visits and model of care are presented in . The units and total costs for the model estimated using AHF salary scales are presented in the supplementary file 1 in Tables S2‐S4.
Table 1. Unit costs for activities and the model of care*.
Table 2. Unit costs for laboratory tests and medicines the model of care*.
Table 3. Total costs for the visits and model of care for hepatitis C virus infection at tertiary hospital*.
3.3. Sensitivity analysis
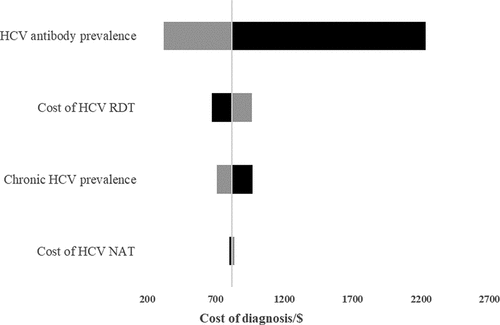
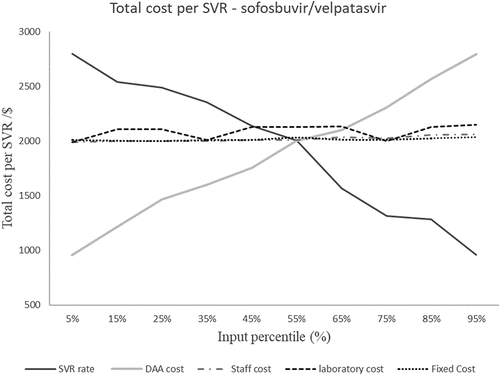
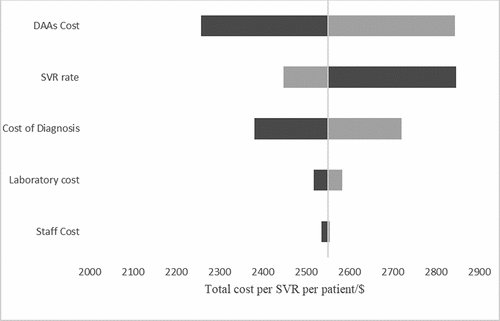
shows the effect of changing the staff cost, laboratory cost, DAAs cost, SVR rate and cost of diagnosis on the total cost per SVR. The total cost per SVR was more sensitive to DAAs cost, cost of diagnosis and SVR rate. Increasing the DAAs cost by 20% resulted in a 11.5% increase in the total cost/SVR as compared to a 0.01% increase due to change of the same magnitude and direction in laboratory costs. From the analysis, there is a direct variation relation between DAA cost and total cost/SVR and inverse variation between SVR rate and total cost per SVR. Changing staff costs, laboratory costs, and fixed cost estimates did not alter the total cost per SVR significantly. shows the impact of changing the inputs used to calculate the cost of diagnosis. The HCV antibody prevalence was the most influential input on the total cost of diagnosis. Changing the HCV antibody prevalence from 3% to 2% resulted in a 172% increase in the cost of diagnosis. shows the sensitivity spider diagram demonstrating that DAA cost and SVR rate were the most influential inputs on the total cost per SVR.
Figure 2. Tornado diagram showing the impact of changes in parameters on the total cost of care in a deterministic sensitivity analysis. SVR-sustained virologic response, DAAs-directly acting antivirals.
4. Discussion
Our study provides the first estimate of the cost of screening and treating chronic HCV infection using pan genotypic DAAs in Zimbabwe. Scaling up integrated screening and treatment of HCV is listed as one of the key activities of the strategic plan to eliminate viral hepatitis in Zimbabwe [Citation15]. This estimate is important for projecting the required resources to implement the strategic plan. The estimated cost of screening and treatment is out of reach for many Zimbabweans. A low health insurance coverage of 12% [Citation28] and high unemployment rate 35% [Citation29] result in many families not being able to afford the HCV screening and treatment services.
The estimated costs of screening and treating HCV infection using sof/vel or sof/dcv were $2448(SD = 290) and $2364 (SD = 263) respectively. These figures are substantially higher than those reported by studies from Cambodia [$925(IQR $638–$1631)] [Citation13], Pakistan [$717 (SD $336)] [Citation30] and Myanmar [$1229(95%CI 848–1829)] [Citation9]. We found DAA cost to be the major contributor to the total cost per patient in this study, and this is consistent with results from other LMICs [Citation9,Citation13,Citation30]. The differences in costs can be explained by the fact that the studies in Cambodia and Pakistan used negotiated prices for DAAs whilst our study used prices from private wholesalers in Zimbabwe. The prices of generic DAAs remain very high compared to other African countries [Citation31]. This is despite a significant decrease of 75% from 2016 in the global prices of both originator and generic DAAs [Citation31]. Public health sectors in South Africa, Rwanda and Egypt are paying $216, $60, $51 for sof/dcv 12-week supply as compared to $1326 reported in this study [Citation31]. This is potentially due to exorbitant in-country mark-ups from logistics costs and distributor mark-ups. The Zimbabwean government can benefit from reduced prices offered by pooled procurement facilities in the United Nations Development Programme (UNDP) and The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM). Through GFATM facility 12 weeks supply of sof/dcv and sof/vel can be procured at $75and s $270 respectively [Citation31]. Acquiring DAAs at these low prices will reduce the total cost of treatment significantly.
We proposed a simplified model of care that was ratified by expert opinion. Specialist-driven HCV care is difficult to implement in Zimbabwe due to the limited numbers of gastroenterologists. Simplified models of care make it possible to use junior medical officers and nurses [Citation8,Citation32–34] to manage non-complicated HCV cases and refer patients with decompensated cirrhosis, renal impairment, and treatment-experienced patients to the specialist.
In the model of HCV care presented in this paper, we proposed universal screening. Although universal HCV screening has been reported to be cost-effective [Citation35–37] in high-income countries, it remains very unaffordable for LMICs. In this study, we reported a cost per case detected of $819 which is very high as compared to $283 reported by a study in Cambodia [Citation38]. A risk-based screening strategy can be useful to reduce the cost of case finding. An example is a study in Iran where a risk-based screening questionnaire was administered among prisoners before HCV testing [Citation39]. The drug use question in the questionnaire was a positive predictor of positive HCV antibody and RNA test [Citation39]. Risk-based screening results in more individuals screened testing positive for HCV and that reduces the cost per case detected and the cost per patient treated. Anecdotal data in Zimbabwe suggests that people who received blood and blood products before isolation of HCV, HIV-infected, and people who inject drugs are the high-risk populations.
The main strength of the study was that it provided a method of estimating costs of treatment from guidelines. When treatment guidelines are published by WHO, policy-makers at the national level are not aware of the cost implications of the recommended changes. The method of cost estimation used in this study may be useful to aid future decision-making before implementation of treatment recommendations.
The cost analysis presented in this paper has potential limitations. First, the analysis focused on one site which was a tertiary hospital. A tertiary hospital is better staffed and has better access to services like laboratories. The cost estimates and implementation of the model of care may be different in district hospitals. The cost estimates reported in this study cannot be generalized to other LMICs due to differences in unit costs such as staff salaries and prices of DAAs. However, the simplified model of care for screening and treatment of HCV provides valuable information that can be shared and implemented by LMICs. The second limitation of this study is the lack of real patient-level data. Treatment of chronic HCV by DAAs was not included in the local treatment guidelines before 2020 revisions, hence very low numbers of HCV patients on DAA-based treatment. We estimated costs based on ideal adherence to the proposed model of care. Real patients may have comorbidities, bring variability in adherence, loss of follow up and clinic visits which may bring about changes in costs. Real-world patient data allows stratification of costs according to disease states (F0, F1, F2, F3, F4, DC, HCC) which is useful in health economic evaluations. However, even with such limitations, cost estimates from this study are a useful input in costing the national HCV elimination strategy.
5. Conclusions
Screening and treating chronic HCV infected individuals using pan genotypic DAAs require substantial financial investment. Further research is required to determine the benefits of such investment will be high enough for intervention to be cost-effective in Zimbabwe.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers in this manuscript have no relevant financial or other relationships to disclose.
Author contributions
B Dzingirai, N Mafirakureva, L Katsidzira and M van Hulst were involved in the conception and design of the study. B Dzingirai and V Mwanesani were involved in the data collection. B Dzingirai, N Mafirakureva, M van Hulst and MJ Postma were involved in the analysis and interpretation. B Dzingirai drafted the manuscript, and all authors reviewed the manuscript contributing to the intellectual content. All authors approved the final version for publication and agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (27.3 KB)Acknowledgments
The authors would like to thank the staff at the Parirenyatwa Opportunistic Infections (OI) Clinic.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2024.2348055
Additional information
Funding
References
- World Health Organization. Global health sector strategies on respectively, HIV, viral hepatitis, and sexually transmitted infection for the period 2022-2030. Geneva (Switzerland): World Health Organization (WHO); 2022.
- World Health Organization. Updated recommendations on treatment of adolescents and children with chronic HCV infection, and HCV simplified service delivery and diagnostics. Geneva (Switzerland): WHO; 2022.
- Graham CS, Swan T. A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Res. 2015;119:89–96. doi: 10.1016/j.antiviral.2015.01.004
- The Lancet Gastroenterology & Hepatology. Access to HCV treatments: hurdles not barriers. The Lancet Gastroenterology & Hepatology. 2017;2(1):1. doi: 10.1016/S2468-1253(16)30175-3
- The Lancet Gastroenterology & Hepatology. Crunching the numbers for viral hepatitis. Lancet Gastroenterol Hepatol. 2021;6:875. doi: 10.1016/S2468-1253(21)00350-2
- Dahl EH, Zahid H, Aslam K, et al. Leaving no one behind: towards equitable global elimination of hepatitis C. J Glob Health. 2020;10:010308. doi: 10.7189/jogh.10.010308
- Lombardi A, Mondelli MU. ESCMID Study group for viral hepatitis (ESGVH). Hepatitis C: is eradication possible? Liver Int. 2019;39:416–426. doi: 10.1111/liv.14011
- Zhang M, O’Keefe D, Craig J, et al. Decentralised hepatitis C testing and treatment in rural Cambodia: evaluation of a simplified service model integrated in an existing public health system. Lancet Gastroenterol Hepatol. 2021;6(5):371–380. doi: 10.1016/S2468-1253(21)00012-1
- Marquez LK, Chaillon A, Soe KP, et al. Cost and cost-effectiveness of a real-world HCV treatment program among HIV-infected individuals in Myanmar. BMJ Glob Health. 2021;6:e004181. doi: 10.1136/bmjgh-2020-004181
- Min Thaung Y, Chasela CS, Chew KW, et al. Treatment outcomes and costs of a simplified antiviral treatment strategy for hepatitis C among monoinfected and HIV and/or hepatitis B virus‐co‐infected patients in Myanmar. J Viral Hepat. 2021;28(1):147–158. doi: 10.1111/jvh.13405
- Mushtaq S, Akhter TS, Khan A, et al. Efficacy and safety of generic sofosbuvir plus daclatasvir and sofosbuvir/velpatasvir in HCV genotype 3-infected patients: real-world outcomes from Pakistan. Front Pharmacol. 2020:11. doi: 10.3389/fphar.2020.550205
- Sonderup MW, Gogela N, Nordien R, et al. Direct-acting antiviral therapy for hepatitis C: the initial experience of the University of Cape Town/Groote Schuur Hospital Liver Clinic, South Africa. S Afr Med J. 2020;110:112. doi: 10.7196/SAMJ.2020.v110i2.14195
- Walker JG, Mafirakureva N, Iwamoto M, et al. Cost and cost‐effectiveness of a simplified treatment model with direct‐acting antivirals for chronic hepatitis C in Cambodia. Liver Int. 2020;40:2356–2366. doi: 10.1111/liv.14550
- Asker B, Jawad R, Asreah R, et al. Cost effectiveness of screening for hepatitis C virus in Iraq in the era of simplified testing and treatment. PharmacoEconomics. 2021;39:1327–1341. doi: 10.1007/s40273-021-01064-z
- Ministry of Health and Child Care Zimbabwe. Strategic plan for the control and elimination of viral hepatitis in Zimbabwe 2019-2022. Government of Zimbabwe; 2019.
- Saayman E, Hechter V, Kayuni N, et al. A simplified point-of-service model for hepatitis C in people who inject drugs in South Africa. Harm Reduct J. 2023;20(1):27. doi: 10.1186/s12954-023-00759-0
- Markby J, Shilton S, Sem X, et al. Assessing the impact of simplified HCV care on linkage to care amongst high-risk patients at primary healthcare clinics in Malaysia: a prospective observational study. BMJ Open. 2021;11:e055142. doi: 10.1136/bmjopen-2021-055142
- Boyer S, Baudoin M, Nishimwe ML, et al. Cost-utility analysis of four WHO-recommended sofosbuvir-based regimens for the treatment of chronic hepatitis C in sub-saharan Africa. BMC Health Serv Res. 2022;22:303. doi: 10.1186/s12913-021-07289-0
- Assefa Y, Hill PS, Ulikpan A, et al. Access to medicines and hepatitis C in Africa: can tiered pricing and voluntary licencing assure universal access, health equity and fairness? Global Health. 2017;13:73. doi: 10.1186/s12992-017-0297-6
- Hendriks ME, Kundu P, Boers AC, et al. Step-by-step guideline for disease-specific costing studies in low- and middle-income countries: a mixed methodology. Glob Health Action. 2014;7: doi: 10.3402/gha.v7.23573
- Costa N, Derumeaux H, Rapp T, et al. Methodological considerations in cost of illness studies on Alzheimer disease. Health Econ Rev. 2012;2(1):18. doi: 10.1186/2191-1991-2-18
- Bertram MY, Lauer JA, Stenberg K, et al. Methods for the economic evaluation of health care interventions for priority setting in the health system: an update from WHO CHOICE. Int J Health Policy Manag. 2021;10:673–677. doi: 10.34172/ijhpm.2020.244
- Larson BA, Ngoma T, Silumbe K, et al. A framework for evaluating the costs of malaria elimination interventions: an application to reactive case detection in southern province of Zambia, 2014. Malaria j. 2016;15(1):408. doi: 10.1186/s12936-016-1457-5
- World Health Organization. WHO-Choosing health interventions that are cost effective. 2005 [cited 2020 Dec 18]. Available from: https://www.who.int/news-room/questions-and-answers/item/who-choice-frequently-asked-questions
- Reserve Bank of Zimbabwe. Exchange rates. [cited 2019 Sep 6]. Available from: https://www.rbz.co.zw/index.php/research/markets/exchange-rates
- Rao VB, Johari N, Du Cros P, et al. Hepatitis C seroprevalence and HIV co-infection in sub-saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:819–824. doi: 10.1016/S1473-3099(15)00006-7
- Mafirakureva N, Stone J, Fraser H, et al. An intensive model of care for hepatitis C virus screening and treatment with direct‐acting antivirals in people who inject drugs in Nairobi, Kenya: a model‐based cost‐effectiveness analysis. Addiction. 2022;117:411–424. doi: 10.1111/add.15630
- Mhazo AT, Maponga CC, Mossialos E. Inequality and private health insurance in Zimbabwe: history, politics and performance. Int J Equity Health. 2023;22(1):54. doi: 10.1186/s12939-023-01868-9
- Zimbabwe National Statistics Agency. First Quarter labour force survey report 2022. Zimbabwe; 2022.
- Mafirakureva N, Lim AG, Khalid GG, et al. Cost‐effectiveness of screening and treatment using direct‐acting antivirals for chronic hepatitis C virus in a primary care setting in Karachi, Pakistan. J Viral Hepat. 2021;28:268–278. doi: 10.1111/jvh.13422
- Clinton Health Access Initiative (CHAI). HCV Market Intelligence Report 2021 and Preliminary HBV market Insights. Clinton Health Access Initiative. 2021.
- Kapadia SN, Marks KM. Hepatitis C management simplification from test to cure: a framework for primary care providers. Clin Ther. 2018;40:1234–1245. doi: 10.1016/j.clinthera.2018.05.010
- Radley A, Robinson E, Aspinall EJ, et al. A systematic review and meta-analysis of community and primary-care-based hepatitis C testing and treatment services that employ direct acting antiviral drug treatments. BMC Health Serv Res. 2019;19:765. doi: 10.1186/s12913-019-4635-7
- Dieterich DT. A simplified algorithm for the management of hepatitis C infection. Gastroenterol Hepatol. 2019;15(5 supp 3):1–12.
- Zhou H, Yan M, Che D, et al. Universal screening for HCV infection in China: an effectiveness and cost-effectiveness analysis. JHEP Rep. 2024;6:101000. doi: 10.1016/j.jhepr.2024.101000
- Eckman MH, Ward JW, Sherman KE. Cost effectiveness of universal screening for hepatitis C virus infection in the era of direct-acting, pangenotypic treatment regimens. Clin Gastroenterol Hepatol. 2019;17(5):930–939.e9. doi: 10.1016/j.cgh.2018.08.080
- Kim H-L, Kim K-A, Choi GH, et al. A cost-effectiveness study of universal screening for hepatitis C virus infection in South Korea: a societal perspective. Clin Mol Hepatol. 2022;28:91–104. doi: 10.3350/cmh.2021.0236
- Han SM, Por I, Samley K, et al. Costing analysis of field implementation of hepatitis C case detection in rural Maung Russey operational district, Cambodia. WPSAR. 2021;12:17–24. doi: 10.5365/wpsar.2020.11.3.006
- Hariri S, Sharafkhah M, Alavi M, et al. A simple risk-based strategy for hepatitis C virus screening among incarcerated people in a low- to middle-income setting. Harm Reduct J. 2020;17(1):56. doi: 10.1186/s12954-020-00400-4