ABSTRACT
Introduction
Patients with epilepsy can experience seizure clusters (acute repetitive seizures), defined as intermittent, stereotypic episodes of frequent seizure activity that are distinct from typical seizure patterns. There are three FDA-approved rescue medications, diazepam rectal gel, midazolam nasal spray, and diazepam nasal spray, that can be administered to abort a seizure cluster in a nonmedical, community setting. Despite their effectiveness and safety, rescue medications are underutilized, and patient/caregiver experiences and perceptions of ease of use may constitute a substantial barrier to greater utilization.
Areas covered
The literature on rescue medications for seizure clusters is reviewed, including the effectiveness and safety, with an emphasis on ease and timing of treatment and associated outcomes. Barriers to greater utilization of rescue medication and the role of seizure action plans are discussed.
Expert opinion
Intranasal rescue medications are easier to use and can be administered more rapidly than other routes (rectal, intravenous). Importantly, rapid administration of intranasal rescue medications has been associated with shorter durations of seizure activity as compared with rectal/intravenous routes. Intranasal rescue medications are also easy to use and socially acceptable. These factors potentially remove or reduce barriers to use and optimize the management of seizure clusters.
1. Introduction
Some patients with epilepsy may experience seizure clusters (also called acute repetitive seizures), which are generally considered to be acute episodes of increased seizure activity that differ from a patient’s usual seizure pattern [Citation1]. Seizure clusters are typically recognizable to caregivers [Citation2] and may be characterized differently for each patient [Citation3]. Seizure clusters can consist of any seizure type (eg, focal or generalized epilepsy with motor seizures or alteration of awareness) [Citation4] and are more common in patients with pharmacoresistant epilepsy [Citation5]. Prompt treatment is critical to prevent additional seizures in the cluster because seizure clusters can lead to status epilepticus and potentially neuronal damage and death [Citation6–8]. Appropriately dosed benzodiazepines remain the mainstay of treatment for seizure clusters, with the first treatment, diazepam rectal gel, being approved by the US Food and Drug Administration (FDA) in 1997 [Citation8].
Intramuscular and buccal administration of benzodiazepines are sometimes used for the treatment of seizure clusters in prehospital seizure emergencies [Citation9], and there are various other antiseizure medications that show promise for the treatment for various epilepsy conditions including rescue therapy for seizure clusters, although so far these are unapproved by the FDA [Citation10]. Diazepam rectal gel, midazolam nasal spray, and diazepam nasal spray are approved by the FDA for acute treatment of intermittent, stereotypic episodes of frequent seizure activity (seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy [Citation11–13]. Diazepam rectal gel is approved for use in patients ≥2 years of age and diazepam nasal spray is approved for patients ≥6 years, while midazolam nasal spray is approved for patients ≥12 years [Citation11–13]. A second dose of diazepam rectal gel or diazepam nasal spray may be administered 4 h after the first dose [Citation11,Citation13], and a second dose of midazolam nasal spray may be administered 10 min after the initial dose [Citation12]. Administering diazepam rectal gel involves multiple steps [Citation11] and carries privacy considerations, all of which could affect time to treatment [Citation14]. Easy access with fewer steps to administer nasal sprays [Citation12,Citation15] make these formulations attractive alternatives to rectal gel. Least restrictive rescue medications (ie, intranasal) are recommended for treatment in a school setting by the National Association of School Nurses (NASN) [Citation16]. The FDA designated diazepam nasal spray as clinically superior to diazepam rectal gel owing to the inherently easier and less invasive route of administration [Citation17]. In the European Union (EU), seizure clusters are not recognized as a clinical indication [Citation1]. However, buccal midazolam (oromucosal solution) is approved by the EU for treatment of prolonged, acute, convulsive seizures in pediatric patients 3 months to less than 18 years of age [Citation18], and rectal diazepam solution is indicated for treatment of epileptic and febrile convulsions [Citation19–21].
Rescue medications for seizure clusters are underutilized in the community setting [Citation8]. In a survey published in 2017, 20 years after approval of diazepam rectal gel, only 20% of adult patients reported use of rescue medications for seizure-cluster treatment [Citation22]. The objective of this manuscript is to discuss potential barriers to the use of rescue medications for seizure clusters and how the underlying characteristics of rescue medications impact these barriers, including such factors as ease of use, convenience, time to onset, and duration of effectiveness.
2. Barriers to use of rescue medication for seizure clusters
Potential barriers include route of administration (ie, rectal) [Citation22], which is intertwined with the degree of difficulty in administering the treatment, which in turn affects the time to treatment and therefore the time to seizure cessation [Citation23]. Seizure action plans (SAPs) [Citation22], a general resource for care, and acute SAPs (ASAPs), focused on acute treatment during a seizure [Citation3], help to optimize rescue medication use, but these are not, however, utilized by most patients. Lack of access to rescue medications may also be an issue due to lack of insurance coverage, requirement for prior authorization, limited pharmacy availability, or cost.
A potential barrier to use of rescue medication is the absence of a consensus definition for seizure cluster [Citation1]; thus, some patients may not receive a diagnosis and rescue medication. Further, even when rescue medication is available, confusion over identification of a seizure cluster may prevent timely administration [Citation1], which is associated with faster cessation of seizure activity [Citation24]. Additional barriers may include a lack of understanding of the role for rescue treatment (use, effectiveness, and safety) as well as emotional barriers of fear and anxiety [Citation22,Citation25]. Fear and anxiety reflect the stress felt by patients and caregivers when experiencing a seizure cluster and may involve such issues as public embarrassment (particularly with rectal administration), when and if to use a second dose, and length of time needed to recuperate [Citation25].
Improved understanding of rescue medications, including ease of use and administration, and use of SAPs could reduce barriers and lead to greater utilization, which could empower patients/caregivers to optimize management of seizure clusters. This could be especially true for those with excessive seizure worry (anticipatory anxiety of seizures), who have an elevated fear of seizures and could benefit from counseling on treatment options [Citation26]. In general, nonadherence with an epilepsy medication regimen is associated with an external locus of control [Citation27], which is a person’s belief that their life is controlled by outside factors over which they have limited control [Citation28]. A better understanding of easy-to-use options for rescue medication and subsequent increased utilization could potentially improve how patients perceive their level of control over seizures, thereby promoting a patient-centric treatment approach.
Prompt treatment of seizure clusters with rescue medication may prevent negative outcomes (eg, physical injury and quality of life in addition to progression to status epilepticus, emergency-department use, and neuronal injury) [Citation6,Citation7,Citation29]. Moreover, continuous seizure activity is associated with a diminished response to benzodiazepine treatment [Citation30].
3. Rescue medications for seizure clusters
An ideal rescue medication should be easy to use, safe with good tolerability, and potent and have consistent, efficient absorption with high bioavailability, resulting in a rapid onset of action and sufficient duration [Citation8]. The efficacy of approved rescue medications (diazepam and midazolam) to terminate seizure clusters has been established in randomized placebo-controlled trials [Citation2,Citation31,Citation32]. Importantly, because seizure clusters are recognizable to caregivers, administration instructions for these formulations do not require occurrence of a second seizure or waiting a specified amount of time prior to administration [Citation11–13]. However, these rescue medications may differ according to route of administration and formulation, including molecules, excipients, and solvents [Citation8].
The route and steps for administration may affect who can administer treatment and time to treatment, which could influence overall time to seizure cessation and the potential risks of status epilepticus [Citation7,Citation24]. Prompt treatment for seizure clusters is reinforced by guidelines for the treatment of status epilepticus, which recommend treatment initiation well in advance of the 30-minute mark defining onset of status epilepticus [Citation33]. Associated advantages and disadvantages () of different rescue medications may influence ease of use and time to administration.
Table 1. Advantages and disadvantages of FDA-approved rescue medications.
4. Diazepam rectal gel
An open-label, repeat-dose study with 149 treated patients examined safety, efficacy, and tolerance to diazepam rectal gel for treatment of seizure clusters, breakthrough seizures, or prolonged seizures in patients aged ≥2 years [Citation29]. Few adverse events (AEs) related to treatment occurred, with somnolence reported in 17% of patients, although as few as 9% of cases were considered related to treatment. Two patients reported transient hypoventilation; no serious AEs were treatment related. Three patients withdrew from the study owing to AEs that were considered possibly related or related to treatment. A single dose of diazepam rectal gel was sufficient to prevent additional seizures for 77% of seizure episodes during the 12 h following treatment. Time to administration of diazepam rectal gel was not captured in this study [Citation29].
4.1. Ease of use
Relative to an intravenous (IV) treatment, diazepam rectal gel is easy for nonmedical personnel to administer (limited training, no IV preparation needed) [Citation35]. For some, the familiarity/experience with diazepam rectal gel [Citation29,Citation34] might improve comfort with administration. However, diazepam rectal gel has social limitations, particularly at school, work, or public settings [Citation34]. Administration is a 14-step process that requires positioning the patient on their side [Citation11], which may not be easy for some situations (eg, heavier patients or those who utilize wheelchairs).
4.2. Time to administration
The rectal route has been associated with shorter times to treatment for seizure emergencies compared with an IV route [Citation35]. In a study that compared time to administration with diazepam rectal gel vs IV lorazepam for treatment of status epilepticus in adults with developmental disabilities, time to administration was shorter by ~8 min with diazepam rectal gel (12 vs 20 min) [Citation35]. Nurses began rescue treatment after 10 min of seizure activity [Citation35]. The delay in administration with IV lorazepam is likely a product of preparation and infusion [Citation8], which can cause substantial delays in treatment compared with diazepam rectal gel. This is noteworthy as diazepam rectal gel also requires time to prepare the patient (eg, disrobing and turning) for administration [Citation34]. The mean time from administration to seizure cessation was longer with diazepam rectal gel than IV lorazepam (7 vs 4 min), but the total seizure duration was shorter with diazepam rectal gel (18 vs 25 min) [Citation35]. This suggests that the difference between rectal diazepam and IV lorazepam in time to administration has a meaningful effect on the overall treatment outcome.
5. Midazolam nasal spray
An open-label extension study of a phase 3 trial was conducted to examine the safety and efficacy of midazolam nasal spray for treatment of seizure clusters in patients aged ≥12 years (N = 161 treated patients) [Citation36]. Common treatment-emergent AEs were nasal discomfort (12.4%) and somnolence (9.3%). There were no reports of respiratory depression attributed to treatment. (In the test-dose phase of the parent placebo-controlled study, 5 of 292 patients experienced respiratory depression and discontinued the study [Citation32]). Two patients (1.2%) discontinued the study owing to treatment-related AEs (1 case each of somnolence and nasal discomfort). Most seizure-cluster episodes (61.5%) were treated with a single dose of midazolam nasal spray within the protocol-defined period of up to 6 h from seizure onset [Citation36]; notably, this was half the 12-hour period after administration that was used in the diazepam rectal gel study [Citation29]. The median time for patients to return to normal function following treatment was 1.2 h [Citation36]. Time to administration has not been fully characterized and ease of use has not been described with the approved formulation of midazolam nasal spray [Citation36], although early time to treatment has been associated with increased rates of sustained control of seizure clusters (24 h) [Citation37].
5.1. Ease of use
In a survey that compared intranasal administration of an IV formulation of midazolam administered using an atomizer (prior to approved formulations of midazolam and diazepam nasal sprays) with rectal diazepam for treatment of acute seizures, more than two-thirds of caregivers felt that intranasal midazolam was easier to use and explain and was more comfortable to use than rectal diazepam [Citation38]. Another survey of caregivers also reported that administration was easier with atomized midazolam compared with rectal diazepam for treatment of prolonged seizures [Citation39]. Intranasal administration of atomized midazolam has been associated with less use of emergency and urgent care services as compared to rectal diazepam [Citation40], which the authors felt could be attributed, in part, to ease and comfort of use [Citation38].
5.2. Time to administration
Few studies have specifically examined the difference in time to administration between intranasal midazolam and rectal diazepam. One study reported a shorter time from preparation to administration with intranasal midazolam (nasal drops, 50.6 s) than rectal diazepam (68.3 s) for treatment of acute seizures [Citation41]. In contrast, a study of home use of rescue medications for treatment of prolonged seizures did not report a difference in the median time to administration between intranasal, atomized midazolam and rectal diazepam (5 min for both) [Citation39]. However, in addition to differences in intranasal administration (atomized solution vs nasal drops), this study recorded the timing of the administration of atomized solution from caregivers who were instructed to treat seizures lasting >5 minutes [Citation39], while the nasal-drop study was conducted in a medical facility and healthcare professionals administered the rescue medications [Citation41].
A recent systematic review and meta-analysis of rescue medications used in children for treatment of acute seizures reported similar durations from time to administration to seizure cessation between intranasal midazolam (nasal drops or atomized solution) and rectal or IV benzodiazepines [Citation24]. However, the duration from hospital arrival to seizure cessation was shorter with intranasal midazolam, suggesting that intranasal treatment required less time to administer compared with rectal or IV treatment [Citation24].
6. Diazepam nasal spray
The safety and effectiveness of diazepam nasal spray for treatment of seizure clusters was examined in a phase 3, long-term, open-label, repeat-dose, safety study that consisted of 163 treated patients [Citation4]. The most common AEs, aside from seizure, were nasopharyngitis (12.3%) and upper respiratory tract infection (12.3%). Treatment-related nasal discomfort and somnolence occurred in 6.1% and 1.8% of patients, respectively. There were no reports of either respiratory depression or serious treatment-related AEs. No patients discontinued the study owing to a treatment-related AE. A single dose of diazepam nasal spray was used in 87.4% of seizure clusters across 24 h [Citation4] (twice the 12-hour period in the rectal gel study [Citation29]), a surrogate that supports the effectiveness of treatment [Citation4]. Most patients (59.4%) who responded to a patient-experience survey indicated that they returned to their usual self within 1 h of administration [Citation42]. Similarly, more than half of caregivers (59.5%) responding to the survey reported they (caregivers) were able to return to normal activities within an hour of administration [Citation42].
6.1. Ease of use
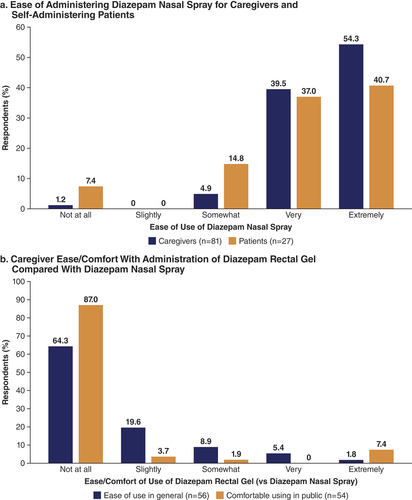
Patient and caregiver experiences with diazepam nasal spray were assessed with surveys administered near the end of the long-term safety study [Citation42]. Administration of diazepam nasal spray was rated extremely or very easy by 93.8% of caregivers (). Nearly two-thirds (64.3%) of caregivers who had used diazepam rectal gel in the past felt that it was not at all easy to use compared with diazepam nasal spray (), and 87.0% were not at all comfortable using diazepam rectal gel in public compared with diazepam nasal spray. As an important indication of ease of use, among 67 patients who responded to the survey, 27 (40.3%) reported participating in their care by self-administering treatment. Three-quarters (77.8%) of this group rated administration extremely or very easy (). In addition, two-thirds (66.7%) were extremely, very, or somewhat comfortable with its use in a public setting. Patients self-administered primarily at first sign of an oncoming seizure (48%) or between seizures (24%), which would suggest ease of use during potentially stressful situations. The rate of medication errors in self-administering patients was low (1.1%), primarily as a result of missing the nostril during administration or misunderstanding directions [Citation42]. The overall rate of medication errors in the safety study was 1.0% [Citation4].
6.2. Time to administration
In the safety study, time to administration of diazepam nasal spray from onset of seizure activity was recorded in seizure diaries [Citation4]. Fifty percent of treated seizure clusters were dosed in ≤2 min (range, 1–750 min) from onset (), and 75% were treated in ≤5 min [Citation43]. In children aged 6–11 years (diazepam nasal spray is the only approved intranasal rescue medication for this age group [Citation12,Citation13]), 50% of treated seizure clusters were treated in ≤2 min from onset, and 75% were treated in ≤5 min [Citation43]. In addition, treatment times in patients who self-administered were comparable to the full cohort () [Citation43].
Figure 2. Time to Diazepam Nasal Spray Administration. Time to administration expressed as cumulative percentiles of patients (eg, 25th, 50th, 75th), from onset of the seizure cluster to treatment for all patients (a: n = 162; 3627 seizures) and self-administrators (b: n = 26; 814 seizures). For example, 75% of all patients (a) received diazepam nasal spray in ≤5 minutes from seizure-cluster onset.
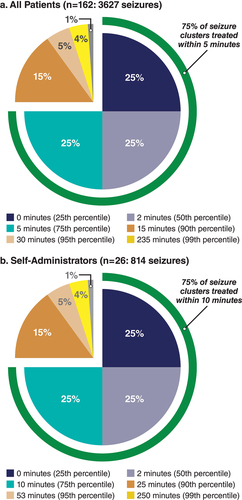
The median time from treatment to seizure-cluster cessation was 4 min in the full cohort [Citation43] and 3 min in the subgroup of patients aged 6–11 years. The median time from seizure onset to cessation was 6 min for both the full cohort [Citation43] and the age 6–11 subgroup. It is worth noting that both diazepam and midazolam intranasal rescue medications utilize a device for intranasal delivery that does not require a particular head position for successful administration, which also aids ease and speed of administration [Citation44].
7. SAPs
Having a written action plan that provides customized treatment information for seizures can also facilitate seizure-cluster management. An SAP is a general resource for care, including patient information and medications, seizure information (eg, triggers), first aid, and contact information [Citation3,Citation45,Citation46]. An acute SAP (ASAP) is a more concise plan focused on acute treatment during a seizure, designed to provide easy-to-understand treatment instructions, individualized to each patient and seizure type [Citation3]. As such, the ASAP may be ideal for improving consistency of management of seizure emergencies in the community and providing caregivers with reassuring guidance during a stressful time. Formalized plans for seizure care could reinforce timely treatment and appropriate dosing. However, SAP use differs across adult and pediatric populations [Citation3], with more pediatric patients utilizing SAPs, possibly because of school requirements [Citation34]. Overcoming barriers to adult use of SAPs and encouraging the use and sharing of information in the workplace might also lead to greater utilization of rescue medication.
8. Conclusions
Prompt administration of rescue medication is a critical component of seizure-cluster treatment. Ease of use and patient satisfaction with intranasal rescue medications suggest that these agents help provide patient-centric management of seizure clusters. Intranasal rescue medications are associated with prompt administration, supporting rapid, effective treatment during seizure clusters. Time to treatment, time to seizure cessation, and ease of use are improved with intranasal rescue medications, with reported differences in duration of action measured across noncomparative studies. In all, the incorporation of easy-to-use and effective intranasal rescue medications into a patient-centric treatment approach along with SAPs/ASAPs could empower patients/caregivers to take on a greater role in the rapid treatment and management of seizure clusters.
8.1. Expert opinion
The unpredictable nature of drug-resistant epilepsy and seizure clusters can create a substantial burden on patients and caregivers [Citation5,Citation47]. Clinicians can help patients and families understand appropriate use of first aid and rescue treatment options, which provide an opportunity to increase adherence and improve overall treatment by empowering patients and caregivers to take a larger role in management of prolonged seizures and clusters.
The introduction of a relatively easy-to-use rectal gel in 1997 made it possible for nonmedical caregivers to treat seizure clusters at home and in the community [Citation8], potentially reducing emergency room visits by half [Citation29]. More than just an advance in convenience, treatment could be provided quickly, without delay for transport to the hospital. Despite this pharmacologic achievement, barriers to treatment remained. For example, one study found that although 4 in 5 physicians advised their patients to use rescue medication for a seizure cluster, only 1 in 5 adults used rescue treatment for seizure clusters [Citation22]. For adult patients, in particular, social considerations may have limited even the consideration of rescue treatment.
The more recent availability of easy-to-use, safe, and effective intranasal rescue medications may encourage their use and promote patient/caregiver management of serious seizure events. This aligns with the recommendations from NASN that support the least restrictive option for rescue medication in school settings [Citation34]. Intranasal therapy may be administered rapidly (no need to position or disrobe the patient), more easily (fewer steps in administration [Citation34], premeasured doses [Citation48], option for self-administration [Citation22]), and with potentially less interpatient variability in bioavailability [Citation49] than rectal therapy. Moreover, intranasal therapies are associated with a rapid onset of action (within minutes) [Citation50]. Formulations that people feel comfortable using in public may result in people talking more openly about their epilepsy and help dispel fear of seizures.
Future developments in clinical use include expanding patient-centric management strategies, such as creating written SAPs with the patients and/or caregivers. This would reinforce consistent and appropriate use of rescue treatment. It could also start a discussion of other self-management strategies and may lead to improved quality of life and reduced need for emergency care [Citation51], further expanding patient and caregiver empowerment. SAPs seem likely to be further incorporated into practice, which is a pressing need for adult patients. Easy-to-use patient- and family-friendly formats could be integrated into electronic medical record systems, for example [Citation3]. Future research should be aimed toward better identification of patients with seizure clusters and strategies to increase the proportion with both SAPs and rescue medication. In addition, further research into what endpoints are important to patients and families is needed. A better understanding of aspects of that burden and the effect of rescue treatment for patients and caregivers (eg, anxiety, social interaction, and quality of life) as well as the healthcare system (eg, need for emergency department visits) could reinforce the value of treatment and minimize underuse.
Another goal of future research would be to expand the benefits of intranasal treatment to younger patients who could benefit from ease and speed of administration, as well as characterizing blood levels of rescue medication (benzodiazepines) in these patients. (Of note, pediatric blood levels have not been assessed for diazepam rectal gel [Citation52]). Stellina (NCT05076838) is an ongoing open-label pharmacokinetic and safety study of diazepam nasal spray in children 2 to 5 years of age with epilepsy needing benzodiazepine intervention for seizure control [Citation52,Citation53].
Together, a more nuanced definition of the burden imposed by seizure clusters, quantification of benefits of treatment, and expansion of treatment options for seizure clusters will help address a range of important yet currently unmet clinical needs for patients, their families and caregivers, and clinicians.
Article highlights
Rescue medications (typically benzodiazepines) can be used to treat seizure clusters in a community setting, with the potential to improve rescue treatment outcomes
FDA-approved rescue medications have shown effectiveness; however, these medications are largely underutilized
Route of administration can differ across rescue medications (intranasal vs rectal), affecting the ease and acceptability of use as well as time to treatment
Individual patient seizure action plans are useful adjuncts to treatment
Rescue medications that are easier to use and implemented with seizure action plans may help address barriers to use
Declaration of interest
K O’Hara is on the advisory board of Greenwich Biosciences (now part of Jazz Pharmaceuticals) and is a speaker for Neurelis, Inc. S Dewar is a consultant to Neurelis, Inc. P Dean is a speaker for Jazz Pharmaceuticals and Neurelis, Inc., and is a consultant for Azurity Pharmaceuticals. S Misra is an employee of and has received stock options from Neurelis, Inc. J Desai has received research funding from Neurelis, Inc,; Novartis; Ovid; Aquestive; and UCB. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial support was provided by Kirk W. Evanson, PhD, of The Curry Rockefeller Group, LLC (Tarrytown, NY), and was funded by Neurelis, Inc. (San Diego, CA).
Additional information
Funding
References
- Haut SR, Nabbout R. Recognizing seizure clusters in the community: the path to uniformity and individualization in nomenclature and definition. Epilepsia. 2022;63(suppl 1):S6–13.
- Dreifuss FE, Rosman NP, Cloyd JC, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338(26):1869–1875. DOI:10.1056/NEJM199806253382602
- Penovich P, Glauser T, Becker D, et al. Recommendations for development of acute seizure action plans (ASAPs) from an expert panel. Epilepsy Behav. 2021;123:108264. DOI:10.1016/j.yebeh.2021.108264
- Wheless JW, Miller I, Hogan RE, et al. Final results from a phase 3, long-term, open-label, repeat-dose safety study of diazepam nasal spray for seizure clusters in patients with epilepsy. Epilepsia. 2021;62(10):2485–2495. DOI:10.1111/epi.17041
- Jafarpour S, Hirsch LJ, Gainza-Lein M, et al. Seizure cluster: definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. DOI:10.1016/j.seizure.2018.05.013
- Dingledine R, Varvel NH, Dudek FE. When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv Exp Med Biol. 2014;813:109–122.
- Haut SR. Seizure clusters: characteristics and treatment. Curr Opin Neurol. 2015;28(2):143–150.
- Cloyd J, Haut S, Carrazana E, et al. Overcoming the challenges of developing an intranasal diazepam rescue therapy for the treatment of seizure clusters. Epilepsia. 2021;62(4):846–856. DOI:10.1111/epi.16847
- Shah MI, Macias CG, Dayan PS, et al. An evidence-based guideline for pediatric prehospital seizure management using GRADE methodology. Prehosp Emerg Care. 2014;18(suppl 1):15–24. DOI:10.3109/10903127.2013.844874
- Pong AW, Ross J, Tyrlikova I, et al. Epilepsy: expert opinion on emerging drugs in phase 2/3 clinical trials. Expert Opin Emerg Drugs. 2022;27(1):75–90. DOI:10.1080/14728214.2022.2059464
- Bausch Health US, LLC. Diastat® C-IV (diazepam rectal gel). Full prescribing information. Bridgewater NJ: Bausch Health US, LLC; 2023.
- UCB, Inc. Nayzilam® (midazolam nasal spray). Full prescribing information. Smyrna GA: UCB, Inc.; 2023.
- Neurelis, Inc. VALTOCO® (diazepam nasal spray). Full prescribing information. San Diego CA: Neurelis, Inc.; 2023.
- Tatum WO. Adult patient perceptions of emergency rectal medications for refractory seizures. Epilepsy Behav. 2002;3(6):535–538.
- Neurelis, Inc. VALTOCO. Instructions for use for 5 mg and 10 mg doses. San Diego CA: Neurelis, Inc; 2022.
- Lepkowski AM. School nursing evidence-based practice clinical guideline: students with seizures and epilepsy. Silver Spring MD: National Association of School Nurses; 2018.
- US Food and Drug Administration. Clinical Superiority Findings [Internet]. cited 2020 Jun 23]. Available from: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/clinical-superiority-findings
- Shire Services BVBA. Buccolam summary of product characteristics (midazolam hydrochloride oromucosal solution). Summary of product characteristics. Brussels Belgium: Shire Pharmaceuticals; 2020.
- Electronic Medicines Compendium. Diazepam RecTubes 10mg rectal solution [Internet]. [cited 2021 Sep 7]. Available from: https://www.medicines.org.uk/emc/product/6799/smpc
- Electronic Medicines Compendium. Stesolid rectal tubes 10mg [Internet]. [cited 2021 Sep 10]. Available from: https://www.medicines.org.uk/emc/product/104/smpc
- Electronic Medicines Compendium. Diazepam desitin 5 mg rectal solution [Internet]. [cited 2021 Sep 8]. Available from: https://www.medicines.org.uk/emc/product/2997/smpc
- Penovich PE, Buelow J, Steinberg K, et al. Burden of seizure clusters on patients with epilepsy and caregivers: survey of patient, caregiver, and clinician perspectives. Neurologist. 2017;22(6):207–214. DOI:10.1097/NRL.0000000000000140
- Haut SR, Seinfeld S, Pellock J. Benzodiazepine use in seizure emergencies: a systematic review. Epilepsy Behav. 2016;63:109–117.
- Chhabra R, Gupta R, Gupta LK. Intranasal midazolam versus intravenous/rectal benzodiazepines for acute seizure control in children: a systematic review and meta-analysis. Epilepsy Behav. 2021;125:108390.
- Kapur J, Long L, Dixon-Salazar T. Consequences: bench to home. Epilepsia. 2022;63(suppl 1):S14–24.
- Ertan D, Hubert-Jacquot C, Maillard L, et al. Anticipatory anxiety of epileptic seizures: an overlooked dimension linked to trauma history. Seizure. 2021;85:64–69.
- Gopinath B, Radhakrishnan K, Sarma PS, et al. A questionnaire survey about doctor-patient communication, compliance and locus of control among south Indian people with epilepsy. Epilepsy Res. 2000;39(1):73–82. DOI:10.1016/S0920-1211(99)00112-6
- Boddu VK, Rebello A, Chandrasekharan SV, et al. How does “locus of control” affect persons with epilepsy? Epilepsy Behav. 2021;123:108257.
- Mitchell WG, Conry JA, Crumrine PK, et al. An open-label study of repeated use of diazepam rectal gel (Diastat) for episodes of acute breakthrough seizures and clusters: safety, efficacy, and tolerance. North American Diastat Group. Epilepsia. 1999;40(11):1610–1617. DOI:10.1111/j.1528-1157.1999.tb02047.x
- Naylor DE. Treating acute seizures with benzodiazepines: does seizure duration matter? Epileptic Disord. 2014;16(Spec No 1):S69–83.
- Cereghino JJ, Mitchell WG, Murphy J, et al. Treating repetitive seizures with a rectal diazepam formulation: a randomized study. The North American Diastat Study Group. Neurology. 1998;51(5):1274–1282. DOI:10.1212/WNL.51.5.1274
- Detyniecki K, Van Ess PJ, Sequeira DJ, et al. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters—a randomized, double-blind, placebo-controlled trial. Epilepsia. 2019;60(9):1797–1808. DOI:10.1111/epi.15159
- Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61. DOI:10.5698/1535-7597-16.1.48
- Dean P, O’Hara K, Brooks L, et al. Managing acute seizures: new rescue delivery option and resources to assist school nurses. NASN Sch Nurse. 2021;36(6):346–354. DOI:10.1177/1942602X211026333
- Fitzgerald BJ, Okos AJ, Miller JW. Treatment of out-of-hospital status epilepticus with diazepam rectal gel. Seizure. 2003;12(1):52–55.
- Wheless JW, Meng TC, Van Ess PJ, et al. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters: an open-label extension trial. Epilepsia. 2019;60(9):1809–1819. DOI:10.1111/epi.16300
- Wheless JW, Brunnert M, Floricel F, et al. Early intervention with midazolam nasal spray and efficacy in patients with seizure clusters: post-hoc analysis of an open-label extension trial (P13-8.007). Neurology. 2022;98(18 suppl):707. https://n.neurology.org/content/98/18_Supplement/707
- Nunley S, Glynn P, Rust S, et al. A hospital-based study on caregiver preferences on acute seizure rescue medications in pediatric patients with epilepsy: intranasal midazolam versus rectal diazepam. Epilepsy Behav. 2019;92:53–56.
- Holsti M, Dudley N, Schunk J, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164(8):747–753. DOI:10.1001/archpediatrics.2010.130
- Nunley S, Glynn P, Rust S, et al. Healthcare utilization characteristics for intranasal midazolam versus rectal diazepam. J Child Neurol. 2018;33(2):158–163. DOI:10.1177/0883073817744696
- Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34(5):355–359.
- Penovich P, Wheless JW, Hogan RE, et al. Examining the patient and caregiver experience with diazepam nasal spray for seizure clusters: results from an exit survey of a phase 3, open-label, repeat-dose safety study. Epilepsy Behav. 2021;121(Pt A):108013. DOI:10.1016/j.yebeh.2021.108013
- Wheless J, Peters J, Misra SN, et al. Comment on “Intranasal midazolam versus intravenous/rectal benzodiazepines for acute seizure control in children: a systematic review and meta-analysis”. Epilepsy Behav. 2022;128:108550.
- Aptar Pharma. Aptar brochure: nasal spray device with uni-dose and bi-dose systems. Crystal Lake, IL: The corporate headquarters; 2016. https://www.aptar.com/wp-content/uploads/2020/07/Brochure-Nasal-spray-device-with-Uni-dose-and-Bi-dose-systems.pdf
- Gidal B, Klein P, Hirsch LJ. Seizure clusters, rescue treatments, seizure action plans: unmet needs and emerging formulations. Epilepsy Behav. 2020;112:107391.
- Neville KL, McCaffery H, Baxter Z, et al. Implementation of a standardized seizure action plan to improve communication and parental education. Pediatr Neurol. 2020;112:56–63.
- Dewar SR, Ranit L, Pieters HC. Reciprocal burden: adults with drug-resistant epilepsy reflect upon informal caregiver support. Seizure. 2021;89:85–92.
- Terry D, Patel AD, Cohen DM, et al. Barriers to seizure management in schools: perceptions of school nurses. J Child Neurol. 2016;31(14):1602–1606. DOI:10.1177/0883073816666738
- Hogan RE, Gidal BE, Koplowitz B, et al. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61(3):455–464. DOI:10.1111/epi.16449
- Almohaish S, Sandler M, Brophy GM. Time is brain: acute control of repetitive seizures and status epilepticus using alternative routes of administration of benzodiazepines. J Clin Med. 2021;10(8):1754.
- Barrecheguren M, Bourbeau J. Self-management strategies in chronic obstructive pulmonary disease: a first step toward personalized medicine. Curr Opin Pulm Med. 2018;24(2):191–198.
- Lopez-Toledano MA, Guerra C, Misra SN, et al. Study design of an open-label pharmacokinetic and safety trial of diazepam nasal spray (Valtoco®) in children 2–5 years old with seizure clusters [poster #1.287]. Presented at: American Epilepsy Society; 2021 December 3-7; Chicago IL
- ClinicalTrials.gov. Pharmacokinetics study of VALTOCO® in pediatric subjects with epilepsy (NCT05076838) [Internet]. [cited 2022 Aug 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT05076838