ABSTRACT
Introduction
This systematic review and meta-analysis evaluates the evidence from randomized controlled trials (RCTs) involving pharmacological interventions for improving sleep in people with Alzheimer’s disease (AD).
Methods
A systematic literature search in eight databases from January 2000 to July 2023 focusing on RCTs that compared a pharmacological intervention with a placebo for enhancing sleep in people with AD. The authors registered the study protocol at Prospero, followed the PRISMA guidelines, and produced the pooled estimates using random-effect or IVhet models.
Results
Eight different interventions and 29 different sleep outcomes were examined in 14 RCTs included in this review. Eszopiclone positively affected sleep efficiency, as did orexin antagonists. However, there was no difference when melatonin was used. The interventions demonstrated low discontinuation rates and a few adverse drug reactions.
Conclusion
Although melatonin was the most investigated intervention, the evidence for its efficacy is inconclusive. On the other hand, trazodone and orexin receptor antagonists showed promising results; however, more RCTs are needed for definite answers.
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative condition characterized by a gradual decline in memory and cognitive function [Citation1]. The prevalence of Alzheimer’s dementia increases significantly with advancing age [Citation2,Citation3]. A systematic review conducted in 2015 estimated a global prevalence of 5.2% among adults over 60 who had various forms of dementia [Citation4]. Predictions indicate that the number of individuals diagnosed with AD is expected to triple by the year 2050, potentially reaching a staggering 115 million cases worldwide [Citation5]. AD ranked the fifth leading cause of death globally [Citation6]. Interestingly, certain regions across the world exhibit a higher incidence of AD in women, particularly after reaching the age of 80 [Citation7].
One of the early manifestations of AD is sleep disturbances, notably alterations in sleep patterns, with nighttime sleep disturbance affecting 64.2% of individuals and impacting sleep quality in 58.3% of cases [Citation8]. Disruption in the sleep-wake cycle, a fundamental component of the circadian rhythm governing our 24-hour internal clock, is notable in people with AD [Citation9]. Circadian rhythms are endogenous, self-sustaining near 24-hour rhythms generated by autonomous (internal) molecular clocks, which play a pivotal role in this context [Citation10]. The sleep-wake cycle is a behavioral manifestation of the circadian system and one of the main circadian rhythms – numerous such rhythms exist [Citation9]. The regulation of the sleep-wake cycle is severely disrupted in people with AD, and critical brain regions such as the cerebral cortex, basal forebrain, locus coeruleus, hippocampus, and hypothalamus are implicated in both the sleep-wake cycle and the progression of the disease [Citation9].
Research has unveiled a bidirectional relationship between sleep disturbances, the severity of cognitive impairment, and cognitive decline in people with AD [Citation10,Citation11]. The disrupted sleep-wake cycle is often a potential diagnostic marker for AD. Various sleep-related issues are prevalent among people with AD, with many linked to amyloid-beta (Aβ) pathology and its impact on cognitive function [Citation12]. Fragmented sleep increased daytime napping, and other sleep disturbances emerge as some of the earliest symptoms of AD, often preceding cognitive impairment [Citation13]. Furthermore, the elderly population is generally susceptible to reduced sleep duration, potentially increasing their vulnerability to developing AD [Citation14].
There is a potential connection between the duration of nighttime sleep and the risk of developing AD, with longer sleep duration associated with reduced Aβ levels [Citation15]. Notably, a 2014 study involving healthy participants revealed that increased nocturnal sleep time was associated with a 6% decrease in Aβ levels, suggesting that this phenomenon extends beyond people with AD [Citation16]. Sleep breathing disorders, such as obstructive sleep apnea (OSA), have also been linked to higher Aβ deposition in specific brain regions. Research spanning from 2003 to 2011 found that continuous positive airway pressure (CPAP) treatment in individuals with sleep apnea syndrome (SAS) and mild to moderate AD slowed down cognitive deterioration [Citation17].
Treatment for sleep disorders in people with AD typically begins with non-pharmacological interventions, including bright light therapy (BLT), exercise, and cognitive behavioral therapy. When medication is deemed necessary, options such as benzodiazepines, GABAergic drugs, sedating antidepressants, melatonin, and non-benzodiazepine hypnotics are commonly prescribed [Citation8]. Sleep medications have been shown to reduce the incidence of sleep disturbances [Citation8]. However, it’s important to note that there is still uncertainty regarding the safety and efficacy of these treatments for sleep disturbances in people with AD. The Cochrane review, published in 2014, included only a few randomized controlled trials (RCTs) in this area [Citation18]. The research question addressed in the current review deals with various pharmacological interventions to improve sleep in people with AD. Based on the findings, we highlight the safety and efficacy of pharmacological interventions and discuss relevance for policy and further research directions.
2. Methods
The systematic review undertaken in this study adhered to the PRISMA guidelines [Citation19], and its protocol was registered with Prospero (CRD42023420089). Eight scientific databases, namely Medline, PsychInfo, ClinicalTrials.gov, CENTRAL, CINAHL, ICTRP, ETHOS, and EMBASE, were scrutinized (from January 2000 to July 2023) to compile a comprehensive body of evidence.
2.1. Search strategy and study selection
Our systematic search strategy thoroughly examined all eight selected databases, employing comprehensive search terms. These terms encompassed a wide range of sleep-related concepts, including ‘sleep,’ ‘insomnia,’ ‘circadian,’ ‘hypersomnia,’ ‘parasomnia,’ ‘somnolence,’ ‘sundowning,’ as well as dementia-related terms like ‘dementia,’ ‘Alzheimer’s,’ ‘cognitive impairment,’ ‘memory loss,’ and ‘delirium.’ We specifically focused on identifying randomized controlled trials (RCTs) that investigated the efficacy of pharmacological interventions in improving sleep among people with AD (see supplementary Table S1).
We included RCTs that compared pharmacological interventions against placebos intending to enhance sleep quality in people with AD. Conversely, we excluded trials that solely compared non-pharmacological interventions to placebos or those lacking a pharmacological group. In cases where both the intervention and placebo groups underwent identical non-pharmacological interventions, we included these trials in our review.
To ensure the accuracy and completeness of our data, we tracked and recorded duplicate records encountered throughout the screening process. This approach contributed to the overall reliability of our findings.
Screening titles and abstracts were carried out systematically, adhering to predefined inclusion and exclusion criteria (see supplementary Table S2). Filters were applied to each database to identify studies meeting these criteria accurately. In a structured manner, we initially screened the titles from the initial search, applying the established inclusion and exclusion criteria. This initial step allowed us to identify abstracts that warranted further examination. Subsequently, we screened the abstracts with the same rigor, using the same criteria. This systematic process culminated in determining the number of full-text articles that required thorough review. From this review, we generated a final count of relevant RCTs found within each respective database.
2.2. Data collection/data items
Upon identifying the final set of articles, we compiled the results from these studies. This process involved the collaboration of four independent reviewers, namely AB, HD, SS, and JK, who conducted data retrieval individually. A comparative analysis was performed at the conclusion of this data extraction phase to ensure data accuracy and consistency. Each reviewer employed separate templates for data extraction, and the extracted information was entered accordingly. In cases where discrepancies arose among the four reviewers, these were discussed, and any necessary data adjustments were made by consensus, with the active involvement of SSH.
The data extracted from eligible studies encompassed a comprehensive range of outcomes assessed from objective and subjective perspectives. Specifically, these outcomes included actigraphy, polysomnography (PSG), the Pittsburgh Sleep Quality Index (PSQI), electroencephalogram (EEG) data, as well as discontinuation rates and adverse drug reactions (ADRs) as reported within the randomized controlled trials (RCTs) under review. As part of this systematic review, the following list outlines the sleep-related outcomes that were documented in Box 1:
Box 1. Sleep outcomes.
Total sleep time (TST) in minutes represents the total sleep duration during a 24-hour period.
Nocturnal/night-time total sleep time/ main nocturnal sleep duration (NTST/MNSD) in minutes, signifying the total sleep time, specifically during nighttime.
Total PSQI scores, quantifying sleep quality through the Pittsburgh Sleep Quality Index questionnaire.
The number of daytime naps or daytime sleep episodes is expressed as the total count of daytime napping occurrences.
Wakefulness after sleep onset (WASO) in minutes indicate the duration of time spent awake after the onset of sleep.
Sleep efficiency as a percentage representing the proportion of time spent asleep while in bed.
Daytime total sleep time (DTST) in minutes or as a percentage, reflecting the overall duration of daytime sleep.
Latency to persistent sleep (LPS) in minutes, denoting the time elapsed from getting into bed to falling asleep.
Number of nighttime awakenings, expressed as the total count of awakenings during nighttime sleep.
Rapid Eye Movement sleep (REM) as a percentage or in minutes, reflecting the stage of sleep characterized by increased brain activity and memory restoration.
Latency to rapid eye movement sleep (LREM) in minutes, signifying the time from sleep onset to the onset of the first REM sleep episode.
Non-rapid eye movement (NREM) in minutes represents the phase of deep sleep.
Daytime-to-nighttime sleep ratio (NTST: DTST) illustrates the duration of nighttime sleep compared to daytime sleep.
Interdaily stability mean as a mean value, assessing the strength and consistency of the circadian rhythm.
Intradaily variability mean as a mean value, measuring disturbances in the circadian rhythm.
Relative rhythm amplitude is a score indicating the amplitude of the circadian rhythm.
Night sleep bout duration in minutes, representing the duration of sleep bouts during nighttime.
Day sleep bout duration in minutes, signifying the duration of sleep bouts occurring during daytime.
Number of day wake bouts, expressed as a count, reflecting continuous periods of wakefulness lasting 10 minutes or longer that occur throughout the day.
Day wake bout duration in minutes, denoting the cumulative duration of wake bouts during daytime.
The number of wake bouts during nighttime, expressed as a count, indicates the number of times participants were awake for 10 minutes or longer.
Unless an included study has investigated participants under a constant routine or forced desynchrony protocol, the described metrics do not describe an individual’s circadian rhythm: they represent their sleep-wake rhythm or, as another description, their day-night or diurnal rhythm. Beyond the sleep-related outcomes detailed above, our data collection process encompassed recording several supplementary factors. These included documenting adverse events, comorbid conditions, mean participant age, gender distribution among male and female participants, and any instances of participant discontinuation within the reviewed studies.
In most instances within the final review, the effect sizes were gauged through means and standard deviations (SD), medians, or mean values along with corresponding 95% confidence intervals (CI).
2.3. Risk of bias assessment
We employed the revised Cochrane risk of bias tool, as Sterne et al. [Citation20] outlined, to evaluate the potential bias risk within each study included in our final analysis. This tool comprises five distinct domains, each demanding a comprehensive assessment for any indications of bias.
Randomization Process (Domain 1): This domain scrutinized the risk of bias from the randomization procedure.
Deviations from Interventions (Domain 2): Domain 2 focused on assessing bias risk related to deviations from the intended interventions.
Missing Data (Domain 3): The risk of bias associated with missing data was explored in Domain 3.
Outcome Measurement (Domain 4): Domain 4 delved into the potential bias risk linked to how outcomes were measured within the studies.
Selection of Reported Results (Domain 5): This domain evaluated the bias risk associated with selecting results reported within the study.
Specific questions were considered for each domain, with responses documented. Subsequently, after a comprehensive evaluation across all domains, we derived an overall judgment regarding the level of bias within each of the studies included in our final review. This process ensured a robust assessment of potential bias risk within the reviewed studies, contributing to the overall credibility of our analysis.
2.4. Quantitative synthesis
Our research rigorously employed a meta-analysis to evaluate whether discernible differences existed in treatment responses between the intervention and placebo groups. Furthermore, our objective was to determine whether the desired outcomes favored the intervention or placebo.
We utilized both the random-effects model and the inverse variance heterogeneity (IVhet) model to achieve this. These methodologies harnessed data from individual trials to compute pooled effect sizes accompanied by 95% CIs. The effect sizes were presented in two formats: the weighted mean difference (WMD) and the standardized mean difference (SMD), each complemented by its respective 95% CI.
Assessing heterogeneity was a pivotal aspect of our analysis, and we utilized the I2 statistic and Cochran Q tests for this purpose. Substantial heterogeneity was predetermined at 50%, with a significance level of p < 0.10. The I2 value guided our choice between the random-effects model and the IVhet model; the random-effects model was employed if I2 exceeded 50%, while the IVhet model was applied when I2 was below this threshold. Moreover, we visually examined funnel plots to explore potential publication bias.
Our meta-analysis encompassed a comprehensive array of critical sleep-related outcomes, including total sleep time, total nocturnal sleep time, daytime total sleep time, sleep efficiency, REM latency, sleep latency, wakefulness after sleep onset, number of daytime naps, and number of nighttime awakenings. To ensure consistency in measurement, we converted values originally expressed in hours to minutes, ensuring data accuracy.
An Microsoft Excel spreadsheet collated values related to each outcome to facilitate data management and visualization. This approach enabled the generation of forest plots for each sleep-related parameter, providing a clear and comprehensive representation of our meta-analysis results. All analyses were conducted using Meta XL, version 5.3 [Citation21], ensuring a robust and systematic evaluation of the selected outcomes.
3. Results
3.1. Study selection
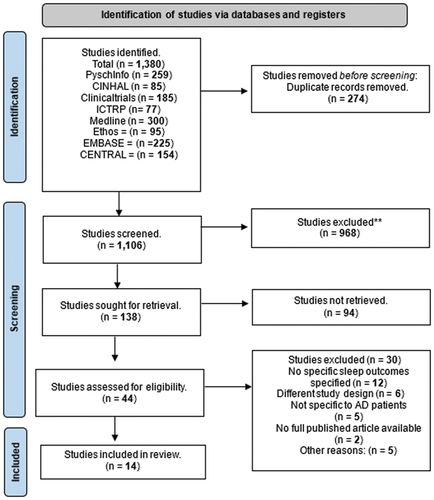
In the initial search, a total of 1,380 trials were identified. After eliminating 274 duplicate entries across various databases, 1,106 trials remained for screening. Upon reviewing the titles, 968 trials were excluded. This initial screening left us with 138 trials that met our inclusion and exclusion criteria. Further assessment of abstracts led to identifying 44 trials eligible for review. Subsequently, the full texts of these 44 trials were evaluated, including 14 trials in our review that passed the authors’ selection criteria. This selection process is visually summarized in .
3.2. Characteristics of included trials
The final review encompassed randomized controlled trials (RCTs) conducted in diverse settings, as shown . Five RCTs were carried out globally (multi-country), with participation from multiple centers [Citation1,Citation25,Citation30,Citation32,Citation33]. Brazil was the location for four RCTs [Citation27,Citation29,Citation31,Citation35], while the United States hosted two [Citation26,Citation28]. There was one RCT each conducted in China [Citation34], Japan [Citation23], and the United Kingdom [Citation22].
Table 1. Study characteristics of the 14 RCTs included in our review.
These 14 studies exhibited variations in sample size, ranging from 20 to 285 participants. Globally conducted trials tended to have larger sample sizes, with Herring et al. [Citation32], and Markowitz et al. [Citation24] featuring 285 and 261 participants, respectively. Singer et al. [Citation25], enrolled 157 participants, Wade et al. [Citation30], had 73, and Moline et al. [Citation33], included 62. Huo et al. [Citation34], also had a sizable sample of 96 participants. In contrast, the remaining studies had smaller participant pools, with Louzada et al. [Citation35], involving 59 participants, Dowling et al. [Citation26], including 50, Serfaty et al. [Citation22], having 44, and Gehrman et al. [Citation28], with 41 participants. Camargos et al. [Citation29], and Grippe et al. [Citation31], both randomized 36 participants, Moraes et al. [Citation27], had 23, and Asayama et al. [Citation23], had 20 participants.
The mean age of participants across these 14 studies spanned from 69.1 to 86 years. Actigraphy was the primary technique utilized in eight studies [Citation22,Citation23,Citation26,Citation28,Citation29,Citation31,Citation33,Citation35]. The Pittsburgh Sleep Quality Index (PSQI) was employed in two studies [Citation24,Citation30], while polysomnography (PSG) was the chosen method in two others [Citation27,Citation32]. Several studies used a combination of techniques, including but not limited to electroencephalogram (EEG), PSG, actigraphy, PSQI, and sleep diary [Citation25,Citation34]. In all 14 studies, the Mini-Mental State Examination (MMSE) was used to assess the severity of AD in participants, with scores ranging from 5.6 to 26. While not all RCTs reported comorbidities, some recorded information about other medications taken by patients. Supplementary Tables 3a to 3c present the complete list of sleep outcomes from each included RCT.
3.3. Risk of bias assessment
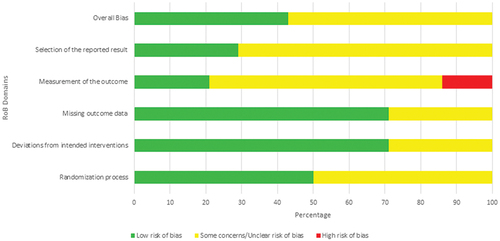
The risk of bias in all 14 RCTs was assessed using the RoB 2 assessment tool [Citation20]. Six of these studies were determined to have a low risk of bias [Citation22,Citation25,Citation30,Citation32,Citation33,Citation35]. However, in the remaining eight studies, certain concerns were identified when evaluating the risk of bias [Citation23,Citation24,Citation26–29,Citation31,Citation34] ( & Table S5).
3.4. Pharmacological interventions
In the 14 RCTs analyzed, a total of eight different interventions were employed (see supplementary Figure S1). It is worth noting that lemborexant and suvorexant, while distinct drugs, belong to the same class of orexin antagonists and were utilized as interventions by Moline et al. [Citation33], and Herring et al. [Citation32]. Additionally, melatonin was administered in the study by Asayama et al. [Citation23] and was also used by Dowling et al. [Citation26], Gehrman et al. [Citation28], Serfaty et al. [Citation22], Singer et al. [Citation25], and Wade et al. [Citation30]. Galantamine was the intervention of choice in the study conducted by Markowitz et al. [Citation24], while eszopiclone, a sedative-hypnotic, was employed by Huo et al. [Citation34]. Sedative hypnotics, specifically zolpidem and zopiclone, were used by Louzada et al. [Citation35]. Trazodone was the intervention in studies by Camargos et al. [Citation29], and Grippe et al. [Citation31], while donepezil was administered in the study conducted by Moraes et al. [Citation27].
3.5. Discontinuation and adverse drug reactions
Safety data indicated that most interventions were well tolerated, with limited adverse drug reactions (ADRs) and low discontinuation rates (see supplementary Table S4).
3.6. Sleep outcome measures
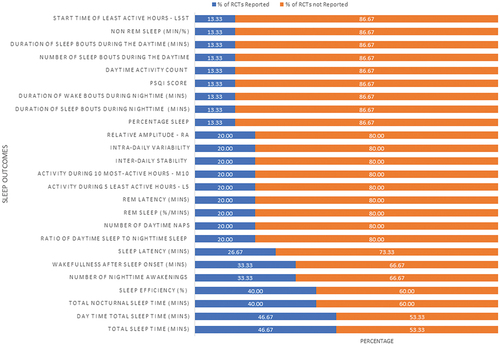
A total of 29 sleep outcome measures were reported across the 14 studies included in the final review. illustrates the percentage of studies that measured each listed outcome. Among these, sleep efficiency and daytime total sleep time emerged as the most frequently assessed sleep outcomes. However, it is important to note that not all of these outcomes were addressed in every study.
3.7. Effects of pharmacological interventions on sleep outcomes
The meta-analysis focused on several key sleep outcome measures, including Total Sleep Time (TST), Nocturnal Total Sleep Time (NTST), Daytime Total Sleep Time (DTST), Sleep Efficiency, REM Latency, Sleep Latency, Wakefulness After Sleep Onset (WASO), Daytime Naps, and Nighttime Awakenings.
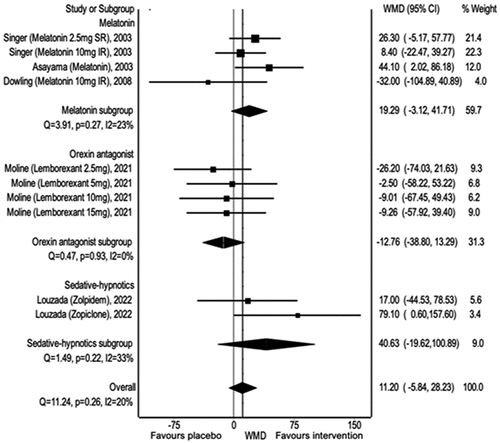
3.8. Sleep Efficiency
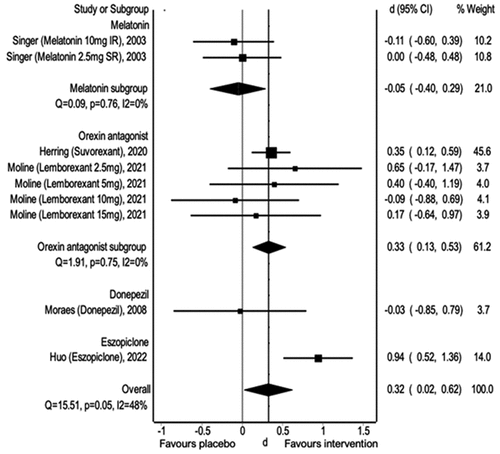
There was a noticeable difference between the intervention and placebo groups when examining sleep efficiency overall (Weighted Mean Difference (WMD): 0.32, 95% CI 0.02, 0.062). Eszopiclone positively affected sleep efficiency (WMD: 0.94, 95% CI 0.52, 1.36), as did orexin antagonists (WMD: 0.33, 95% CI 0.13, 0.53). However, no difference was observed when melatonin was used (WMD −0.05, 95% CI −0.40, 0.29) ().
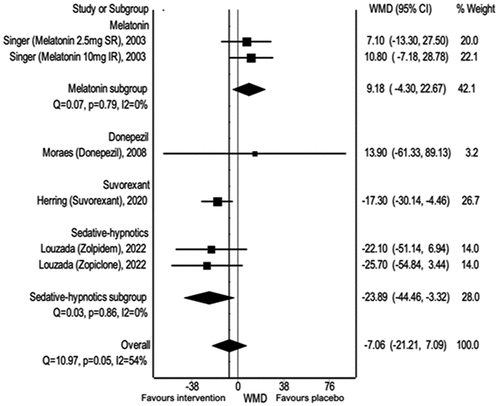
3.9. Wakefulness after sleep onset (WASO)
Overall, there was a minimal effect of pharmacological treatment on WASO across the four studies included in the meta-analysis (WMD: −7.06, 95% CI −21.21, 7.09). Notably, sedative-hypnotics were preferred over placebo, as demonstrated by Louzada et al. [Citation35] (WMD: 23.89, 95% CI −44.46, −3.32) ().
Figure 5. Pharmacological Interventions versus placebo: wakefulness after sleep onset (WASO), using weighted mean difference.
The funnel plot used to detect publication bias revealed gross asymmetry, potentially indicating limited publication of studies for specific sleep outcomes and interventions.
3.10. REM Latency
Placebo was favored overall when measuring REM latency (WMD: 12.82, 95% CI 8.11, 17.53). This trend was consistent in the study where eszopiclone was used (WMD: 13.73, 95% CI 8.85, 18.61) (see supplementary Figure S2).
3.11. Total sleep time (TST)
A significant difference was observed between the intervention group and placebo for TST (WMD: 15.77, 95% CI 2.88, 28.65). Orexin antagonists positively impacted TST (WMD: 28 minutes, 95% CI 9.70, 46.30), as did eszopiclone (WMD: 10.00, 95% CI (−3.65, 23.65)). However, the effect of Donepezil on TST was less reliable (95% CI −60.80, 95.40), and melatonin favored placebo in this regard.
3.12. Nocturnal total sleep time (NTST)
While no significant overall difference was observed, sedative-hypnotics as a subgroup showed positive results (WMD: 40.63, 95% CI −19.62, 100.89) ().
3.13. Daytime total sleep time (DTST)
Overall, there was a slight increase in the placebo groups (WMD: 13.02, 95% CI −5.00, 31.03), which was statistically insignificant.
3.14. Sleep latency
The overall results from the meta-analysis favored intervention in reducing sleep latency (WMD: −4.44, 95% CI −8.93, 0.05). The study by Huo et al.. (2022) [Citation34] also showed intervention favorability and demonstrated reliability due to minimal standard deviation (WMD: −4.29, 95% CI −6.85, −1.73) (see supplementary Figure S3).
3.15. Number of daytime naps
The forest plot indicated a small effect in favor of the intervention group (WMD −0.61, 95% CI −1.30, 0.08) (see supplementary Figure S4).
3.16. Number of nighttime awakenings
Overall, pharmacological intervention did not yield significant effects, with minor differences between intervention and placebo groups (WMD: −0.12, 95% CI −1.72, 1.49) (see supplementary Figure S5).
4. Discussion
The research landscape surrounding sleep disturbances in people with AD is characterized by a conspicuous gap in knowledge and notable challenges in establishing a consensus regarding the most effective and safest pharmacological intervention. The findings extracted from the included Randomized Controlled Trials (RCTs) present a wide spectrum of responses to the various pharmacological interventions employed, reflecting the complexity of addressing sleep disturbances in this population. While some trials reported favorable outcomes with melatonin, it is evident that most did not yield statistically significant therapeutic effects. This diversity in findings underscores the heterogeneity of people with AD and the multifaceted nature of sleep disturbances in this context. It also highlights the pressing need for further research to understand better the underlying factors contributing to these variations and to develop more tailored interventions.
Melatonin is a commonly used treatment for sleep disturbances in people with AD and featured prominently among the 14 RCTs in our review. Intriguingly, our systematic review challenges the prevailing assumption about melatonin’s efficacy, contrasting with findings from the literature review. In a study by Serfaty et al. [Citation22] involving alternating intervention and placebo treatments, sleep efficiency measurements remained similar, suggesting melatonin’s limited impact. Furthermore, a multicenter RCT by Singer et al. [Citation25], involving 176 participants reported no improvement in sleep efficiency with melatonin, regardless of dosage, and no significant difference compared to the placebo group. Although melatonin seemed promising when assessing Nocturnal Total Sleep Time (NTST), another study revealed a decrease in total sleep time and an increase in total day sleep time [Citation28]. These discrepancies in outcomes might stem from variations in Mini-Mental State Examination (MMSE) scores, with Singer et al. [Citation25], having an average MMSE baseline score of 13.9 compared to a lower score of 5.8 in Gehrman et al. [Citation28], indicating more severe AD. Reduced endogenous melatonin levels in people with AD affecting circadian rhythms may necessitate higher doses for those with severe AD, as suggested by Cardinali et al. [Citation36]. Gehrman et al. [Citation28], also suggested that late administration of melatonin at 10 pm might have hampered its therapeutic effect. These studies with low risk of bias, including Serfaty et al. [Citation22], Singer et al. [Citation25], and Wade et al. [Citation30], bolster the reliability of these findings.
Despite uncertainties regarding its efficacy, melatonin remains widely used, partly due to its favorable safety profile. Studies indicate fewer serious side effects compared to alternative interventions. For instance, an add-on prolonged-release melatonin study reported an adverse event profile similar to a placebo.28 Nevertheless, some studies in our systematic review lacked information on adverse events [Citation23,Citation28], underscoring melatonin’s appeal as a safe option for addressing sleep disturbances in people with AD.
Orexin antagonists are less commonly prescribed for people with AD with sleep disturbances. Nonetheless, our systematic review uncovers promising results associated with these interventions. Notably, an RCT by Herring et al. [Citation32], demonstrated that suvorexant had positive effects on various sleep outcomes, particularly Total Sleep Time (TST), Wakefulness After Sleep Onset (WASO), and sleep efficiency when measured using polysomnography (PSG). A previous study also concluded that suvorexant effectively treated insomnia in people with AD and noted no side effects on follow-up [Citation37]. Additionally, a study by Moline et al. [Citation33], investigated lemborexant’s effects on people with AD with irregular sleep-wake rhythm, revealing improvements in specific circadian rhythm parameters, particularly with a 5 mg dosage. Although higher doses led to increased adverse effects, they remained manageable. Orexin receptor antagonists potentially offer a safer profile than other medications, primarily reducing wakefulness rather than promoting sleep. However, next-day somnolence is a reported side effect, possibly linked to a dose-dependent half-life. Investigating the effects of a 20 mg dose, as suggested by Sun et al. [Citation38], for healthy participants could provide valuable insights. Despite some variations in sample sizes and measurement techniques, trials by Moline et al. [Citation33], and Herring et al. [Citation32], conducted globally offer comprehensive assessments of these drugs’ efficacy and safety profiles. The limited use of orexin antagonists in clinical practice could be attributed to several factors. FDA approval of this class of medication as a sedative drug for insomnia only occurred in 2014 [Citation39], implying a relatively short history compared to other commonly used drugs like zopiclone. Furthermore, uncertainty persists regarding the impact of the orexin system on AD disease progression [Citation13].
Sedative hypnotics, such as zopiclone and zolpidem, are frequently prescribed for short-term treatment of sleep disturbances, primarily due to their efficacy. A study by Louzada et al. [Citation35], compared zopiclone and zolpidem to a placebo and found a preference for the intervention in several sleep parameters. Zopiclone notably increased Nocturnal Total Sleep Time (NTST), reduced Wakefulness After Sleep Onset (WASO), and decreased nighttime awakenings. However, the study reported adverse events, including mental confusion, hallucinations, and agitation, in a small number of participants. Additionally, while certain parameters improved, others, such as daytime total sleep time, remained unaffected. The current meta-analysis revealed the favorable outcomes of eszopiclone in a study by Huo et al. [Citation34], where improved sleep efficiency, Rapid Eye Movement (REM) latency, and total sleep time were observed, with fewer participants experiencing side effects than the control group. It is worth noting that zopiclone, like benzodiazepines, is limited to short-term use due to the risk of dependence and abuse and the potential for rebound issues [Citation40].
Trazodone has demonstrated positive effects when used to address insomnia in people with AD. In a trial included in our review, notable improvements were observed in Nocturnal Total Sleep Time (NTST), the number of naps, Wakefulness After Sleep Onset (WASO), Daytime Total Sleep Time (DTST), and the frequency of nighttime awakenings [Citation29]. Importantly, adverse effects were minimal. Other sources also highlight trazodone’s potential to mitigate cognitive decline, with five studies demonstrating cognitive improvements [Citation41]. A study by Karageorgiou et al. [Citation42] concluded that trazodone usage correlated with a delay in cognitive decline.
Two of the 14 RCTs in our systematic review explored the use of acetylcholinesterase inhibitors as interventions compared to placebos. Although they do not directly address sleep disturbances, these inhibitors were found to have neutral or favorable effects on sleep parameters in people with AD. Galantamine, known for its dual action of inhibiting acetylcholine esterase and enhancing receptor effects, was used in a study investigating acetylcholinesterase inhibitors’ effects on sleep quality in AD [Citation24]. The study found that Galantamine’s use in AD did not induce sleep-related adverse effects, highlighting it as a safe treatment option. Similarly, when donepezil was employed in a trial involving people with AD with obstructive sleep apnea, it was associated with an increase in Rapid Eye Movement (REM) sleep, positively affecting memory and cognition. Furthermore, only mild side effects were reported, such as headaches and nausea [Citation27].
4.1. Implications for policy and practice
One of the most significant merits of this systematic review is its role as a critical update to the field. It builds upon and extends the knowledge base established by a prior systematic review conducted in 2014 [Citation18], which identified a limited number of eligible Randomized Controlled Trials (RCTs). This earlier review highlighted the dearth of evidence to guide treatment decisions for addressing sleep disturbances in people with AD. The increase in RCTs in the current review demonstrates substantial progress in research efforts over the intervening years.
Expanding the evidence base by including a more extensive pool of studies underscores the review’s dedication to providing a comprehensive and up-to-date understanding of the subject matter. This updated perspective is particularly valuable given the dynamic nature of scientific research and the evolving landscape of AD management. By offering a more extensive and contemporary synthesis of the available evidence, this systematic review equips clinicians, researchers, and policymakers with the most current insights into sleep disturbance interventions for people with AD.
Furthermore, this review addresses the persistent absence of a definitive consensus on the most effective pharmacological interventions for sleep disturbances in people with AD. The nuanced and variable responses observed across the included RCTs emphasize the multifaceted nature of sleep disturbances in this population. By presenting this diversity of findings, the review encourages a more nuanced and patient-centric approach to addressing sleep disturbances, recognizing that a one-size-fits-all solution may not be appropriate.
The thorough exploration of multiple pharmacotherapies and their respective efficacy and safety profiles adds depth to understanding potential treatment options [Citation43]. While conclusive recommendations may remain elusive, the review provides a valuable resource for clinicians and researchers seeking to make informed decisions based on the available evidence. It offers a foundation for future research, guiding the development of more tailored and effective pharmacological interventions for sleep disturbances in people with AD.
4.2. Limitations
Two limitations of this review are worth discussing: the relatively small sample sizes in most included trials and the number of trials available for individual drugs. These limitations could raise concerns about the reliability and generalizability of the results. AD is a heterogeneous disease, and individual variations in disease progression and sleep patterns may not be fully captured in smaller sample sizes. Although the number of trials included in this review is much greater than in the previous Cochrane review,16 the findings from included trials may not fully represent the broader AD population, and caution should be exercised when extrapolating the results to a broader patient population.
The persisting uncertainties in the field are partly attributable to the variations in sample sizes across the included studies. Inconsistencies in patient characteristics and the absence of uniformity in Mini-Mental State Examination (MMSE) scores and diagnoses further compound these uncertainties. These variations can affect the comparability of results across trials and hinder the establishment of a cohesive body of evidence. Confounding factors, often inherent in studies involving people with AD, can also impact the interpretation of results. Factors such as comorbidities, concomitant medication use, and individual differences in disease progression may introduce confounding variables that are challenging to control in clinical trials. Acknowledging and addressing these confounders is essential for drawing meaningful conclusions from the available evidence.
There is a pressing need for larger-scale RCTs featuring multiple arms. In addition to larger RCTs, conducting international trials (multi-country) can significantly contribute to refining our understanding of pharmacological sleep treatments in people with AD. These trials should be designed with a particular emphasis on stratifying patients based on disease severity and age. Such stratification would help elucidate the varying responses to interventions and facilitate the development of precise and individualized treatment recommendations. Understanding how different interventions affect patients at different stages of AD can be invaluable in tailoring therapeutic approaches to meet the specific needs of each subgroup.
5. Conclusion
While our systematic review has shed light on various aspects of sleep disturbances in people with AD, several critical gaps in knowledge remain, necessitating focused attention and future research endeavors. Melatonin emerged as the most commonly chosen intervention in treating sleep disturbances among people with AD. This preference likely stems from the drug’s relatively favorable safety profile, although its efficacy is somewhat limited. Our review revealed a nuanced picture, with some RCTs supporting melatonin’s use while others did not demonstrate significant therapeutic effects. Among the newer drugs, orexin receptor antagonists showed promise in addressing sleep disturbances in people with AD. The systematic review demonstrated their effectiveness and tolerability in the trials, yet further RCTs are essential to solidify their position as a viable treatment option. Other medications, such as zopiclone, exhibited improvements in sleep parameters, but their applicability remains constrained by the risk of dependence, relegating them to short-term use. Trazodone, on the other hand, displayed a noteworthy therapeutic effect with minimal adverse effects, though the available evidence remains somewhat limited.
By embracing rigorous research methodologies and considering the diversity inherent in people with AD, the scientific community can move closer to enhancing the quality of life for individuals with AD who are struggling with sleep disturbances. These endeavors will contribute to refining treatment strategies and alleviate the considerable burden of sleep disturbances in this vulnerable population.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author’s contributions
All authors contributed to this paper. SS Hasan conceptualized the research idea. A Bedward, J Kaur, S Seedat, and H Donohue were involved in screening, data extraction, interpretation, and writing the first draft. SS Hasan was involved in data analysis, interpretation, and manuscript revision. MK Rasheed, A Javed, and CS Kow were involved in interpreting results and manuscript revision.
Supplemental Material
Download MS Word (4.7 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737175.2024.2341004
Additional information
Funding
References
- Zhang Y, Ren R, Yang L, et al. Sleep in Alzheimer’s disease: a systematic review and meta-analysis of polysomnographic findings. Transl Psychiatry. 2022;12(1):136. doi: 10.1038/s41398-022-01897-y
- Caglayan C, Demir Y, Kucukler S, et al. The effects of hesperidin on sodium arsenite-induced different organ toxicity in rats on metabolic enzymes as antidiabetic and anticholinergics potentials: a biochemical approach. J Food Biochem. 2019;43(2):e12720. doi: 10.1111/jfbc.12720
- Özbey F, Taslimi P, Gülçin I, et al. Synthesis of diaryl ethers with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. J Enzyme Inhib Med Chem. 2016;31(sup 2):79–85. doi: 10.1080/14756366.2016.1189422
- Joe E, Ringman JM. Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ (Online). 2019;367:l6217–l6217. doi: 10.1136/bmj.l6217
- Defrancesco M, Marksteiner J, Fleischhacker WW, et al. Use of benzodiazepines in Alzheimer’s Disease: a systematic review of literature. Int J Neuropsychopharmacol. 2015;18(10):yv055–pyv055. doi: 10.1093/ijnp/pyv055
- Hampel H, Hardy J, Blennow K, et al. The amyloid-β pathway in Alzheimer’s disease. Mol Psychiatry. 2021;26(10):5481–5503. doi: 10.1038/s41380-021-01249-0
- Mielke MM, Aggarwal NT, Vila‐Castelar C, et al. Consideration of sex and gender in Alzheimer’s disease and related disorders from a global perspective. Alzheimer’s Dementia. 2022;18(12):2707–2724.
- Zhou G, Liu S, Yu X, et al. High prevalence of sleep disorders and behavioral and psychological symptoms of dementia in late-onset Alzheimer disease. Medicine. 2019;98(50):e18405. doi: 10.1097/md.0000000000018405
- Lim MM, Gerstner JR, Holtzman DM. The sleep–wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag. 2014;4(5):351–362.
- Suni E, Singh A. Circadian rhythm, what it is, what shapes it and why its fundamental to getting quality sleep. Sleep Foundation 2023 [cited Feb 16]. Available from: www.sleepfoundation.org/circadian-rhythm.
- Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s Disease. CNS Drugs. 2001;15(10):777–796. doi: 10.2165/00023210-200115100-00004
- Vanderheyden WM, Lim MM, Musiek ES, et al. Alzheimer’s disease and sleep–wake disturbances: amyloid, astrocytes, and animal models. J Neurosci. 2018;38(12):2901–2910. doi: 10.1523/JNEUROSCI.1135-17.2017
- Gao F, Liu T, Tuo M, et al. The role of orexin in Alzheimer disease: from sleep-wake disturbance to therapeutic target. Neurosci Lett. 2021;765:136247–136247. doi: 10.1016/j.neulet.2021.136247
- Carvalho DZ, St Louis EK, Knopman DS, et al. Association of excessive daytime sleepiness with longitudinal β-amyloid accumulation in elderly persons without dementia. JAMA Neurol. 2018;75(6):672–680. doi: 10.1001/jamaneurol.2018.0049
- Brzecka A, Leszek J, Ashraf GM, et al. Sleep disorders associated with Alzheimer’s disease: a perspective. Front Neurosci. 2018;12:330–330. doi: 10.3389/fnins.2018.00330
- Ooms S, Overeem S, Besse K, et al. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971–977. doi: 10.1001/jamaneurol.2014.1173
- Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85(12):1405–1408.
- McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in Alzheimer’s disease. Cochrane Database Syst Rev. 2014;21(3):CD009178. doi: 10.1002/14651858.CD009178.pub2
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:4898. doi: 10.1136/bmj.l4898
- MetaXL Version 5.3. 2019. https://www.epigear.com/index_files/metaxl.html
- Serfaty M, Kennell-Webb S, Warner J, et al. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17(12):1120–1127. doi: 10.1002/gps.760
- Asayama K, Yamadera H, Ito T, et al. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J Nippon Med Sch. 2003;70(4):334–341. doi: 10.1272/jnms.70.334
- Markowitz JS, Gutterman EM, Lilienfeld S, et al. Sleep-related outcomes in persons with mild to moderate Alzheimer disease in a placebo-controlled trial of galantamine. Sleep. 2003;26(5):602–606. doi: 10.1093/sleep/26.5.602
- Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s Disease. Sleep. 2003;26(7):893–901. doi: 10.1093/sleep/26.7.893
- Dowling GA, Burr RL, Van Someren EJW, et al. Melatonin and bright-light treatment for rest–activity disruption in institutionalized patients with Alzheimer’s Disease. J Am Geriatr Soc. 2008;56(2):239–246.
- Moraes W, Poyares D, Sukys-Claudino L, et al. Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study. Chest. 2008;133(3):677–683. doi: 10.1378/chest.07-1446
- Gehrman PR, Connor DJ, Martin JL, et al. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Ame J Geriatr Psychiatry. 2009;17(2):166–169. doi: 10.1097/JGP.0b013e318187de18
- Camargos EF, Louzada LL, Quintas JL, et al. Trazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled study. Am J Geriatr Psychiatry. 2014;22(12):1565–1574. doi: 10.1016/j.jagp.2013.12.174
- Wade AG, Farmer M, Harari G, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–961. doi: 10.2147/CIA.S65625
- Grippe TC, Goncalves BS, Louzada LL, et al. Circadian rhythm in Alzheimer disease after trazodone use. Chronobiol Int. 2015;32(9):1311–1314.
- Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimer’s Dementia. 2020;16(3):541–551.
- Moline M, Thein S, Bsharat M, et al. Safety and efficacy of lemborexant in patients with irregular sleep-wake rhythm disorder and Alzheimer’s Disease dementia: results from a phase 2 randomized clinical trial. J Prev Alzheimers Dis. 2021;8(1):1–12.
- Huo S, Cheng L, Li S, et al. Effects of eszopiclone on sleep quality and cognitive function in elderly patients with Alzheimer’s disease and sleep disorder: a randomized controlled trial. Brain Behav. 2022;12(2):e2488. doi: 10.1002/brb3.2488
- Louzada LL, Machado FV, Quintas JL, et al. The efficacy and safety of zolpidem and zopiclone to treat insomnia in Alzheimer’s disease: a randomized, triple-blind, placebo-controlled trial. Neuropsychopharmacology. 2022;47(2):570–579.
- Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Curr Neuropharmacol. 2010;8(3):218–227. doi: 10.2174/157015910792246209
- Hamuro A, Honda M, Wakaura Y. Suvorexant for the treatment of insomnia in patients with Alzheimer’s disease. Aust NZJ Psychiatry. 2018;52(2):207–208. doi: 10.1177/0004867417747402
- Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–267.
- Banerjee I. Orexin receptor competitive antagonists: a novel target of the sedative and hypnotics drugs for the pharmacotherapy of Insomnia. Nepal J Epidemiol. 2018;8(1):713–715. doi: 10.3126/nje.v8i1.21139
- Lader M. Zopiclone: Is there any dependence and abuse potential? J Neurol. 1997;244(S1):S18–S22. doi: 10.1007/BF03160567
- Gonçalo AMG, Vieira-Coelho MA. The effects of trazodone on human cognition: a systematic review. Eur J Clin Pharmacol. 2021;77(11):1623–1637. doi: 10.1007/s00228-021-03161-6
- Karageorgiou E, La A, Krystal A, et al. F2‐05‐04: slow wave sleep enhancers as a potential therapeutic approach in delaying cognitive decline in Alzheimer’s disease: the trazodone paradigm. Alzheimers Dement. 2019;15(7S_Part_10):524–P524.
- Javed B, Javed A, Kow CS, et al. Pharmacological and non-pharmacological treatment options for sleep disturbances in Alzheimer’s disease. Expert Rev Neurother. 2023;23(6):501–514. doi: 10.1080/14737175.2023.2214316