ABSTRACT
Background
This study aimed to analyze the adverse events to bendamustine using data obtained from the Food and Drug Administration open public data project (openFDA) and to provide a reference for its use in clinical practice.
Research design and methods
Adverse events (AEs) due to bendamustine usage reported from 1 January 2008 to 31 March 2023 were collected from the FDA Adverse Event Reporting System (FAERS). The reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian plausible propagation neural network (BCPNN), and multinomial gamma-Poisson distribution shrinking (MGPS) algorithms were used to identify signs of adverse reactions caused by bendamustine.
Results
A total of 4214 AE reports where bendamustine was considered as the first suspected drug were obtained from FAERS. The analysis revealed 214 AE risk signals, among which 141 met the criteria but they were not listed as possible side effects on the drug information sheet provided in the package.
Conclusion
Our findings identified numerous common AEs with previously reported clinical observations. We also identified some signs of potential new AEs, indicating the need of careful clinical monitoring of patients treated with bendamustine and further risk identification research about this drug.
1. Introduction
Bendamustine is a bifunctional nitrogen mustard derivative containing a purine-like benzimidazole ring, which may enhance its clinical efficacy [Citation1]. The chemical structure of bendamustine comprises a nitrogen mustard moiety, benzimidazole ring, and n-butyric acid side chains. Bendamustine is active against both quiescent and dividing cells and exerts its antitumor effects through the alkylation of deoxyribonucleic acid (DNA), causing longitudinal and transverse crosslinking of single or double strands and interfering with further DNA synthesis and repair. Preclinical studies suggest that bendamustine can cause cell death through apoptosis and other pathways that disrupt normal cell division. Clinical trials have shown that bendamustine significantly reduces recurrence and mortality rates in cancer patients.
Bendamustine was approved by the U.S. Food and Drug Administration (FDA) for the treatment of chronic lymphocytic leukemia and inert B-cell non-Hodgkin’s lymphoma that progressed after treatment with rituximab or a rituximab-containing regimen in March 2008 and October 2008, respectively. In December 2018, it was approved for marketing in China.
The FDA’s open public data program, openFDA, was established in June 2014 that allowed the public to retrieve and access public data from FDA databases. In this paper, we retrieved the AE reports of bendamustine and its characteristics from the data obtained from openFDA, analyze the reports from multiple perspectives to provide reference for prescribing clinical medication, and promote the rational application of drugs. The management procedures for adverse drug reactions mainly include monitoring and identifying side effects. Relevant personnel such as healthcare providers and patients closely observe any potential adverse drug reactions, assess and categorize them, promptly report them through the adverse reaction reporting system, and take corresponding measures.
2. Methods
2.1. Data sources
The data were obtained from the Adverse Drug Events (ADR) database on the openFDA platform. Data were extracted using the Open-Vigil-FDA analysis tool, which directly extracts structured ADR information from the openFDA database using an application program interface (API) in an efficient and accurate manner. AEs were described using preferred terms (PT) included in the Medical Dictionary of Regulatory Activities Adverse Drug Event Thesaurus, and AEs were classified using the System Organ Classification (SOC). The reports recorded from January 2008 to March 2023 were analyzed.
2.2. Statistical analysis
Disproportionality analysis was performed by combining four algorithms: reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian plausible propagation neural network (BCPNN), and MGPS methods. ROR and PRR have high sensitivity, whereas BCPNN and MGPS have satisfactory adverse drug reaction (ADR) prediction ability. These were used to quantify the signs of bendamustine-associated adverse events (AEs). The used calculation formulas, detection indices, and thresholds are shown in . Cases where AE criteria were met with at least one of the four algorithms were considered as positive drug-associated signals. In this study we selected AE signals that simultaneously met the criteria of the four algorithms to make the results more convincing.
Table 1. Four main algorithms used to assess the potential association between bendamustine and AEs.
3. Results
3.1. General characteristics
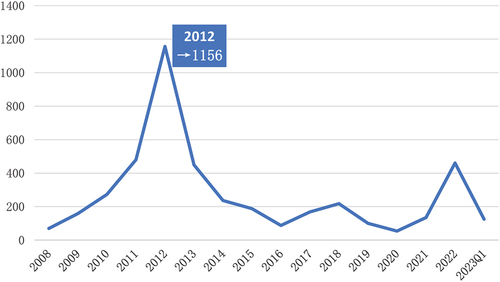
A total of 4214 AE reports for bendamustine were found from 1 January 2008 to 31 March 2023. The annual distribution, as shown in , increased year by year from 2008 to 2012, reaching a peak in 2012 with a total of 1156 cases, and then gradually decreased and stabilized. The characteristics of bendamustine-associated AEs are shown in . Except for unspecified reported cases, the proportion of AE reports due to bendamustine was higher among males than among females (49.88% vs. 32.25%). In terms of age distribution, the highest number of reports were recorded for the elderly (aged >65 years; 35.86%; n = 1511), followed by adults (aged 18–65 years; 22.62%; n = 953), with a small number of reports being reported in minors. Bendamustine-associated adverse reactions were primarily reported from the United States, accounting for > 55% reports, followed by Japan (12.72%), Germany, France, and Italy. Overall, 2,340 reports of serious adverse reactions (including hospitalization, death, life-threatening, and disability) were found, accounting for 55.53% reports.
Table 2. Clinical characteristics of bendamustine use reported in the FAERS Database (January 2008 to March 2023).
3.2. Adverse reaction risk signal analysis results
illustrates the annual distribution of adverse drug reactions to bendamustine, peaking in 2012 and gradually decreasing thereafter, stabilizing over time. With the increasing use of bendamustine, there might be an enhanced awareness of its potential adverse reactions, leading to proactive measures for their prevention. The details of 10 most common types of adverse reaction to bendamustine after removing logically implausible adverse reactions are listed in . Our analysis showed that the PTs for the top 10 AEs reported were fever, rash, neutropenia, febrile neutropenia, pancytopenia, malignant progression, infectious pneumonitis, thrombocytopenia, reduced platelet count, and anemia. SOC categorizes systemic diseases and various reactions by the site of administration, skin and subcutaneous tissue diseases, blood and lymphatic system diseases, benign, malignant, and tumors of undetermined nature, respiratory, thoracic, and mediastinal disorders, and various investigations. The number of cases for these 10 AEs was 1,835, accounting for 43.55% adverse event reports.
Table 3. Top 10 types of adverse reactions to bendamustine.
ADR risk signal analysis was performed for the 4214 adverse reaction reports. A total of 214 adverse reaction risk signals were detected, among which 141 were not listed as AEs in the drug specification, involving 20 system organ classifications, with variations in each system organ ().The detailed results of the PTs of the AEs involved in the 214 risk signals are shown in .
Table 4. ADR events involving system organs.
Table 5. Comparison of Signal Intensity Reported for Bendamustine at preferred term levels by SOC classification.
4. Discussion
4.1. Analysis of ADR composition
The acquisition of the highest number of reports from the United States (55.67%) can be attributed to the fact that FDA database is a U.S.A.-based resource. The time of commercialization of bendamustine in different countries could also be considered as a dictating factor. One may assume similar guideline-based treatments in US and the reporting countries in CLL and NHL. The fact that Centres in Japan and Europe contribute to the database is a strength, making results more representative. ADR were mainly reported in patients aged >65 years, which may be related to the predominance of elderly patients. The high proportion of serious ADR events (including hospitalization, death, life-threatening, and disability) (55.83% of all reports) suggests that more attention should be paid while clinically using this drug, more related ADRs should be explored, and clinicians should increase alertness toward the occurrence of serious ADRs.
4.2. Analysis of screened ADR signals
Based on a disproportionality analysis, this study found that ADRs to bendamustine primarily occurred in patients with blood and lymphocyte disorders, skin and subcutaneous tissue disorders, systemic disorders, infections, and reactions at the site of administration. The ROR, PRR, and EBGM05 were > 70.0 for ADRs including but not limited to infusion site phlebitis, humoral immunodeficiency, injection site phlebitis, infusion site irritation, Listeria sepsis, extravasation, cytomegalovirus small intestinal colitis, cytomegalovirus choroidal retinitis, and cytomegalovirus-associated viremia. The distribution of adverse reactions, mostly consistent with the indications, indicates a certain representativeness of the clinical studies, revealing the most significant as well as new severe adverse reactions.
Bendamustine is a bifunctional mescaline-alkylating agent that is used to treat patients with hematologic malignancies. We found that hematologic AEs [Citation2,Citation3] such as neutropenia, decreased lymphocyte count [Citation4], pancytopenia, thrombocytopenia, and anemia frequently occurred after bendamustine administration. In patients with prolonged myelosuppression, delayed dosing is required [Citation5,Citation6]. This myelosuppressive and cytotoxic drug reduces the number of CD4+ lymphocytes [Citation7] and the CD4/CD8 ratio, causing dose-dependent immunosuppression. This increases the risk of infection-related complications in patients. Previous research has demonstrated the need for careful monitoring of lymphocyte counts during clinical treatment with bendamustine [Citation8,Citation9]. A meta-analysis that presented data on the AEs associated with bendamustine-containing regimens revealed infections as the most common AE, followed by neutropenia and lymphocytopenia. This study, based on data obtained from openFDA, identified the signs of blood and lymphocyte ADRs and diseases that developed due to bendamustine administration with significant signal intensity. These signs included pancytopenia, lymphopenia, hemolysis, myelodysplastic syndromes, and acute myeloid leukemia of AEs (). This finding was in line with the drug specifications. Although autoimmune hemolytic anemia and aplastic anemia were reported, these were not listed in the drug specifications. These newly identified ADRs will be valuable for clinicians. Drug-induced immune hemolytic anemia is a rare and serious complication in the treatment of blood disorders [Citation10,Citation11]. More than 100 drugs have been associated with immune hemolytic anemia [Citation12]. Neta reported that 5 (16%) of 31 patients with chronic lymphocytic leukemia receiving bendamustine developed some degree of hemolysis. This is possibly related to the combined alkylating and purine analogue properties of the drug. Immune hemolytic anemia previously associated with fludarabine may be a risk factor for bendamustine-induced hemolysis.
Notably, long-term use of bendamustine has been associated with an increased risk of cytomegalovirus (CMV) infection and reactivation of a previous CMV infection. A retrospective study observed a frequency of CMV viral reactivation of up to 19% [Citation13]. This may be attributed to the attenuation of immune response by bendamustine, making patients vulnerable to opportunistic infections, including cytomegalovirus reactivation. However, another study found no difference in the incidence of CMV reactivation at any stage in patients with lymphoma who received a bendamustine-containing regimen as a first-line therapy [Citation14]. This supports the likelihood that viral reactivation is related to immune damage induced by chemotherapeutic agents rather than by the disease itself. Moreover, the incidence of CMV reactivation is high among older adults (aged >60 years) with newly diagnosed and relapsed/refractory inert and invasive disease treated with bendamustine-containing regimens and CMV lesions in the liver, gastrointestinal tract, lungs, or retina [Citation15]. Therefore, early and close monitoring of clinical and laboratory findings in elderly hematologic patients treated with bendamustine may improve the clinical management and prognosis of these patients. In addition to viral infections [Citation16], invasive fungal and bacterial infections have been reported to be caused by bendamustine [Citation13,Citation17]. This study supported this finding and reported that AEs such as fungal pneumonitis, bronchopulmonary aspergillosis, Aspergillus infections, and bacterial infections are not documented in the drug package inserts. This highlights the need for clinical attention to the signs of infection and use of appropriate anti-infective prophylaxis when necessary.
Bendamustine has potent immunosuppressive effects and can lead to reactivation of hepatitis B virus (HBV) [Citation18,Citation19]. Yoshiki has reported two cases of lymphoma patients with reactivation of preexisting HBV infections due to bendamustine chemotherapy, In these patients, prompt treatment with entecavir resulted in a rapid turnaround of HBV DNA levels and prevented its progression [Citation20]. However, HBV reactivation has also been reported due to bendamustine and prophylactic entecavir administration, and entecavir has been associated with mortality in patients with HBV reactivation due to increased lactic acidosis and encephalopathy [Citation21]. This suggests that caution should be exercised when using entecavir as preemptive therapy. This study found AE-related signs of HBV and HBV reactivation among the AE reports, consistent with the drug specifications, suggesting the need for close clinical monitoring of HBV DNA levels during bendamustine treatment.
Cutaneous AEs are concern during bendamustine treatment. A clinical trial showed that the occurrence of bendamustine-related skin AEs, in descending order of frequency, are infusion-related reactions (ROR = 5.708), herpes zoster (ROR = 4.658), allergy (ROR = 3.271), and rash (ROR = 1.472) [Citation22]. In this study, the strongest associations found were infusion site vesicles (ROR = 63.66), infusion site discoloration (ROR = 49.27), infusion site erythema (ROR = 17.81), and Stevens – Johnson syndrome (ROR = 12.83), in that order. It was considered to be related to the extraction of the database and the number of reported cases. Lysis of toxic skin necrosis has also been reported in clinical trials and post-commercialization [Citation23]. No signal intensity for toxic skin necrolysis was found in our study. We believe that it reflects underreporting of life-threatening toxic reactions such as Stevens – Johnson syndrome and toxic epidermal necrolysis and this question needs to be explored further.
Of the respiratory, thoracic, and mediastinal diseases found in our analysis, Pneumocystis jirovecii pneumonia, and diffuse alveolar injury were mentioned in the drug specifications. A retrospective study found that, among adult cases of NHL treated with bendamustine and a CD20-targeted monoclonal antibody (rituximab or ofatumumab), the Pneumocystis jirovecii pneumonia infection rate was 6% [Citation2]. Another single-center retrospective cohort study [Citation24] showed a cumulative incidence of Pneumocystis carinii pneumonia of 1.7% (95%CI 0.8%–3.3%; maximum follow-up of 2.5 years) after initiation of bendamustine. Additionally, pleural effusion, coronavirus infection, occlusive bronchiectasis, Pseudomonas aeruginosa-induced pneumonia, pneumococcal pneumonia, and spontaneous pneumothorax were identified in this analysis. These are newly identified serious AEs to bendamustine that are not documented in the drug specifications. Among these, pleural effusion had the highest incidence (n = 48) and was highly correlated with the signal intensities of ROR 4.75 (3.54–6.37), PRR 4.71 (3.52–6.3), IC025 2.23 (1.28), and EBGM 4.7 (3.5).
Other severe AEs identified for the first time in this study were progressive multifocal leukoencephalopathy (PML), Guillain – Barré syndrome, and demyelinating polyneuropathy. PML [Citation25] is a rare and severe central nervous system disease [Citation26,Citation27] caused by John Cunningham (JC) virus infection [Citation28]. High-intensity signals of JC virus infection were consistently found in our analysis in infectious and invasive diseases with an ROR of 18.36 (6.86–49.11), PRR 18.34 (6.86–49.02), IC025 4.19 (1.52), and EBGM 18.21 (6.81). T-cell defects are a major contributor to drug-associated PML and a common risk factor [Citation29]. Such T-cell defects may result from decreased T-cell counts due to bendamustine. CD4+ T-cells play a central role in the control of intracerebral JC virus [Citation30]. CD4+ T-cells and Th1 cells act synergistically to control the dissemination of intracerebral JC virus infection by driving the activation and proliferation of CD8+ T-cells, which ultimately direct the production of JC virus-specific effector cells [Citation29,Citation31]. Therefore, when patients treated with bendamustine develop unexplained neurologic symptoms, they should undergo prompt magnetic resonance imaging to facilitate the swift diagnosis of JC virus in the cerebrospinal fluid by polymerase chain reaction.
5. Limitations
Our study had several limitations. First, FAERS data comes from a voluntary reporting system, which may be subject to incomplete reports and biases. Second, because some drugs are used more often than others, reports of AEs associated with them will show corresponding differences in frequency, resulting in the potential over- and underestimation of the incidences of AE. Third, there is no comparison or control group, making it difficult to determine whether the ADRs associated with bendamustine use are higher than the expected baseline risk or not. Fourth, the data provided by FAERS show only correlations between the occurrence of ADRs and the use of a particular drug, they do not prove the direct causal relationship. Demonstrating causality will require further clinical studies and evaluation. Fifth, in clinical practice, most adverse drug reactions, especially rare complications and infectious complications, occur during the combined treatment with bendamustine. Therefore, it is difficult to clearly separate them, and further clinical research is needed to confirm this.
6. Conclusion
This study scientifically and quantitatively analyzed the AE risks of bendamustine using reports from the FAERS database. Unexpected and previously unidentified major AEs were identified, including autoimmune hemolytic anemia, pleural effusion, PML, and JC virus. Hematologic and cutaneous reactions, infections, and infusion reactions were found to be common AEs that should be of great concern. This research provides a reference for clinicians and a springboard for further research. It is recommended to closely monitor the infusion sites and skin changes of patients during the clinical use of bendamustine. Dynamic monitoring of blood routine, coagulation function, inflammatory markers, ultrasound, and imaging examinations is essential to promptly detect possible adverse drug reactions caused by the medication. Early intervention should be taken to minimize the associated risks that may occur during the course of treatment.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution
Q Huang: conceptualization, methodology, execution, acquisition of data, analysis and interpretation, writing original and revised draft; Y Wu: methodology, formal analysis, Validation, draft proofreading; H Li: conceptualization, study design, supervision, Writing original and revised draft.
Additional information
Funding
References
- Dennie TW, Kolesar JM. Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-Hodgkin lymphoma. Clin Ther. 2009;31(2):2290–2311. doi: 10.1016/j.clinthera.2009.11.031
- Hakamifard A, Mardani M, Nasiri MJ, et al. Bendamustine and pneumocystis pneumonia: a systematic review. Health Sci Rep. 2022;5(3):e610. doi: 10.1002/hsr2.610
- Sakai R, Ohmachi K, Sano F, et al. Bendamustine-120 plus rituximab therapy for relapsed or refractory follicular lymphoma: a multicenter phase II study. Ann Hematol. 2019;98:2131–2138. doi: 10.1007/s00277-019-03750-7
- Satoshi Y, Takumi M, Mariko M, et al. Clinical impact of bendamustine exposure on lymphopenia risk after bendamustine and rituximab combination therapy for follicular lymphoma: a single-institute retrospective study. Ann Hematol. 2022;101(1):209–211. doi: 10.1007/s00277-020-04388-6
- Tageja N. Bendamustine: safety and efficacy in the management of indolent non-Hodgkin's lymphoma. Clin Med Insights Oncol. 2011;5:145–156. doi: 10.4137/cmo.S6085
- Klippstein A, Schneider CP, Sayer HG, et al. Pneumocystis carinii pneumonia as a complication of bendamustine monotherapy in a patient with advanced progressive breast cancer. J Cancer Res Clin Oncol. 2003;129(5):316–319. doi: 10.1007/s00432-003-0441-y
- Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma. 2016;57(3):512–519. doi: 10.3109/10428194.2015.1110748
- Brugger W, Ghielmini M. Bendamustine in indolent non-Hodgkin’s lymphoma: a practice guide for patient management. Oncology. 2013;18(8):954–964. doi: 10.1634/theoncologist.2013-0079
- Gafter-Gvili A, Gurion R, Raanani P, et al. Bendamustine-associated infections—systematic review and meta-analysis of randomized controlled trials. Hematol Oncol. 2017;35(4):424–431. doi: 10.1002/hon.2350
- Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24(4–5):143–150. doi: 10.1016/j.blre.2010.06.004
- Ahrens N, Genth R, Kiesewetter H, et al. Misdiagnosis in patients with diclofenac-induced hemolysis: new cases and a concise review. Am J Hematol. 2006;81(2):128–131. doi: 10.1002/ajh.20494
- Goldschmidt N, Gural A, Ben-Yehuda D, et al. Short communication: bendamustine-related hemolytic anemia in chronic lymphocytic leukemia. Cancer Chemother Pharmacol. 2013;72(3):709–713. doi: 10.1007/s00280-013-2243-5
- Wu TKY, Tang KHK, Hwang YY, et al. Bendamustine treatment of haematological malignancies: significant risks of opportunistic viral, fungal and bacterial infections. Hematology. 2022;27(1):535–542. doi: 10.1080/16078454.2022.2072065
- Luca P, Valentina G, Bianca S, et al. Real-world evidence of cytomegalovirus reactivation in non-Hodgkin lymphomas treated with bendamustine-containing regimens. Open Med (Wars). 2021;16(1):672–682. doi: 10.1515/med-2021-0274
- Noriyoshi I, Katsuhiro M, Hiromichi T, et al. Clinical entity of cytomegalovirus disease in patients with malignant lymphoma on bendamustine therapy: a single-institution experience. Leuk Lymphoma. 2023;64(1):171–177. doi: 10.1080/10428194.2022.2131426
- Anat G, Elena R, Nadav M, et al. Infections associated with bendamustine containing regimens in hematological patients: a retrospective multi-center study. Leuk Lymphoma. 2016;57(1):63–69. doi: 10.3109/10428194.2015.1046862
- Fung M, Jacobsen E, Freedman A, et al. Increased risk of infectious complications in older patients with indolent non-Hodgkin lymphoma exposed to Bendamustine. Clin Infect Dis. 2019;68(2):247–255. doi: 10.1093/cid/ciy458
- Tapan U, May SK, Fiore J, et al. Reactivation of hepatitis B virus following bendamustine-containing chemotherapy in a patient with multiple myeloma. Leuk Lymphoma. 2011;52(5):916–918. doi: 10.3109/10428194.2010.551573
- Lock G, Helmich F, Bertram M. Impending liver failure after chemoimmunotherapy-induced reactivation of hepatitis B - successful treatment with entecavir. Dtsch Med Wochenschr. 2012;137(23):1248–1250. doi: 10.1055/s-0032-1305038
- Yoshiki H, Akira K, Hirohisa S, et al. [Two cases of malignant lymphoma with reactivation of resolved hepatitis B virus infection after bendamustine hydrochloride monotherapy]. Nihon Shokakibyo Gakkai Zasshi. 2016;113(9):1582–1587. doi: 10.11405/nisshoshi.113.1582
- Angela R, Lorenzo R, Miriam L, et al. Hepatitis B reactivation despite entecavir prophylaxis in a patient with chronic lymphocytic leukaemia receiving bendamustine. J Antimicrob Chemother. 2012;67(2):510–511. doi: 10.1093/jac/dkr486
- Uchida M, Kawashiri T, Maegawa N, et al. Pharmacovigilance evaluation of bendamustine-related skin disorders using the Japanese adverse drug event report database. J Pharm Pharm Sci. 2021;24:16–22. doi: 10.18433/jpps31597
- Rosen AC, Balagula Y, Raisch DW, et al. Life-threatening dermatologic adverse events in oncology. Anticancer Drugs. 2014;25(2):225–234. doi: 10.1097/cad.0000000000000032
- Rice MBJ, Thompson CA, Barreto JN, et al. Incidence of pneumocystis jirovecii pneumonia utilizing a polymerase chain reaction-based diagnosis in patients receiving bendamustine. Cancer Med. 2021;10(15):5120–5130. doi: 10.1002/cam4.4067
- Bernard-Valnet R, Koralnik IJ, Pasquier DR. Advances in treatment of progressive multifocal leukoencephalopathy. Ann Neurol. 2021;90(6):865–873. doi: 10.1002/ana.26198
- Trociukas I, Zirnis AE, Beļajeva L, et al. Progressive multifocal encephalopathy in a patient with non-Hodgkin follicular lymphoma. Exp Oncol. 2020;42(3):238–241. doi: 10.32471/exp-oncology.2312-8852.vol-42-no-3.15198
- Lane MA, Renga V, Pachner AR, et al. Late occurrence of PML in a patient treated for lymphoma with immunomodulatory chemotherapies, bendamustine, rituximab, and Ibritumomab Tiuxetan. Case Rep Neurol Med. 2015;2015:1–4. doi: 10.1155/2015/892047
- Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021;17(1):37–51. doi: 10.1038/s41582-020-00427-y
- Pavlovic D, Patel MA, Patera AC, et al. T cell deficiencies as a common risk factor for drug associated progressive multifocal leukoencephalopathy. Immunobiology. 2018;223(6–7):508–517. doi: 10.1016/j.imbio.2018.01.002
- Aly L, Yousef S, Schippling S, et al. Central role of JC virus-specific CD4+ lymphocytes in progressive multi-focal leucoencephalopathy-immune reconstitution inflammatory syndrome. Brain. 2011;134(9):2687–2702. doi: 10.1093/brain/awr206
- Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011;85(14):7256–7263. doi: 10.1128/jvi.02506-10