Abstract
Earlier studies Citation showed that of the glycolytic enzymes, the muscle isozymes PFK-1, LDH, and AK were inhibited by ascorbic acid. These studies on the characteristics of the inhibition of RMAK by ascorbate are part of a hypothesis [Citation] that ascorbate facilitates the storage of skeletal muscle glycogen by inhibiting glycolysis when the muscle is at rest. These studies examine conditions for RMAK inhibition, prevention of inhibition, and reversal of ascorbate inhibition. We found that the concentration of RMAK was an important condition for inhibition. Above 200 nM RMAK, inhibition by ascorbate could not be demonstrated and below that concentration RMAK became increasingly sensitive to ascorbate inhibition. Associated with increased sensitivity to inhibition by ascorbate is a deviation from a linear to a concave relationship between low RMAK concentrations and enzyme activity. At low RMAK concentrations, the concave relationship becomes convex in the presence of muscle aldolase. In addition, aldolase reverses inhibitions by ascorbate. A comparison of inhibition of RMAK by ascorbate and inhibition of LDH-m4 [Citation] is discussed. Other proteins prevent RMAK inhibition but do not reverse inhibition by ascorbate. The role of RMAK as a factor in the control of the rate of glycolysis is presented as is the role of compartmentalization with respect to the proposed role for ascorbate inhibition.
Introduction
Previous reports Citation1-3 showed that normally found ascorbate concentrations specifically inhibited muscle phosphofructokinase-1 (mPFK-1), muscle lactate dehydrogenase (LDH-m4), and muscle adenylate kinase (AK-1), isozymes facilitating or essential for anaerobic glycolysis. We also showed [Citation3] that either muscle aldolase or G-actin protected LDH-m4 from inhibition by ascorbate and that muscle aldolase had the additional property of reversing ascorbate inhibitions. Based on these observations, the hypothesis was developed [Citation2] that when at rest, ascorbate facilitates muscle glycogen storage from glucose by inhibiting isozymes involved in glycolysis and, when the muscle is active, these isozymes are protected from inhibition by aldolase and/or other proteins. This paper reports on characteristics of rabbit muscle adenylate kinase (RMAK) inhibition by ascorbate and protection from inhibition by muscle aldolase and G-actin and other proteins.
We found inhibition by ascorbate depended upon rabbit muscle AK-1 (RMAK) concentration; μmolar ranges of RMAK are not inhibited by ascorbate while low nmolar ranges are extensively inhibited. Low RMAK concentrations also show a diminished activity similar to mPFK-1 [Citation4]. We found muscle aldolase prevented diminished RMAK activity due to low concentrations and also resulted in recovery of activity due to ascorbate inhibition. G-actin prevented diminished activity due to low RMAK concentrations and terminated inhibitions by ascorbate in progress but unlike aldolase resulted in no recovery of activity due to ascorbate inhibition. Aldolase and G-actin exhibited these characteristics for inhibition of LDH-m4 by ascorbate [Citation3]. The characteristics of RMAK activity losses are discussed in relation to the hypothesis.
Materials and methods
Materials
These studies used enzymes from rabbit unless stated otherwise. The following materials were obtained commercially from Sigma-Aldrich Co: rabbit muscle adenylate kinase (RMAK, EC 2.7.4.3); muscle pyruvate kinase (PK, EC 2.7.1.40); and muscle and heart lactate dehydrogenase (LDH-m4 and LDH-h4, EC 1.1.1.27); and ascorbic acid (A 5960). Sigma-Aldrich Co was also the source of enzymes used in the assay systems.
Methods
All operations were at 25 °C unless otherwise stated.
When G-actin was present, all final RMAK solutions and reagents were in 20 mM potassium phosphate buffer, pH 8.0 to avoid polymerization to F-actin.
Adenylate kinase (AK) assay
Normal assay conditions
We measured AK activity, AMP + MgATP = ADP + MgADP, according to Adam [Citation5]. Other kinases and reactions producing ADP were appropriately coupled and adapted to the same pyruvate kinase-lactate dehydrogenase assay system as AK. A 1 mL assay mixture contained 0.3 mM phosphoenolpyruvate, 0.4 mM NADH, 8.0 mM ATP, 8.1 mM MgCl2 and 20 mM potassium phosphate or Tris–HCl buffer, pH 8. There were sufficient amounts of LDH-h4 and pyruvate kinase (PK) so the coupling system was not rate limiting. Reactions were initiated by additions of test enzymes. Initial reaction rates were determined by measuring decreased absorbance of NADH at 340 nM with time. A molar absorptivity value of 6,220 was used to convert NADH absorbance changes to μmoles of product formed. One AK enzyme unit (eu) of activity is defined as formation 1 μmole of NAD+ formed per min.
Assay conditions for dilute RMAK solutions
When 100 μL sample aliquots measured below 0.05 absorbancy units/min for uninhibited, dilute RMAK solutions under normal assay conditions, the RMAK sample was made up to 0.9 mL and assay components were concentrated 10 times the normal assay concentrations into 0.1 mL so that final concentrations were identical to the normal AK assay.
Aldolase assay
Aldolase activity was measured using modifications of a method previously published [Citation9]. All solutions were made up in 100 mM Tris-potassium phosphate buffer, pH 8. The assay medium contained the following concentrations: 2 mM fructose 1,6-bisphosphate, 0.13 mM NADH and 1.7 and 18 eu/mL of glyceraldehyde 3-phosphate dehydrogenase and triose phosphate isomerase, respectively. A molar absorptivity value of 6,220 was used to convert NADH absorbance changes to μmoles of product formed. One enzyme unit (eu) of aldolase activity is defined as 1 μmole of NAD+ formed per min.
Pre-incubations of low RMAK concentrations
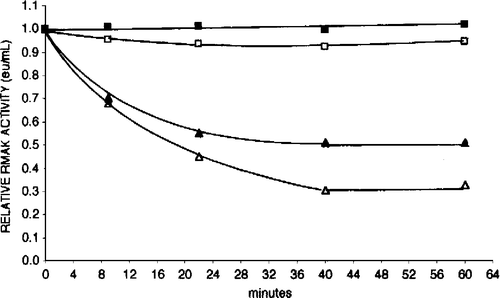
Unless stated otherwise, inhibitions with ascorbate were with low RMAK concentrations that lost activity as a result of the dilution. The magnitude of activity loss was related to RMAK concentration and stabilized in about 40 min (see ). Unless stated otherwise, a routine inhibition procedure was to allow diluted RMAK to stand for 1 h before ascorbate additions.
Figure 1 Effect of dilution on RMAK activity. A 2000 nM RMAK solution in 0.1 M potassium phosphate, pH 8.0 (▪) was diluted with buffer to 200 nM RMAK (▪), 25 nM RMAK (▴) and 6 nM RMAK (▵). Activities were measured after dilution and then with time. The relative activities of 1.0 at 0 min were estimated as the determined activity of 2000 nM RMAK stock solution divided by the appropriate dilution.
Inhibition assays
Three inhibition conditions were used in these studies.
Time course of ascorbate inhibition
Ascorbate and RMAK were mixed and aliquots removed to assay activity remaining at indicated times. Under our conditions, it was determined that no significant changes in ascorbate inhibition occurred after 0.5 h of incubation.
Ascorbate titrations against a constant RMAK concentration
Fixed RMAK concentrations with varying ascorbate concentrations were incubated for at least 0.5 h. The RMAK activity remaining was then determined.
RMAK titrations against a constant ascorbate concentration
Fixed concentrations of ascorbate against varying RMAK concentrations were incubated for 0.5 h or more. The RMAK activity remaining was then determined.
Measurements of protein concentrations
Protein concentrations were measured using the following formula: mg protein/mL = 1.55 A280 − 0.76 A260, where A280 and A260 are absorbencies at 280 nm and 260 nm, respectively [Citation6]. The spectrophotometric protein determinations were comparable to the Bradford method [Citation7].
Criteria of purity
The purity of proteins was determined using SDS polyacrylamide gel electrophoresis (PAGE, not shown). SDS PAGE used 12 percent cross-linked gels and the Mini-Protean II Cell assembly and the Bio-Rad gels were silver stained for proteins using the procedure of Morrissey [Citation8]. Rabbit muscle, G-actin (Sigma, A 2522) and rabbit muscle aldolase (A 8811) were shown free of AK activity under our conditions.
Results
Effect of RMAK concentration on activity
Low RMAK concentrations showed non-linear relationships with activities. shows RMAK activity with time at concentrations ranging from 2000 nM to 6.7 nM. Essentially no changes in activity occurred after 40 minutes up to an additional 24 hours (not shown). The final stable activities are lower than accounted for by dilution alone. The effects of dilutions on activities were similar to those reported for muscle PFK-1 [Citation4] where evidence was presented that the activity losses were due to dissociation of an active tetramer to a dimer that was either not active or had a lower activity.
Non-linear relationship between RMAK concentration and activity
shows a concave relationship between activity and low RMAK concentrations, resulting in activities that are lower than predicted by dilution alone. The presence of aldolase results in a slightly convex relationship. Above 400 nM RMAK the relationship between concentration and activity appears rectilinear and extrapolates through zero.
Figure 2 Dilution and aldolase effect relationships between RMAK concentrations and activity. A 2000 nM RMAK solution in 0.1 M potassium phosphate, pH 8.0 was diluted to concentrations shown, incubated at 25 °C for 1 h and activities then determined (▪). Similar dilutions and conditions were made except that they also were made 500 nM aldolase (▪). The dotted line is the expected linear relationship.
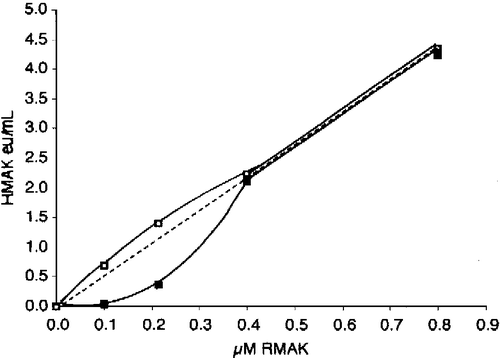
shows that the presence of aldolase increases activity about 10 percent on average at higher RMAK concentrations. Below 400 nM RMAK activities are less than expected and the presence of aldolase increases the activity to more than expected values. At 100 nM RMAK, for example, the presence of aldolase brings about a 6-fold increase above measured RMAK activity.
Table I. Effect aldolase on RMAK concentrations/activity ratios
The relative activities in show that aldolase enhances activity below 200 nM RMAK but has no such effect at higher concentrations. From similar studies at lower RMAK concentrations (not shown), the intersections of the plateaus and the ascending slopes yield estimated aldolase/RMAK ratios between 2 and 4, suggesting that low concentrations of RMAK have a high affinity for ascorbate.
Figure 3 The effect of aldolase concentration on RMAK activity. Dilutions of RMAK were made with aldolase in 0.1 M potassium phosphate, pH 8. The values in brackets show control RMAK activities after dilution and after 1 h incubation with no aldolase. Values in the brackets are stable RMAK activities after 1 h incubation following dilution. A 2000 nM RMAK solution was diluted to 25 nM RMAK, incubated for 1 h, and activity determined (▵) [0.03 eu/mL]. Similar procedures were performed for, 50 nM (▪) [0.07 eu/mL], 100 nM (▪) [0.33 eu/mL], 200 nM (▴) [0.460 eu/mL], and 400 nM RMAK (•) [1.18 eu/mL].
![Figure 3 The effect of aldolase concentration on RMAK activity. Dilutions of RMAK were made with aldolase in 0.1 M potassium phosphate, pH 8. The values in brackets show control RMAK activities after dilution and after 1 h incubation with no aldolase. Values in the brackets are stable RMAK activities after 1 h incubation following dilution. A 2000 nM RMAK solution was diluted to 25 nM RMAK, incubated for 1 h, and activity determined (▵) [0.03 eu/mL]. Similar procedures were performed for, 50 nM (▪) [0.07 eu/mL], 100 nM (▪) [0.33 eu/mL], 200 nM (▴) [0.460 eu/mL], and 400 nM RMAK (•) [1.18 eu/mL].](/cms/asset/eae70325-55c5-4bad-a4de-eaa1fb02b7c8/ienz_a_104320_f0003_b.gif)
Inhibition of RMAK by ascorbate
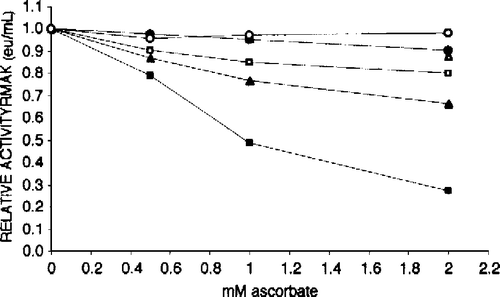
Low RMAK concentrations are subject to ascorbate inhibition; high concentrations are not. shows that above 100 nM RMAK are not extensively inhibited by ascorbate, if at all. At concentrations below 100 nM RMAK the inhibitions by ascorbate become substantial. These losses of activity due to ascorbate inhibitions are in addition to losses due to low RMAK concentrations that stabilized well before 1 hour of prior incubation (see and Materials and methods).
Figure 4 Effect of RMAK concentration on inhibition by ascorbate. Each RMAK concentration shown was diluted from 2000 nM RMAK in 0.1 M potassium phosphate, pH 8.0. Controls without ascorbate were incubated 1 h and activities determined are given within parentheses. Following that 1 h incubation aliquots of each RMAK concentration was made to ascorbate concentrations indicated, and activities remaining determined after an additional 1 h incubation. RMAK activities just prior to the ascorbate additions are with the symbols in parentheses. The RMAK concentrations were 400 nM (○, 1.51 eu/mL); 300 nM (▵, 1.13 eu/mL); 200 nM (•, 0.76 eu/mL); 100 nM (▪, 0.39 eu/mL); 50 nM (▴, 0.21 eu/mL); and 25 nM (▪, 0.08 eu/mL).
Aldolase prevents activity losses at low RMAK concentration
The report [Citation4] that muscle aldolase prevented muscle PFK-1 activity losses due to low concentrations led to consideration that muscle aldolase might also protect RMAK from similar activity losses and from ascorbate inhibitions Citation1-3. In similar studies [Citation3] with LDH-m4 inhibition by ascorbate, there were no activity losses in LDH-m4 concentration ranges used in this present study. shows 20 nM RMAK activity losses are terminated by 5 μM aldolase at various times of addition, again a similarity with muscle PFK–1 studies [Citation4]. Hiowever, there are differences from muscle PFK-1. In the presence of aldolase, the initial rapid RMAK activity loss partially rebounds with a slower convergence of activity towards about half the initial activity. These effects are more evident at 6.7 nM RMAK.
Figure 5 Aldolase protects RMAK from dilution effects. A 2000 nM RMAK in 0.1 M potassium phosphate, pH 8.0 was diluted to 20 nM RMAK as given in and then made 5 μM aldolase by additions to aliquots of the 0 time control (▪) at times indicated and activities remaining were determined. Each addition was then followed with time up to 120 min. The times of aldolase additions were 5 min (▪), 10 min (▴), 20 min (▵), 30 min (•) and 60 min (○). Additions of concentrated aldolase solutions to aliquots were less than 5 percent of the total aliquot volume.
In the presence of 5 μM aldolase, shows a rapid loss of activity and slow restoration at 6.7 nM RMAK up 60 minutes after dilution, followed by a slow activity decline thereafter. The activity losses due to low RMAK concentrations are similar to reports of activity losses at low muscle PFK-1 concentrations [Citation4] with some differences. PFK-1 showed high specificity for muscle aldolase that prevented and terminated activity losses but did not restore activity. Other proteins, including bovine serum albumin, had no effect [Citation4]. Activity losses due to low PFK-1 concentrations were attributed to dissociations of PFK-1 tetramers to less active or inactive dimers. It was proposed that stabilization of activity losses with aldolase was due to formation of a stable PFK-1 dimer-aldolase complex [Citation4].
Figure 6 Aldolase protects RMAK from dilution effects. A 2000 nM RMAK solution in 0.1 M potassium phosphate, pH 8.0 was diluted to 6.7 nM RMAK with the buffer. After 0 min (▪), aliquots were removed at 5 min (▪), 10 min (▴) and 60 min (○), the aliquots were made 5 μM aldolase without significant dilution and the activity remaining was followed with time.
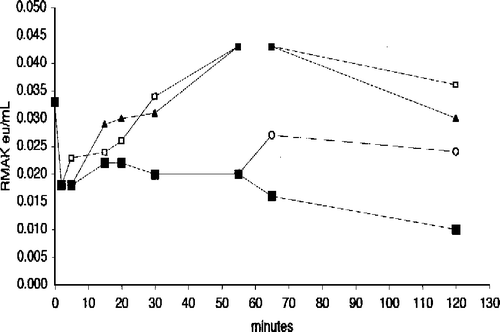
An interpretation of the pattern shown in with RMAK is a rapid dissociation of a RMAK dimer to a less active RMAK monomer followed by a slower formation of a more active RMAK monomer-aldolase complex that slowly loses activity after 60 minutes. We also gave thought to a stable RMAK dimer resistant to ascorbate inhibition and a less active RMAK monomer sensitive to ascorbate inhibition, especially since there is evidence that rat liver AK exists as a monomer and a dimer [Citation9]. We were unsuccessful, however, in identifying dimeric or other polymeric RMAK forms or aldolase-RMAK complexes using gel filtration chromatography with Sephadex [Citation10,Citation11]. The problems were extreme instability of RMAK below 200 nanomolar under size exclusion chromatography conditions. While there were occasional hints of RMAK dimers and of RMAK-aldolase complexes, we lack confidence in the findings because of consistent RMAK activity losses of more than 90 percent. At 200 nM RMAK or higher, we found no evidence of dimers or of RMAK-aldolase complexes.
Specificity of aldolase effects at low RMAK concentrations
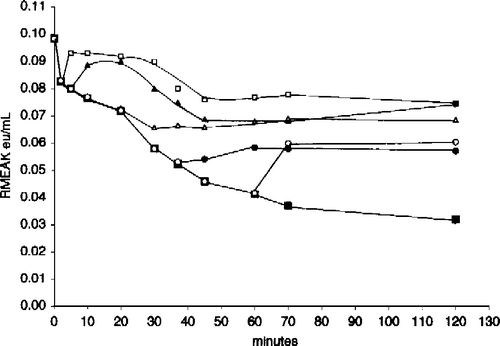
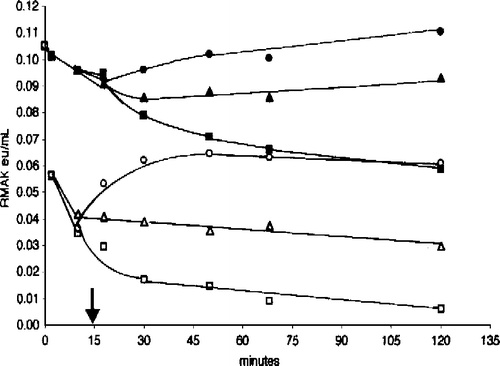
We were interested in the effects of aldolase and G-actin on low RMAK concentrations in the absence and presence of ascorbate. is a composite of two experiments. The filled symbols show activity losses due to dilution alone to 20 nM RMAK (▪) that terminate when G-actin (▴) or muscle aldolase (•) are added 15 minutes (arrow) after dilution. Under similar conditions the open symbols show activity losses due to dilution in the presence of 2 mM ascorbate present at 0 minutes (▪), The addition of muscle aldolase at 15 minutes (arrow) after dilution (○) resulted in recovery of activity losses due to ascorbate inhibition while the addition of G-actin at 15 minutes (▵) only terminated additional activity losses. The termination of activity losses due to the low RMAK concentration by additions of G-actin or the recovery activity losses by muscle aldolase due to ascorbate inhibition were obtained at any point along the time line.
Figure 7 Effect of muscle aldolase and G-actin on 20 nM RMAK in the presence and absence 2 mM ascorbate. A 2000 nM RMAK solution was diluted to 20 nM RMAK with 0.02 M potassium phosphate, pH 8.0 buffer (▪) and activity followed with time. At 15 min (arrow), aliquots were made 5 μM muscle aldolase (•) or 5 μM G-actin (▴) and activity was followed with time. Also at 0-min, a 2000 nM RMAK solution was diluted to 20 nM RMAK in buffer and 2 mM ascorbate (▪) and at 15 min (arrow), aliquots were made 5 μM muscle aldolase (○) or 5 μM G-actin (▵) and activity was followed with time.
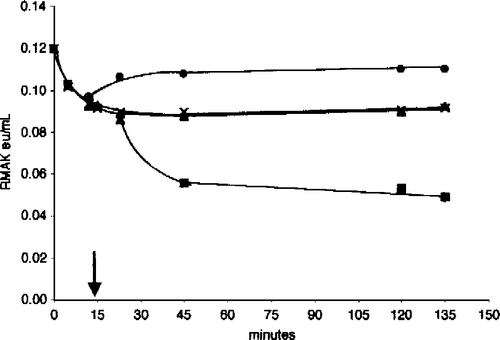
Preventions of enzyme activity losses at 20 nM RMAK by G-actin shown in appear to be a non-specific effect of proteins on dilute enzyme solutions. shows bovine serum albumin and myosin have the same effects as G-actin; they protect from activity losses but do not restore activity. It can also be shown that dilution to 20 nM RMAK with bovine serum albumin and myosin are also similar to G-actin in that they protect from ascorbate inhibition at zero-time and terminate additional ascorbate inhibitions when added at any point along the time line but do not restore activity.
Figure 8 Effect of muscle aldolase, bovine serum albumin, and myosin on 2 mM ascorbate inhibition of 20 nM RMAK. A 2000 nM RMAK in 0.02 M potassium phosphate, pH 8.0, was diluted to 20 nM RMAK. 1 h later the 20 nM RMAK was made 2 mM ascorbate and activities were followed with time (▪). At 15 min, aliquots were made 5 μM muscle aldolase (•) or 5 μM myosin (▴) or 5 μM bovine serum albumin (X) and activities were followed with time.
Effect of substrate on ascorbate inhibitions
It was previously shown Citation11-13 that sulfhydryl compounds such as DTT and GSH restored RMAK activity losses incurred by a variety of methods [Citation2].
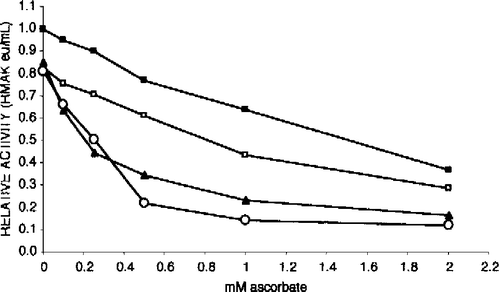
We were also interested in determining the affect of substrates. shows substrates increased RMAK sensitivity to inhibition by ascorbate at low RMAK concentrations that begin to result in activity losses. It can be shown that at 200 nM RMAK and above, (concentrations not inhibited by ascorbate) substrates have no effect.
Figure 9 Substrates increase 25 nM RMAK sensitivity to ascorbate inhibition. The activity of the control, 25 nM RMAK in 0.1 M potassium phosphate, pH 8.0, stabilized by 1 h (▪, 0.07 eu/mL). At that time aliquots of the control were made to ascorbate concentrations indicated. In addition to ascorbate, solutions contained the following, 0.45 mM AMP (▪); 0.5 mM ATP (▴); and 0.5 mM ATP.Mg (○) and were made to ascorbate concentrations indicated, then incubated for an additional 1 h when activities remaining were determined.
Discussion
In previous papers Citation1-3 we proposed that ascorbate facilitates glycogen storage in resting rabbit muscle by inhibiting three muscle isozymes (PFK-1, LDH-m4, RMAK) that promote muscle glycolysis. The hypothesis proposes further that during contraction when these muscle isozymes attach to contractile proteins Citation14-17 they are both protected and released from ascorbate inhibition and glycolysis proceeds unhindered.
Glycolysis serves as an almost immediate, rapid source of ATP during contractions, as does the creatine kinase reaction [Citation18] (creatine phosphate + ADP = creatine + ATP). During glycolysis, PFK-1 forms fructose 1,6-bisphosphate and that putatively commits to pyruvate formation, giving rapid rise to four ATP's. Equally important to pyruvate formation is regeneration of NAD+ by LDH for anaerobic oxidation of 3-phosphoglyceraldehyde.
The role of the AK-1 reaction in muscle glycolysis is complex and less direct than PFK-1 and LDH-m4. During muscle contraction ATP is spent and an ADP is produced. Through the AK-1 reaction, two ADP's generate one AMP and one ATP and the three adenosine nucleotides are maintained close to equilibrium concentrations [Citation19]. Coupled with the creatine kinase reaction, muscle ATP concentrations remain relatively constant and small decreases in the ATP concentration result in large changes in AMP concentrations [Citation20]. At the same time, the flux of ATP through the AK-1 reaction can increase by as much as 100-fold during muscle contraction and increases in the flux of ATP result in relatively large AMP concentration increases; a 40 percent decrease in ATP can be associated with more than a 100-fold increase in AMP concentration [Citation20].
The AK reaction is central to the control of AMP concentrations [Citation21]. As an oversimplification, AMP stimulates glycolysis as a positive allosteric effector of the PFK-1 reaction, competing with the opposing effects of ATP. Separate from the c-AMP cascade, studies and discoveries in the past decade of 5′ AMP-activated protein kinases and 5′ AMP-protein kinase kinases suggest a very important, complex, widespread influence of AMP concentration on cellular energy status [Citation22,Citation23]. In the resting state, ATP concentrations are slightly higher and AMP concentrations are lower relative to active states. Decreased AK-1 activity contributes to decreased glycolytic activity.
We show that AMP and in particular ATP and MgATP increased the sensitivity of RMAK to ascorbate inhibition (). This increased sensitivity to ascorbate is consistent with the proposal that higher ATP concentrations in resting muscle [Citation20] diminish RMAK activity, which decreases AMP concentration. Decreased glycolytic activity is associated with decreased AMP concentration that facilitates glycogen synthesis on the basis of a decreased competing process. While the effects of ascorbate, aldolase, and G-actin on RMAK are consistent with the proposed hypothesis, incorporation into the hypothesis is complicated by RMAK concentration constraints not observed for LDH-m4 inhibitions by ascorbate [Citation3].
The effects of aldolase, actin, myosin or bovine serum albumin on activity losses at low RMAK concentrations in these studies are the same as their effects on activity losses due to ascorbate inhibition. At low RMAK concentrations at which the loss of activity has stabilized () aldolase both protects and restores RMAK activity losses (, ); G-actin and other proteins tested terminate further activity losses but do not restore activity (not shown).
The same effects of aldolase, actin, myosin or bovine serum albumin apply to activity losses due to ascorbate inhibition. After the activity losses due to low RMAK concentration stabilize (), aldolase protects against and restores activity losses due to ascorbate inhibition; the other proteins tested terminate ascorbate inhibition whenever added but do not restore lost activity ( and ).
The focus of the hypothesis is on the role of ascorbate in the synthesis of muscle glycogen. Of total glycogen storage, skeletal muscle accounts for more than 60% and liver another 30%. The functions of glycogen from these two major sources are very different—skeletal muscle glycogen is an immediate source of energy and liver glycogen is a source of blood glucose. It is interesting that while liver isozymes of PFK-1 and AK are not inhibited by ascorbate [Citation1], LDH-m4, the major isozyme in liver, is inhibited by ascorbate Citation1-3.
The concentration requirements are not yet understood. Preliminary studies with rabbit muscle PFK-1 indicate ascorbate inhibition characteristics very similar to the RMAK isozyme shown here rather than LDH-m4 [Citation3]. It appears that the inhibitions of muscle PFK-1 by ascorbate also have specific concentration requirements. Increasing evidence in the literature of sequestered and compartmentalized proteins and enzymes [Citation4] in various cell environments is a complex issue that raises questions of the role of partitions on intracellular enzyme concentrations and function [Citation24].
Acknowledgements
The authors appreciate the following for support of this work: HCOE Grant 5 D34 MB02060; EXPORT Grant 1 PD60 M00622; and UCSD NIH Scholars Program Grant.
References
- Russell PJ, Williams A, Austin T. J Enz Inhib 2000; 15: 283–296
- Russell PJ, Williams A, Gapuz D. Biochem Biophys Res Commun 1997; 233: 386–388
- Russell PJ, Williams A, Xavier A, Vargas R. J Enz Inhib Med Chem 2004; 19: 91–98
- Räis B, Ortega F, Puigjaner J, Comin B, Orosz F, Ovádi J, Cascante M. Biochim Biophys Acta 2000; 1479: 303–314
- Adam H. Methods of enzymatic analysis. Academic Press, New York 1963; 145–152
- Wilson K, Walker J. Principles and techniques of practical biochemistry. Cambridge University Press, Cambridge 2000
- Bradford MM. Anal Biochem 1976; 72: 248–254
- Morrissey JH. Anal Biochem 1981; 117: 307–310
- Criss WE, Sapico V, Litwack GJ. Biol Chem 1970; 245: 6346–6351
- Akers GK. Biochemistry 1964; 3: 723–730
- Russell PJ, Chinn E, Williams A, David-DiMarino C, Taulane JP, Lopez R. J Biol Chem 1990; 265: 11804–11809
- Williams A, Taulane JP, Russell PJ. Comp Biochem Physiol 1994; 107B: 489–494
- Russell PJ, Williams A, Avila D, Chinn E, Taulane JP. J Enz Inhib 1995; 9: 179–194
- Walsh TP, Winzor DJ, Clarke FM, Masters CJ, Morton DJ. Biochem J 1980; 186: 89–98
- Méjean C, Pons F, Benjamin Y, Roustan C. Biochem J 1989; 264: 671–677
- Arnold H, Henning R, Pette D. Eur J Biochem 1971; 22: 121–126
- Clarke FM, Masters CJ. Biochim Biophys Acta 1975; 381: 37–46
- Balschi JA, Shen H, Madden MC, Hai JO, Bradley EL, Jr, Wolkowicz PE. J Mol Cell Cardiol 1997; 29: 3123–3133
- Fredrich M, Balschi JA. J Biol Chem 2002; 277: 1929–1932
- Hochachka PW, McCelland GB. J Exp Biol 1997; 200: 381–386
- Hardie DG, Hawley SA. BioEssays 2001; 23: 1112–1119
- Hardie DG, Carling D. Eur J Biochem 1997; 246: 259–273
- Hardie DG, Scott JW, Pan A, Hudson ER. FEBS Lett 2003; 546: 113–120
- Savabi F. Mol Cell Biochem 1994; 133/134: 145–152
