Abstract
In vitro effects of omeprazole, morphine sulphate, remifentanyl, ketamine and vankomycin were investigated on human red blood cell glucose-6 phosphate dehydrogenase (G-6PD) (E.C. 1.1.1.49) enzyme activity purified from human red blood cell by 2′, 5′ADP-Sepharose 4B affinity gel. The obtained I50 values of omeprazole, morphine, remifentanil, ketamine and vankomycin were 3.24, 43.58, 97.6, 64.16 and 0.903 mM, respectively and the Ki constants for omeprazole, morphine and vankomycin were 8.22 ± 2.055, 25.93 ± 6.482, and 2.71 ± 0.677 mM, respectively and they were non competitive inhibitors.
Introduction
Glucose-6 phosphate dehydrogenase (G-6PD) catalyzed the first step of pentose phosphate metabolic pathway and this is unique source for NADPH synthesis in red blood cells [Citation1,Citation2]. These reactions appear to be necessary for maintenance of cellular integrity. NADPH production is decreased in G-6PD deficiency. The most important role of NADPH is reduction of glutathione [Citation3,Citation4].
Omeprazole is a proton-pump inhibitor for acid-related diseases, which is the world's largest-selling gastrointestinal product [Citation5]. Vankomycin has been an important drug against bacterial pathogens [Citation6]. Anesthetics are widely used in dental pain, and in the therapy of chronic and pain and ketamine, or ketamine hydrochloride, has been used in human medicine for pediatric burn cases and dentistry [Citation7]. Morphine is a naturally occurring substance in the opium poppy and it is a potent narcotic analgesic, its primary clinical use being in the management of moderately severe and severe pain. After heroin, morphine has the greatest dependence liability of the narcotic analgesics in common use [Citation8]. Remifentanyl is a new opioid of the fentanyl family being an analog of fentanyl (4-piperidyl anilide) with a methyl-ester group that allows the molecule to be hydrolyzed by esterases in plasma and tissues. Rapid onset and metabolism make it an easy drug to control for achieving the desired depth of anesthesia, although these aspects are also the drug's main drawbacks [Citation9].
Omeprazole, morphine sulphate, remifentanyl, ketamine and vankomycin are widely used world-wide as drugs but their inhibition kinetics on human G-6PD have not been reported in detail. Here we report for the first time effects on human G-6PD activity of these drugs. In conclusion, when these drugs are used in patients with G-6PD deficiency, their inhibitory effects on this enzyme must be taken into account.
Materials and methods
Materials
2′, 5′ ADP-Sepharose 4B was purchased from Pharmacia. NADP+, glucose 6-phosphate, protein assay reagent and chemicals for electrophoresis were purchased Sigma Chem. Co. All other chemicals used were analytical grade and purchased from either Sigma or Merck.
Preparation of affinity gel
2′, 5′ ADP-Sepharose 4B (2 g dried) gel used for 10 mL column volume was washed with distilled water to remove foreign bodies and air eliminated. Gel was suspended in 0.1 M K-acetate/0.1 M K-phosphate buffer (pH 6.0) and then packed in a small column (1 × 10 cm) and equilibrated with the same buffer. The gel was washed with equilibration buffer by peristaltic pump. The flow rates for washing and equilibration were 50 mL/h [Citation10].
Preparation of hemoIysate
Fresh human blood collected in ependorph tubes with EDTA was centrifuged at 2500 × g for 15 min and the plasma and leukocyte coat removed by drip. The packed red cells were then washed with KCl solution (0.16 M) three times, the samples centrifuged at 2500 × g on each occasion and the supernatants removed. The erythrocytes were hemolysed with 5 volumes of ice-cold water and centrifuged at 4°C, 10 000 × g for 30 min to remove the ghosts and intact cells [Citation5,Citation6]. Ammonium sulphate (35–65%) precipitation was made on the hemolysate, the ammonium sulphate being slowly added for completely dissolution, and the mixture was centrifuged at 5000 × g for 15 min. The precipitate was dissolved in 50 mM of phosphate buffer (pH 7.0), then dialysed at 4°C in 50 mM K-acetate/50 mM K-phosphate buffer (pH 7.0) for 2 h with two changes of buffer [Citation10].
Purification of G-6PD by affinity chromatography
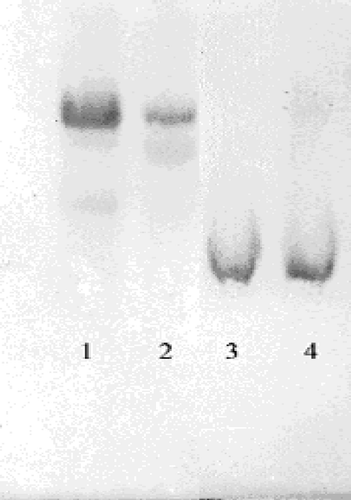
The dialysed sample was loaded on a 2′, 5′ ADP-Sepharose 4B affinity column and the gel was washed with 25 mL of 0.1 M K-acetate/0.1 M K-phosphate (pH 6.0), with 25 mL of 0.1 M K-acetate/0.1 M K-phosphate (pH 7.85), and finally, with 0.1 M KCl/0.1 M K-phosphate (pH 7.85). Elution was carried out with 80 mM K-phosphate +80 mM KCl +0.5 mM NADP++10 mM EDTA (pH 7.85) solution at 20 mL/h flow rate. Eluates were collected in 2 mL tubes and their activity was separately determined. All of the procedures were performed at 4°C [Citation11]. Quantitative protein determination was measured spectrophotometrically at 595 nm according to Bradford's method, with bovine serum albumin as a standard [Citation12]. SDS Polyacryamide Gel Electrophoresis was conducted on the purified enzyme according to Laemmli's method using 4% and 10% acrylamide concentration for stacking and running gel respectively, containing 0.1% SDS () [Citation13].
Measurements of G-6PD activity
G-6PD activity was measured spectrophotometrically at 25°C according to Beutler's method which depends on the reduction of NADP+ by G-6PD in the presence of glucose 6-phosphate. The reaction medium was maintained at 25°C and contained 0.1 mM Tris-HCl (pH = 8.5) with 0.5 mM EDTA, 10 mM MgCl2, 0.2 mM NADP+, and 0.6 mM (G6-P) in a total volume of 1 mL. One unit of enzyme (EU) activity was defined as the amount of enzyme reducing 1 μmol NADP+ per 1 min at pH 8.5. [Citation14].
Inhibition studies
For I50 values, omeprazole, morphine sulphate, remifentanyl, ketamine and vancomycin were studied as inhibitors. Activies were measured for omeprazole (0.12–5.79 mM cuvette concentration), morphine sulphate (0.66–52.8 mM), remifentanyl (41.28–206.4 mM), ketamine (4.557–91.15 mM) and vancomycin (0.344–3.44 mM). Drugless cuvette activity was accepted as 100%. For each drug a [Drug]-activity % graph was drawn at five different inhibitor concentrations (). Drug concentrations which produce 50% inhibition (I50) were calculated from the graphs.
Figure 2 Activity % -[Omeprazole] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different omeprazole concentrations.
![Figure 2 Activity % -[Omeprazole] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different omeprazole concentrations.](/cms/asset/4268a14e-16bd-45a6-b746-18f68285b007/ienz_a_135662_f0002_b.gif)
Figure 3 Activity % -[Morphine Sulphate] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different morphine sulphate concentrations.
![Figure 3 Activity % -[Morphine Sulphate] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different morphine sulphate concentrations.](/cms/asset/569a6864-eca9-4ad5-ac70-d83917428dc4/ienz_a_135662_f0003_b.gif)
Figure 4 Activity % -[Vankomycin] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different vankomycin concentrations.
![Figure 4 Activity % -[Vankomycin] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different vankomycin concentrations.](/cms/asset/584dda9f-3062-4a3e-8f05-8696c08a5450/ienz_a_135662_f0004_b.gif)
Figure 5 Activity % -[Ketamine] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different ketamine concentrations.
![Figure 5 Activity % -[Ketamine] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different ketamine concentrations.](/cms/asset/5ea5ce13-46b4-42b2-b1f9-1296d82e7eb5/ienz_a_135662_f0005_b.gif)
Figure 6 Activity % -[Remifentanyl] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different remifentanyl concentrations.
![Figure 6 Activity % -[Remifentanyl] regression analysis graphs for human erythrocytes G-6PD in the presence of 5 different remifentanyl concentrations.](/cms/asset/d5859a1f-d2de-4d3d-850a-5938b916204b/ienz_a_135662_f0006_b.gif)
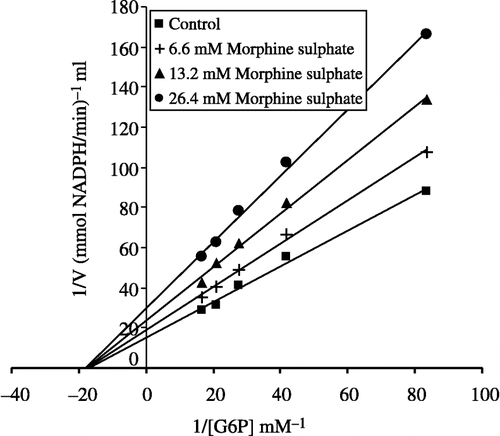
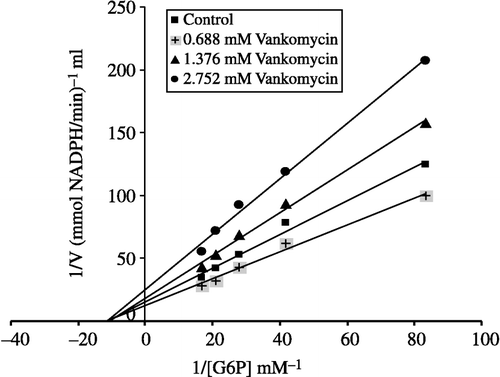
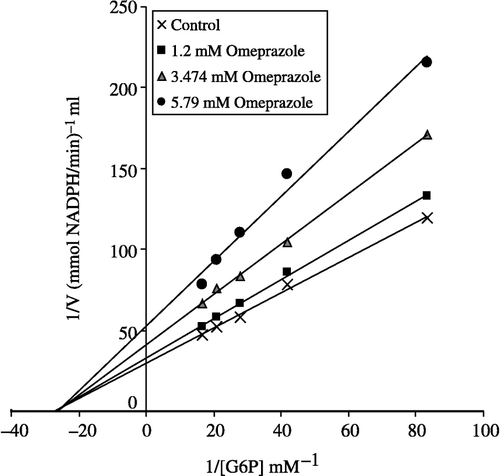
To calculate Ki values for omeprazole, morphine and vancomycin the substrate concentrations between were 0.012–0.060 mM. Inhibitor solutions were added to the reaction medium, resulting in three different fixed concentrations of inhibitors in 2.5 mL total reaction volume as well as five substrate concentrations. Using 1/V vs 1/[S] values, regression analysis was carried out and the equations obtained from the analysis were used to draw Lineweaver-Burk graphs for each fixed inhibitor concentration. Ki values were calculated from these Lineweaver-Burk graphs (, and ).
Figure 7 Lineweaver–Burk graphs for G-6PD in presence of three constant omeprazole and five different substrate concentrations.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Three parallel measurements were analysed by Student's t test.
Results
Human G-6PD was purified by 2′, 5′ ADP-Sepharose 4B gel affinty column. As shown in , specific activity was calculated for the hemolysate and purified enzyme solution as 62.96 EU/mg protein, a yield of 28.33% and a purification coefficient of 2248-fold. Purification steps were controlled by SDS-PAGE. Serum albumin was used as standard (). As shown and , the obtained I50 values for omeprazole, morphine, remifentanyl, ketamine and vankomycin were 3.24 mM, 43.58 mM, 97.6 mM, 64.16 mM, and 0.903 mM, respectively. Ki values (Tables ) were calculated from Lineweaver–Burk graphs ( and ) and the Ki constants for omeprazole, morphine and vankomycin were 8.22 mM, 25.93 mM, and 2.71 mM, respectively. These three drugs were found to be non competitive inhibitors.
Table I. Purification scheme of glucose 6-phosphate dehydrogenase from human erythrocytes.
Table II. Ki and I50 values obtained from Lineweaver–Burk graphs for G-6PD in the presence of three fixed inhibitors and five substrate concentrations for omeprazole, morphine sulphate, and vankomycin. I50 values for remifentanyl and ketamine.
Discussion
Currently, there many patients with G-6PD deficiency disorder in some regions of Turkey and world-wide G-6PD deficiency is frequently seen in African, Mediterranean, Middle Eastern and Far Eastern nations and their lineages with a frequency ranging from 5%–40% [Citation11,Citation15].
Pamaquine used in malaria therapy resulted in some severe side-effects in some patients [Citation16] i.e. dark colored urine, jaundice and anemia. Later, G-6PD deficiency was found in these individuals. Use of this and some other drugs cause hemolysis and are connected with complication. The importance of G-6PD in metabolism has been well known for many years. GSH is used as an antioxidant defense mechanism and its production requires NADPH to be synthesized in the pentose phosphate metabolic pathway in which G-6PD and 6PGD participate [Citation17]. For this reason, G-6PD and 6PGD have been considered as antioxidant enzymes [Citation18].
Inhibitory effects of many drugs on G-6PD enzyme activity in different animal species and human beings have been reported in many investigations [Citation19]. For example, it has been reported that thiamphenicol, amikacin, gentamicin, netilmicin, chloramine-T and CuSO4 inhibit rainbow trout erythrocyte G-6PD [Citation20]. Effects of many drugs such as antibiotics, analgesic and anesthetic have been investigated on human G-6PD [Citation21], sheep erythrocyte G-6PD [Citation22] and sheep liver G-6PD [Citation23]. Several study reports concerning strong inhibitory effects of antibiotic and anaesthetic drugs on erythrocytes G-6PD enzyme have been published [Citation21,Citation24]. Some anesthetics have been reported to inhibit Ca2+-ATP as and Na+/Ca2+ exchanger of the synaptosomal plasma membrane [Citation25]. The an other study, anesthetics inhibited Na+,K+-ATPase, Mg2+-ATPase and acetylcholinesterase activity from rat cerebral cortex [Citation24].
In the present study, an investigation of the effects of some selected drugs on human erythrocyte G-6PD was proposed. For this purpose, G-6PD was purified 2248-fold from human erythrocytes in 28.36 yield by ammonium sulfate precipitation and then 2′,5′-ADP Sepharose 4B affinity chromatography.
Omeprazole is a proton-pump inhibitor for acid-related diseases. Morphine is a narcotic and anesthetics and is widely has been used since the nineteenth century [Citation5,Citation9]. Ketamine is a non-barbiturate, rapid-acting disassociate anesthetic used on both animals and humans [Citation7]. Remifentanyl is a short-acting, esterase-metabolized opioid analgesic drug [Citation9]. Vankomycin is a glycopeptide antibiotic and but has some side effects such as neurotoxicity and nephrotoxicity [Citation6]. The effects on G-6PD activity of these drugs (omeprazole, morphine, remifentanyl, ketamine and vankomycin) was investigated in vitro and the kinetic constants (Ki and I50 values) reported.
Ki values were calculated from Lineweaver–Burk graphs ( and ). Ki constants for omeprazole, morphine and vancomycin were 8.22 mM, 25.93 mM, and 2.71 mM, respectively (). In addition to Ki constants, the types of inhibition of the three drugs were obtained the Lineweaver–Burk graphs, all three drugs showed non competitive inhibition. As shown and , for all these drugs the inhibitor concentrations causing 50% inhibition were determined from Activity%- [Drugs] graphs. The obtained I50 values of omeprazole, morphine, remifentanyl, ketamine and vankomycin were 3.24 mM, 43.58 mM, 97.6 mM, 64.16 mM, and 0.903 mM, respectively. As evident from I50 values, G-6PD inhibition by vankomycin is higher than that observed for the other four compounds. In previous study, it was shown that vankomycin (I50 = 8 mM) have an inhibitory effect on sheep lens G-6PD activity in vitro [Citation25]. In this study we found that human G-6PD enzyme activity was inhibited by vankomycin.
I50 values of omeprazole, morphine and vankomycin approximate the obtained Ki values as shown in . Vancomycin, omeprazole and morphine may worsen the health of patients who have a deficiency of the glucose 6-phosphate dehydrogenase enzyme. As shown in , the inhibitory effects of ketamine and remifentanyl on human glucose 6-phosphate dehydrogenase enzyme is lower than the others drugs.
Vankomycin was found to be the most potent inhibitor although all of these drugs show inhibitory effects on G-6PD enzyme activity. Owing to the wide use of these drugs in both human and animals, their effect on G-6PD activity is important. For this reason, if these drugs are given to a patient with G-6PD deficiency, their dosages should be carefully considered to prevent their side-effect on red blood cells.
References
- Kuo W, Lin J, Tang TK. Human Glucose-6-phosphate dehydrogenase (G-6PD) gene transforms nih 3t3 cells and induces tumors in nude mice. Int J Cancer 2000; 85: 857–864
- Shereve DS, Levy HR. On the molecular weight of human glucose-6 phosphate dehydrogenase. Biochem Biophy Commun 1977; 78: 1369–1375
- Lehninger AL, Nelson DL, Cox MM. Principles of Biochemistry2nd. Worth Publishers Inc., New York 1993; 436–437
- Kanji MI, Toews ML, Carpar WR. A kinetic study of glucose 6-phosphate dehydrogenase. J Biol Chem 1976; 25: 2258
- Bradley D. Taking the stress out of stomach disorder treatments. Pharm Sci Technol 1999; 2: 4–5
- Jacquin C, Nasica X, Guillard D, Aujoulat O, Stoeckel C. Intravitreal treatment of endophthalmitis. Médecine et Maladies Infectieuses 2001; 31(7)493–497
- Dalgarno PJ, Shewan D. Illicit use of ketamine in Scotland. J Psychoactive Drugs 1996; 28: 191–199
- Wenk HN, Nannenga MN, Honda CN. Effect of morphine sulphate eye drops on hyperalgesia in the rat cornea. Pain 2003; 105: 455–465
- Kayaalp SO: Rasyonal tedavi yonunden tibbi farmakoloji. Ankara, Hacettepe-Tas 2002. Yayincilik (Turkish)
- Ninfali P, Orsenigo T, Barociani L, Rapa S. Rapid purification of glucose 6-phosphate dehydrogenase from mammal's erythrocytes. Prep Biochem 1990; 20: 297–309
- Morelli A, Benatti U, Gaetani GF, De Flora A. Biochemical mechanisms of glucose-6-phosphate dehydrogenase deficiency. Proc Natl Acad Sci USA 1978; 75: 1979–1983
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–251
- Laemmli DK. Clevage of structural proteins during in assembly of the head of bacteriophage T4. Nature 1970; 227: 680–683
- Beutler E. Red cell metabolism manual of biochemical methods. Academic Press, London 1971; 68–70
- Laurence DR, Bennett PN, Brown MJ. Clinical Pharmacology8th. Churchill Livingstone, Singapore 1997
- Keha EE, Kufrevioglu, Biyokimya. Istanbul, Aktif Yayinevi 2000; 338–349, (Turkish)
- Gaetani GF, Galiano S, Canepa L, Ferraris AM, Kirkman HN. Catalase and glutathione peroxidase are equally active indetoxification of hydrogen peroxide in human erythrocytes. Blood 1989; 73: 334
- Deutsch J. Glucose-6-phosphate dehydrogenase. Bergmeyer J, Bergmeyer HU. Methods of enzymatic analysis, Verlagsgerellschaff, VCH 1983; 3: 190–196
- Ciftci M, Kufrevioglu OI, Gundogdu M, Ozmen I. Effects of some antibiotics on enzyme activity of glucose-6-phosphate dehydrogenase from human erythrocytes. Pharmacol Res 2000; 41: 109–113
- Erdogan O, Ciftci M, Ciltas A, Hisar O. Inhibition Effects of some antibiotics on the activity of glucose 6-phosphate dehydrogenase enzyme from rainbow trout (oncorhynchus mykiss walbaum, 1792) Erythrocytes. Turk J Vet Anim Sci 2004; 28: 675–681
- Altikat S, Ciftci M, Büyükokuroglu ME. In vitro effects of some anesthetic drugs on enzymatic activity of human red blood cell glucose 6-phosphate dehydrogenase. Polish J Pharmacol 2002; 54: 67–71
- Beydemir S, Ciftci M, Kufrevioglu OI. Purification and characterization of glucose 6- phosphate dehydrogenase from sheep erythrocytes, and inhibitory effects of some antibiotics on enzyme activity. J Enz Inhib Med Chem 2002; 17(4)271–277
- Turkoglu V, Aldemir S, Ciftci M. Purification and characterization of glucose-6 phosphate dehydrogenase from sheep liver. Turk J Chem 2003; 27: 395–402
- Sidek HM, Nayquist-Battie C, Vanderkooi G. Biochim Biophys Acta 1984; 801: 26–33
- Beydemir S, Kulacoglu DN, Ciftci M, Kufrevioglu OI. The effects of some antibiotics on sheep lens glucose 6-phosphate dehydrogenase in vitro. Eur. J Ophthalmol 2003; 13(2)155–161