Abstract
Arylsulfatase B (ASB) hydrolyzes the desulfation of N-acetylgalactosamine-4-sulfate at the non-reducing terminal of glycosaminoglycans. This enzyme activity was found to be elevated in mice schistosomiasis. In the present study, the catalytic and immunological properties of purified ASB from the liver of Schistosoma-infected mouse was investigated in the presence and absence of the schistosomicidal drugs praziquantel and Commiphora extract. The in vitro effect of praziquantel was found to be inhibitory with a Ki value of 5.5 × 10− 4 M while that of commiphora extract was as an activator. Furthermore, these drugs did not have an observed effect on the immunological properties of ASB with regard to its binding to its polyclonal rabbit antibody. These results indicate that some schistosomicidal drugs may reverse the alteration of the catalytic properties of the enzyme in schistosomiasis.
Introduction
Schistosomiasis (Bilharzia) is a debilitating disease and infects millions of people in many parts of South America, Africa and Asia. It is associated with various degree of hepatic dysfunction, liver cirrhosis and spleenomegaly and severe cases of the infection can be fatal [Citation1,Citation2]. The infection may occur by one of several species: Schistosoma hematobium, Schistasoma mansoni, Schistosoma Japonicum, etc [Citation3] and may affect the liver, intestine, spleen and urinary bladder [Citation4]. The chemotherapeutic treatment of schistosomiasis occurs with praziquantel or oxamniquine [Citation5]. Praziquantel is the drug of choice because it is more effective than oxamniquine, especially for the treatment of the infection by Schistasoma mansoni [Citation6]. Alternatively, extracts from herbal products are used as effective schistosomicidal drugs [Citation7] due to drug resistance to praziquantel [Citation8]. An important herbal antischistosomal therapy is derived from myrrh from the stem of the plant Commiphora molmol [Citation5].
However, a change in various enzymes was observed during Schistasoma mansoni such as arylsulfatase B [Citation9] and many hydrolases Citation10-13. Arylsulfatases A, B and C (arylsulfo-hydrolases) are a group of hydrolytic enzymes that occur in various tissues and fluids [Citation14]. Arylsulfatase B (ASB, EC 3.1.6.9), is a lysosomal hydrolase, which desulfates the non-reducing terminal N-acetylgalactosamine-4-sulfate residues present in glycosaminoglycan [Citation14]. Since this enzyme is significantly increased in schistosomiasis [Citation10] and its properties in this disease is still obscure, the present study was undertaken to investigate the interaction of some drugs that are used for the treatment of schistosomiasis with this enzyme. The effect of drugs in vitro on the purified enzyme from the liver of the infected mouse is examined to find their influence on its catalytic and immunological properties. The binding properties between this enzyme and its specific antibody are studied in a comparative manner and both kinetic and immunological properties are investigated in the absence and presence of the drugs.
Materials and methods
Animals
White albino mice (CD1) (av. 15–20 gm) were obtained from the animal house of Theodore Bilharz Institute, Schistosome Biological Supply Program (SBSP), Cairo, Egypt, and infected with 80 cercariae per mouse. The infection period was 13 weeks. Mice were housed in wire cages in groups of 6–8 mice per cage. They were kept under conventional conditions of temperature and humidity with 12 h photoperiod. New Zealand white rabbits in which the polyclonal antiserum against ASB was raised were obtained from Alexandria farm and had a mean weight of 2.0 kg.
Chemicals and reagents
p-Nitrocatechol sulfate (PNCS), Concanvalin–A Sepharose, bovine serum albumin, peroxidase-conjugated anti-rabbit IgG, methylmannoside and Freund's adjuvant complete and incomplete were purchased from Sigma Chemical Co. (St.Louis, Mo, USA). DEAE-Celluose (DE52) was purchased from Whatman. Sephadex G-150 was purchased from ICN Biochemical Inc. (Ohio,USA). Folin-Cioaclteu, o-phenylenediamine and other chemicals were purchased from Merck. All the other reagents were of analytical grade. Praziquantel was obtained from the Alexandria Company for Pharmaceutical and Chemical Industries. Commiphora extract or Myrrh is an oleo-gum resin obtained from the stem of the plant Commiphora molmol. It is a safe, natural flavoring substance and has been approved by the U.S. Food and Drug Administration [Citation15,Citation16].
Assay of ASB
Enzyme activity was assayed by the method of Baum et al. [Citation17] using PNCS as a substrate. A mixture of 0.2 ml of 50 mM PNCS in 0.5 M acetate buffer, pH 6.0 containing 0.01 M barium acetate and 0.2 ml enzyme was incubated at 37°C for 30 min. The reaction was then stopped by adding 0.6 ml of 1 M NaOH and the absorbance was measured at 515 nm. One unit of enzyme activity refers to 1 nmol of p-nitrocatechol liberated per hour.
Purification of ASB
Mice livers were homogenized in 7 volumes of 10 mM Tris–HCl buffer, pH 7.4 containing 0.05% Triton X-100 in a Brinkmann homogenizer. After centrifugation at 6,000 rpm for 20 min, the supernatant was subjected to purification by different chromatographic techniques on DEAE-Cellulose, Concanvalin A-Sepharose and Sepdadex G-150 as described previously [Citation18].
SDS-PAGE of purified enzyme
The purified enzyme from the liver of normal mice by the above mentioned steps was subjected to SDS-polyacrylamide gel (10%, w/v) in 25 mM Tris/Glycine buffer, pH 8.3 as described previously by Laemmli [Citation19].
Effect of some schistosomicidal drugs on ASB
The initial velocity and kinetic parameters of purified ASB from the liver of Schistosoma-infected mice and control were measured in the absence and presence of different concentrations of praziquantel (4–10 mM). A similar procedure was repeated for different concentrations of Commiphora extract.
Immunization
Rabbit anti-ASB polyclonal antibody was raised in the New Zealand white rabbit by injection of the pure enzyme solution from normal mice in complete Freund's adjuvant. Two boosters of the pure enzyme in incomplete Freund's adjuvant were given in two successive weeks after 14 days of the priming of the rabbits. After one week of the last booster, blood was collected from the marginal ear vein. The presence of anti-ASB polyclonal was detected by the double diffusion technique of Ouchterlony [Citation20] and the titer of the collected sera was determined by ELISA [Citation21].
Immunoprecipatation
Seven different concentrations of antiserum were incubated with the enzyme source for 60 min at 37°C. After the incubation period, the mixture of antiserum and the enzyme were kept at 4°C for 24 h and centrifuged at 12,000 rpm for 20 min. The supernatant was drawn and the enzyme activity of ASB was then assayed.
Enzyme–linked immunosorbent assay (ELISA)
The assay was carried out in polystyrene micro titer plates as described previously [Citation21]. Briefly, wells were coated with antigen (ASB enzyme) in 100 μl of 100 mM sodium carbonate buffer, pH 9.6 and kept overnight at 4°C. The wells were then washed three times with 0.1 M sodium phosphate buffer, pH 7.2, containing 0.15 M NaCl (PBS) plus 0.05% (v/v) Tween 20 (PBST). This was followed by blocking of nonspecific site with 100 μl per well of 2% (w/v) bovine serum albumin in PBS (PBSBSA) for 60 min at room temperature. The plates were washed again with PBST and kept at − 70°C till use. The Pooled rabbit serum was diluted in the range of 1: 25 to 1: l600 with PBS containing 0.5% (w/v) of bovine serum albumin. ASB solution was added to rabbit immune serum (1: 50) to give final serum dilution of 1: 100. A 100 μl/well of the above mentioned preparation were put in micro titer plates in triplicate and incubated at room temperature for 2 h. The plates were washed three times with PBST and 100 μl of peroxidase-conjugate goat anti-rabbit IgG diluted in PBSBSA (1: 2000) were then added to each well and incubated as above for 60 min. After five washing times with PBST, 100 μl 0.002% (w/v) o- phenylenediamine in sodium citrate phosphate buffer, pH 5.0 containing 0.012% (v/v) H2O2 was added to each well. The reaction was interrupted after 30 min at room temperature by the addition of 30 μl of 2 M H2SO4. The absorbance was measured at 450 nm wavelength. The percentage of binding of different serum preparation was calculated by the equation: % of binding = (A of sample/A of positive serum) × 100, where A is the reading absorbance at 450 nm.
Effect of ligands on enzyme–antibody binding
The substrate and the drugs praziquantel and Commiphora extract were added separately as ligands to ASB and the ELISA technique was applied as described above.
Protein determination
Protein concentration was determined according to the method of Lowry et al. using bovine serum albumin as a standard [Citation22].
Results
Purification of ASB from the liver of the control and schistosoma-infected mouse
The enzyme was purified from the liver of the control and Schistosoma-infected mouse to an apparent homogeneity. The purified ASB from the liver of the normal mouse showed a homogeneity by SDS-PAGE as illustrated in . The molecular weight of the ASB band was about 60 KDa. The enzyme was purified to the extent previously described for the enzyme from the control and Schistosoma-infected mouse [Citation10]. The observed Km values of the enzyme toward PNCS are 3.50 ± 0.30 and 3.0 ± 0.40 mM in control and infected cases, respectively. The observed value of Vmax were 140 ± 10.0 and 231 ± 11.50 nmol/min/mg protein for the enzyme from control and infected mouse, respectively. Thus, the kinetic parameters of the purified enzyme from control and infected mouse showed a non-significant change in the Km value, whereas a significant elevation was noticed in the Vmax value of the enzyme from the infected mouse compared to control. This is consistent with previously reported results [Citation10].
Effect of some schistosomicidal drugs on ASB
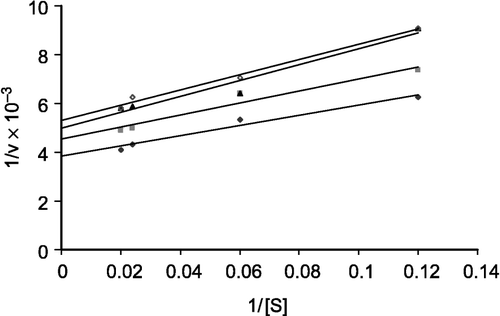
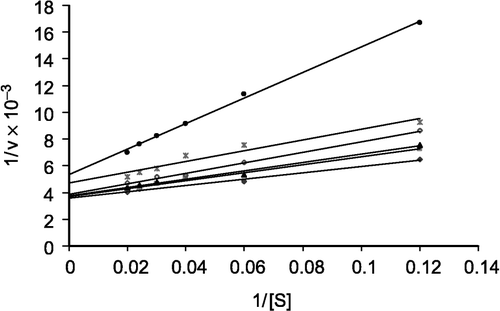
The effect of some schistosomicidal drugs on the kinetic parameters of purified ASB from the infected mouse was studied. The effect of praziquantel on ASB at different concentrations (4.0, 8.0, and 10.0 mM) is shown in by the double reciprocal plot. Praziquantel is an inhibitor of ASB from the Schistosoma-infected mouse compared to control with a Ki value, calculated from the Lineweaver-Burk plot, of 5.5 × 10− 4 M (). Commiphora extract at different concentrations (mg/ml) of the drug has an activation effect on the enzyme from the Schistosoma-infected mouse compared to the control ().
Immunological properties of ASB
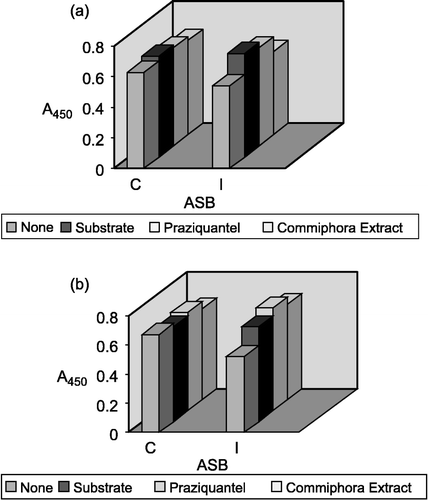
The enzyme was immunoprecipitated by different volumes of ASB antiserum, with incubation and assay at 37°C as shown in , the control and Schistosoma-infected mouse. The effect of some ligands on binding of ASB antiserum to ASB from the control and Schistosoma-infected mouse was measured by ELISA and expressed as absorbance at 450 nm for purified ASB and partially purified ASB from the control and Schistosoma-infected mouse. As shown in and , there was no observed effect of the drugs on the binding activity of both antigen-antibody and enzyme-substrate binding.
Table I. Immunoinhibition of ASB by ASB-antiserum. The hepatic enzyme from the normal and infected mouse was immunoprecipitated by different volumes of ASB antiserum, incubated and assayed at 37°C.
Figure 4 (a) Effect of some ligands on binding of ASB antiserum to the purified ASB from control mice. Binding was measured by ELISA and expressed as Absorbance at 450 nm. Data are average of six measurements. C: ASB antiserum for ASB from control mouse; I: ASB antiserum for ASB from infected mouse. (b) Effect of some ligands on binding of ASB antiserum to the purified ASB from Schistosoma-infected mice. Binding was measured by ELISA and expressed as Absorbance at 450 nm. Data are average of six measurements. C: ASB antiserum for ASB from control mouse; I: ASB antiserum for ASB from infected mouse.
Discussion
In the present study, schistosomiasis is taken as an important disease to investigate its effect on ASB. This enzyme has a highly significant increase in its activity [Citation10,Citation23], which was clearly increased with the progression of the Schistosoma-infection [Citation10]. ASB was purified from crude lysosomal extract to homogeneity. Purification of significant amounts of ASB is necessary for anti-rabbit serum preparation. It was found that praziquantel had an inhibitory effect on the enzyme, but Commiphora extract had an activating effect. A Lineweaver-Burk plot of 1/v against 1/[S] for the inhibitory effect of praziquantel suggested a mixed-type inhibition which suggests that the main bulk of the praziquantel molecule has no structural similarity with the corresponding substrate of the enzyme. The observed Ki value [Citation24] for ASB inhibition by praziquantel was 5.5 × 10− 4 M, indicating a moderate inhibitory effect.
The effect of praziquantel on some lysosomal enzymes in schistosomiasis has been reported [Citation25]. The effect of Schistosoma mansoni infection and the administration of schistosomicidal drugs on the activities of beta-glucuronidase, acid ribonuclease and alpha-naphthyl acetate esterase may be considered as carcinogenicity indices. Moreover, the increase in enzymatic activities in infected animals has been attributed to deranged metabolic function as a result of the injury to liver cells [Citation25].
The effect of ASB-antiserum on the enzyme showed no difference in the activity of the enzyme from the control or infected mouse. This may suggest that the binding of antiserum with ASB occurs at a binding site other than the substrate binding site. Furthermore, in investigating the antiserum raised from the control and infected mouse, it was observed that the effect of substrate on the binding activity of the antigen and its antibody has no detectable effect. Thus, it could be suggested that substrate does not interfere with antigenic determinant of the antibody. There is no clear difference in the antigenisity between ASB from the control mouse and that from the infected one. In the cross reaction test between control and infection cases, there was no cross reaction between them (data not shown) due to common antigenic determinants.
However, antigen binding by antibodies resembles substrate binding by enzymes in several ways. Both involve multiple, weak non-covalent associations, including ionic bonds, hydrogen bonds, Van der Waals' bonds and hydrophobic interactions, which combine to give strong binding [Citation26]. The antigen-antibody reaction had no response to the effect of the PNCS substrate. Both kinetic and immunochemical studies may clarify the mechanism of action of the enzyme and its interaction with either its substrate or its specific antibody [Citation27].
Some biochemical changes including alteration of enzymes have been reported in the host in schistosomiasis. Infection with Schistosoma mansoni induced a decrease in antioxidant enzymes and a drastic reduction of liver glutathione as a result of inhibition of glutathione reductase [Citation28]. Furthermore, the oxidative processes which occurs upon contact with Schistosoma mansoni eggs trapped in the liver seem to go uncontrolled, since the enzymatic activities involved decreased drastically [Citation28].
Beta-glucuronidase was found to be raised in infective hepatitis and toxic hepatitis in patients infected with Schistosoma mansoni [Citation29]. Also, the activity of some hydrolytic enzymes in tissues homogenate such as 5-nucleotidase, alkaline phosphatase, glucose-6-phosphatase and ribonuclease is increased by the effect of the intermediate host in schistosomiasis [Citation12]. Cyclooxygenase is over-expressed in schistosoma-associated bladder cancer [Citation30]. Moreover, the infection with Schistosoma mansoni produced a significant increase in blood pyruvate and hepatic glucose-6-phosphate dehydrogenase activity [Citation31].
In conclusion, the change in the level of ASB and its catalytic properties represents a basis to approach the biochemical changes in schistosomiasis. The effect of some schistosomicidal drugs on hepatic ASB from the Schistosoma-infected mouse indicates that the change of the enzyme is not only a parameter in Schistosoma-infection but also in the treatment by drugs.
Acknowledgements
The authors are grateful to Prof. Dr. Ezzat Mohamed Hassan, Professor of Immunology, Medical Research Institute, Alexandria University for his kind help and sincere advice.
References
- Flores-Villanueva PO, Zheng XX, Strom TB, Stadecker MJ. J Immunol 1996; 156: 3315–3320
- Dalton JP, Lewis SA, Aronstein WS, Strand M. Exp Parasitol 1987; 63: 215–226
- Larotski LS, Davis A. Bull Wld Hlth Org 1981; 59: 115–135
- Mellin TN, Buch RD, Wang CC. J Med Hyg 1993; 32: 83–93
- Sheir Z, Nasr AA, Massoud A, Salama O, Badra GA, EL-Shennawy H, Hassan N, Hammad SM. Am J Trop Med Hyg 2001; 65: 700–704
- Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennet JL. Am J Trop Med Hyg 1996; 55: 214–218
- Stelma FF, Talla I, Sow S, Kong A, Niang M, Polman K, Deelder AM, Gryseels B. Am J Trop Med Hyg 1995; 53: 167–170
- Ndamba I, Nyazyma N, Makaza N, Anderson L, Kaondera K. Ethanopharmacol 1994; 42: 125–132
- Higuchi M, Ito Y, Fukuyama K, Epstein WL. Exp Mol Pathol 1984; 40: 70–78
- Balbaa M, El-Kersh M, Mansour H, Yacout G, Ismail M, Malky A, Bassiouny K, Abdel-Monem N, Kandeel K. J Biochem Mol Biol 2004; 37: 223–228
- Balbaa M, Abbas SE, El-Deen SN, Hassab A, Awad OM. Intern J Environ Studies 2003; 60: 563–573
- Nabih I, el-Ansary A. Cell Mol Biol 1991; 37: 309–314
- Cesari IM, Ballen DE, Perrone T, Oriol O, Hoebeke J, Bout D. J Parasitol 2000; 86: 1137–1140
- Rockfor K, Gary M, John E. Arch Biochem Biophys 1976; 177: 525–538
- Hall BL, Oser BL. Food Technol 1965; 19: 151–197
- Ford RA, Api AM, Letizia CS. Food Chem Toxicol 1992; 30: 91s–92s
- Baum H, Dodgson K, Spencer B. Clin Chim Acta 1959; 4: 53–455
- Gasa S, Balbaa M, Nakamura M, Yonemori H, Makita A. J Biol Chem 1987; 262: 1230–1238
- Laemmli UK. Nature 1970; 227: 680–685, London, UK
- Ouchterlony O. Acta Physiol Microbiol Scand 1949; 20: 507–515
- Gomes M, Lima W, Pesqiero J. J Immunoassay 1994; 15: 157–170
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. J Biol Chem 1951; 193: 265–275
- Khafagy E, Razilky H, Galal A. Egypt J Bilh 1979; 3: 213–219
- Segel IH. Biochemical calculations2nd ed. John Wiley & Sons, New York 1976; 208–323
- El-Sharkawy A, El-Toukhy M, Abdel-Rahman S, El-Kholy Z, Farag H, El-Zoghby S, Gaber N. J Trop Med Hyg 1993; 96: 28–34
- Hood L, Weissman I, Woo W, Wilson H. Immunology2nd ed. 1984; 16–46
- Balbaa M, Noor El-Deen S, Khalifa M, Hassan E, Kandeel K. J Med Res Inst 1999; 20: 1–8
- Gharib B, Abdallahi M, Dessein H, De Reggi M. J Hepatol 1999; 30: 594–602
- Saad A, Ragab M, El-Kholy Z. Bull High Inst Pub Health 1984; 4: 93–98
- El-Sheikh S, Madaan S, Alhasso A, Stamp G, Abel P, Lalani E. BJU Int 2001; 88: 921–927
- Shaheen A, Ebeid F, Fahim A. Pharmacol Res 1989; 21: 263–270