Abstract
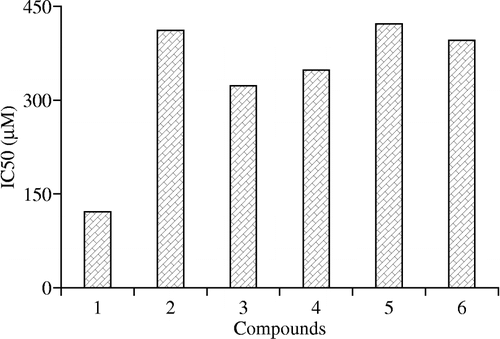
Some 2-aminothiophene analogs 1–6 were synthesized and characterized. Among the tested compounds, compound 1 (IC50 121.47 μM) exhibited highest while the compound 5 showed least anti-inflammatory potential (IC50 422 μM).
Introduction
Inflammation occurs as a defensive response, which induces physiological adaptations to limit tissue damage and remove the pathogenic infections. Such mechanisms involve a complex series of events including venules and capillaries with increased vascular permeability and exudation of fluids including plasma proteins and leukocyte migration into the inflammatory area followed by the loss of tissue function.
Diseases caused by inflammation are an important factor of morbidity and mortality in humans. In certain conditions, there is the appearance of lack of resolution of the acute signs of inflammation and the chronic state develops, that may lead to loss of life of the individual. Inflammatory disorders include rheumatoid arthritis, osteoarthritis, inflammatory bowl diseases, retinitis, multiple sclerosis, psoriasis and atherosclerosis.
Other characteristics, such as erythema and fever can also be observed during inflammatory events. The latter feature occurs when activated macrophages release cytokine (IL-1, TNF-α) which leads to vessel dilation resulting from smooth muscle relaxation followed by an increase in local blood flow (hypothermia) [Citation1].
Acute inflammation is relatively ephemeral (days) and characterized by neutrophils as the primary cellular infiltrate. In contrast, chronic inflammation can continue up to months or longer, and is characterized by infiltration of macrophages and lymphocytes. Chronic inflammation may lead to immune granuloma formation and more serious immunological consequences such as autoimmune disease, i.e. immune response to the body's own constituents with chronic, debilitating and sometimes life threatening tissue and organ injuries.
As a part of our drug discovery programme we screened a focused library of compounds and found 2-aminothiophene and its analogs to have potent anti-inflammatory activity.
Thiophene and its analogs represent an important class of compounds with diverse biological activities. Its derivatives provide intermediates for many products in the fine chemical industries such as pharmaceutics, agrochemicals and dyestuffs.
Substituted thiophenes are also present in natural products. Thienopyrimidinone derivatives have potential radio-protective character [Citation2], analgesic [Citation3], hypocholesterolemic, antitussive [Citation4], and CNS depressant [Citation5], anticonvulsants [Citation6], anti-inflammatory [Citation7], bactericidal [Citation8], fungicidal [Citation9] activities and antifertility [Citation10], sedative effects [Citation11,Citation12], antimalarial Citation13-15 and post-coital antifertility activities in rats [Citation10]. Compounds containing fused pyrimidine rings have attracted great attention in recent years. Many potent drugs in the field of cancer and virus research have been developed Citation16-20. Herein we report 2-aminothiophene and its analogs as a new class of anti-inflammatory compounds.
Methods
Chemistry
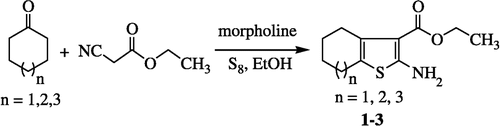
2-Amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid ethyl ester (1)
A mixture of ethyl cyanoacetate (3.1 mL, 29.0 mmol), cyclohexanone (3 ml, 29.0 mmol), sulphur (0.93 g, 29 mmol), morpholine (2.52 mL, 29 mmol) and ethanol (26 mL) was stirred at room temperature. During this exothermic reaction, the reaction mixture was stirred on an ice bath. After 1 h of continuous stirring the reaction was completed and the reaction mixture was evaporated and extracted with ether. The ether extract was dried over anhydrous Na2SO4, evaporated to some extent and kept in the refrigerator to crystallize [Citation21].
Yield = 3.53 g (54%); m.p. = 107.8°C; Rf = 0.5 (9:1, Hexane/EtOAc); UV (methanol) λmax (logε) 209 (IR (KBr): 3408, 2918, 1732, 1608, 1474, 1355, 1145 cm− 1; 1H-NMR (500 MHz, CDCl3): δ 5.91 (br. s, 2H, NH2), 4.24 (q, 2H, OCH2), 2.70–2.67 (m, 2H, H-2), 2.49–2.46 (m, 2H, H-7), 1.77–1.70 (m, 4H, H-5 and H-6), 1.31 (t, 3H, CH3); EI MS: m/z (rel. abund. %) 225 (M+, 67), 179 (100), 151 (61), 123 (30), 91 (41), 65 (40), 51 (22); Anal. Calcd. for C11H15NO2S: C, 58.64; H,6.71, N, 6.22. Found: 58.69; H, 6.66; N, 6.22%.
2-Amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene- 3-carboxylic acid ethyl ester (2)
Yield = 2.24 g (55%); m.p. = 79°C; Rf = 0.5 (9:1, Hexane/EtOAc); UV (methanol) λmax (logε) 211 (I.R. (KBr): 3429, 2973, 1726, 1601, 1470, 1365, 1160 cm− 1; H1-NMR: (400 MHz, CDCl3): δ 5.76 (br. s, 1H, NH2), 4.27 (q, 2H, OCH2), 2.97–2.94 (m, 2H, H-4), 2.57–2.54 (m, 2H, H-8), 1.81–1.76 (m, 2H, H-5), 1.65–1.56 (m, 4H, H-6 and H-7), 1.33 (t, 3H, J = 7.12, CH3); EI MS: m/z (rel. abund. %) 239 (M+, 77), 193 (100), 165 (38), 150 (21), 125 (8), 91 (10); Anal. Calcd for C12H17NO2S: C, 60.22; H, 6.16; N, 5.85;. Found: C, 60.27; H, 6.11; N, 5.87%.
2-Amino-4,5,6,1,8-hexahydrocycloocta[b]thiophene-3-carboxylic acid ethyl ester (3)
Yield = 3.37 g (84%); Rf = 0.6 (9:1, Hexane/EtOAc); UV (methanol) λmax (logε) 207 (IR (KBr): 3421, 2930, 1700, 1601, 1452, 1360, 1109 cm− 1; H1-NMR (400 MHz, CDCl3): δ 5.09 (br. s, 1H, NH), 4.23 (q, J = 7.12, 2H, OCH2), 2.95–2.90 (m, 2H, H-4), 2.73–2.69 (m, 2H, H-9), 2.40–2.35 (m, 2H, H-8), 1.87–1.80 (m, 2H, H-7), 1.33 (t, J = 6.7, 2H, CH3); EI MS: m/z (rel. abund. %) 253 (M+, 67), 208 (13), 179 (29), 139 (28), 107 (11), 83 (100), 55 (87); Anal. Calcd for C13H19NO2S: C, 61.63; H, 7.56; N, 5.53; Found: C, 61.69; H, 6.05; N, 5.94%.
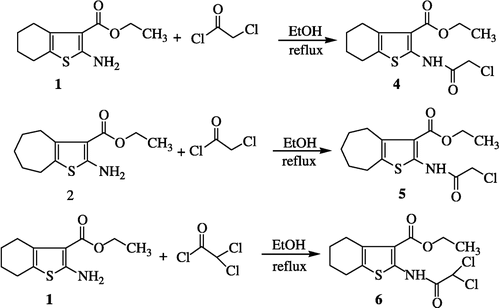
2-[(2-Chloroacetyl)amino]-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylic acid ethyl ester (4)
2-Amino-5,6,7-tetrahydro-1-benzothiophene-3-carboxylic acid ester (0.50 g, 2.2 mmol) (1) was treated with chloroacetylchloride (0.42 mL, 4.4 mmol) in dry chloroform (25 mL). The reaction was stirred at room temperature and was then refluxed for 3 h. When reaction was completed (TLC analysis), it was then fractionally distilled under reduced pressure to afford 4 as an oily product.
Yield = 84%; UV (methanol) λmax (logε) 226 (IR (KBr): 3454 (–NH), 2931 (–CH2–), 1729 (–COO–), 1663 (amide), 1603 and 1472 (C = C aromatic), 764 (C–Cl), 712, 678 cm− 1; 1H-NMR (400 MHz, CDCl3): δ 10.91 (br. s, 1H, NH), 4.28 (q, 2H, OCH2), 4.23 (s, 2H, –CH2Cl), 2.75–2.67 (m, 2H, H-2), 2.47–2.43 (m, 2H, H-7), 1.79–1.73 (m, 4H, H-5 and H-6), 1.31 (t, 3H. CH3); EI MS: m/z (rel. abund. %), 303 (M+2), 301 (M+, 10), 266 (29), 252 (34), 224 (61), 179 (100), 151 (64), 123 (15), 91 (100), 65 (41), 51 (23); Anal. Calcd for C13H16NO3SCl: C, 51.74; H, 5.34; N, 4.64. Found: C, 51.81; H, 5.27; N, 4.68%.
2-[(2-Chloroacetyl)amino]-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylic acid ethyl ester (5)
Yield = 55%; UV (methanol) λmax (logε) 224 (IR (KBr): 3449 (NH), 2927 (–CH2), 1738 (–COO–), 1666 (amide), 1604 and 1471 (C = C, arom.), 771 (C–Cl), 718, 667; 1H-NMR (400 MHz, CDCl3): δ 6.23 (br. s, 1H, NH), 4.49–4.39 (m, 2H, –CH2), 4.38 (s, 2H, –CH2Cl), 4.24 (q, 2H, OCH2), 3.21–3.13 (m, 2H, –CH2), 1.93 − 1.79 (m, 4H, − 2CH2), 1.51 (t, 3H, CH3), 1.32–1.30 (m, 2H, –CH2); EI MS m/z (rel. abund. %): 317 (M+2, 4), 315 (13), 279 (21), 265 (48), 237 (24), 192 (69), 164 (100), 150 (61), 118 (18), 93 (31), 69 (23); Anal. Calcd for C14H18ClNO3S: C, 53.24; H, 5.74; N, 4.44. Found: C, 53.29; H, 5.69; N, 4.38%.
2-[(2,2-Dichloroacetyl)amino]-4,5,6,7-tetrahydro- 1-benzothiophene-3-carboxylic acid ethyl ester (6)
2-Amino-5,6,7-tetrahydro-1-benzothiophene-3-carboxylic acid ester (0.50 g, 2.2 mmol) (1) was treated with dichloroacetylchloride (0.41 g, 4.4 mmol) in dry chloroform (25 mL). The reaction was stirred at room temperature initially and then refluxed for 2 h. When reaction was completed (TLC analysis), it was then fractionally distilled under reduced pressure to afford 6 as an oily product.
Yield = 71%; UV (methanol) λmax (logε) 224 (IR (KBr): 3463 (–NH), 2926 (–CH2), 1736 (–COO–), 1667 (amide), 1605 and 1476 (C = C arom.), 773 (CCl), 719, 663; 1H-NMR (400 MHz, CDCl3): δ 11.01 (br. s, 1H, NH), 4.27 (q, 2H, OCH2), 2.76–2.71 (m, 2H, H-2), 2.48–2.44 (m, 2H, H-7), 1.81–1.75 (m, 4H, H-5/H-6), 1.32 (t, 3H, CH3), 5.49 (s, 2H, –CHCl2); EI MS: m/z (rel. abund. %) 340 (M+4, 4), 338 (M+2, 13), 336 (M+, 21), 252 (37), 224 (53), 179 (69), 151 (38), 136 (21), 91 (100), 65 (43), 51 (28); Anal. Calcd for C13H15NO3SCl2: C, 46.44; H, 4.50; N, 4.17. Found: C, 46.49; H, 4.45; N, 4.11%.
Anti-inflammatory assay protocol
Isolation of human neutrophils
Heparinized fresh venous blood was drawn from healthy volunteers and neutrophils were isolated by the method of Siddiqui et al. [Citation22,Citation23]. Briefly, whole blood was mixed with Ficoll or Dextran 6% in a ratio of 1:3 and allowed to settle down. Buffy coat was collected, layered on the bed of Ficoll (3 mL) and centrifuged at 1500 rpm for 30 min. The pallets were collected and washed with phosphate buffered saline (PBS) buffer (pH 7.4). The RBCs were lysed with ammonium chloride solution and then centrifuged. The pallets were washed with PBS and the cells were resuspended with the same buffer at a concentration of 1 × 106 Cell/mL.
Anti-inflammatory assay
To determine the anti-inflammatory activity of the compounds the modified assay of Berridge et al. was used Citation17-20. This in vitro assay is based on the reduction of highly water-soluble tetrazolium salt (WST-1) in the presence of activated neutrophils. Anti-inflammatory activity was determined in a total volume of 200 μL PBS (pH 7.4) containing 0.5–104 neutrophils/mL, 750 μL WST-1 in various concentrations of test compounds. Control only contains buffer, neutrophils and WST-1. All compounds were equilibrated at 37°C for 10 min and reaction was initiated by adding Zymosan activated serum (ZAS).
Indomethacin was used as a positive control and the absorbance was measured at 450 nm using a spectra MAX 340 microplate reader kinetically for 30 min. Each value was the mean of reactions in six wells for a single compound in a 96-well plate. The IC50 was calculated by comparing with DMSO as blank and expressed as % inhibition of superoxide produced.
Results and discussion
Chemistry
A number of 2-aminothiophenes were synthesised by treating different ketones with ethyl cyanoacetate and sulphur in the presence of morpholine (Scheme ) and some of their derivatives were prepared (Scheme ) [Citation21].
Biological evaluation
Anti-inflammatory activity
Anti-inflammatory activity was done according to the literature protocol of Siddiqui et al. [Citation22,Citation23]. Compound 1 exhibited 61% anti-inflammatory activity with IC50 121 μM, compound 2 showed 94% activity with an IC50 value of 412 μM and compound 3 indicated 30% activity with an IC50 value of 323 μM. Among other thiophene analogs 4, 5, and 6 exhibited 75, 71 and 81% anti-inflammatory activity with IC50 values of 348, 422 and 396 μM, respectively ( and ).
Table I. IC50 μM values of compounds related to 2-aminothiophene.
Acknowledgements
We gratefully acknowledge the Higher Education Commission (HEC) Pakistan for financial assistance under National Research Program for Universities.
References
- De Almeida EC, Menezes H. Anti-inflammatory activity of propolis extracts: A review. J Venom Anim Toxins 2002; 8: 191–212
- Heiba HI, Ghorab MM, El-Gawish MA. Biological and biochemical screening of some new amino acid thienopyrimidinone derivatives for potential radioprotective character. Phosphorus, Sulphur Silicon Relat Elem 1997; 131: 197–205
- Pathak US, Gandhi NV, Singh S, Warde RP, Jain KS. Synthesis of some [1,2,4]triazolo[4,3-a]thieno[3,2-e]pyrimidin-5(4H)-ones. Indian J Chem Sec B 1992; 31: 223–229
- Sauter F. Ger. Offen, 2,210,503. 2-Mercapto-3,4-dihydrothieno[2,3-d]pyrimidin-4-ones Chem Abstr. 1972;77:164752n.
- Kinnard WJ, Carr CJ. A preliminary procedure for evaluation of central-nervous-system depressants. J Pharmacol Exptl Therap 1957; 121: 354–361
- Tripathi S, Ahmad S, Barthwal JP, Tangri KK, Kishore K, Bhargava KP. Anticonvulsant and MAO inhibitory activity of substituted quinozolines. Indian J Exp Biol 1981; 19: 30–33
- Doria G, Passarotti C, Arcari G, Buttinoni A. Belg. BE 893,835, Substituted thiazolo[3,2-a]pyrimidines Chem Abstr, 1983;99:70749u.
- Zhikhareva GP, Mastafanova LI, Evstratova MI, Polukhina LM, Pushkina TV, Pershin GN, Yakhontov LN. Synthesis and chemotherapeutic study of 2-styryl-4-(δ-diethylamino-α-methylbutylamino)quinazolines. Khim-Farm Zh 1982; 16: 199625t, 1982;16:183–188; Chem Abstr
- Zhikhareva GP, Pronina EV, Golovanova EA, Pershin GN, Novitskaya NA, Zykova TN, Guśkova TA, Yakhontov LN. Synthesis and chemotherapeutic study of substituted 2-styryl-4-amino-6-methoxyquinazolines. Khim-Farm Zh 1976; 85: 123848v, 1976;10:62; Chem Abstr
- Manhas MS, Amin SG, Sharma SD, Dayal B, Bose KA. Heterocyclic compounds. XI Potential Post-Coital Antifertility Agent (I). J Heterocyclic Chem 1979; 16: 371–376
- Bosin TR, Campaigne EE. Biologically active benzo[b]thiophene derivatives. Adv Drug Res 1977; 11: 191–232
- Campaigne E, Homfeld E. Benzo[b]thiophene derivatives. XXV. Condensation and reductive Alkylation of 3-aminoalkylbenzo[b]thiophenes with formaldehyde (1). J Heterocyclic Chem 1979; 16: 1321–1324
- Schmidt P, Eichenberger K, Schweizer E, Ger Offen 1969;497:1,908, Pyrimidines. Chem Abstr. 1970;72;31837u
- Eichenberger K, Schweizer E, Schmidt P, U. S. Patent. 2,627,766, December 14, 1971
- Medicinal Chemistry. Medicinal Chemistry3rd ed., A Burgered. Willey-Inter sciences, New York 1970; 72–544 and 719
- Brown DJ. Katritzky and Rees Comprehensive Heterocyclic Chemistry, AJ Boulton, A McKillop. Pergamon Press, Oxford 1983; 3: 57
- Charles H, Fred JA. Experimental and clinical use of fluorinated pyrimidines in cancer chemotherapy. Cancer Res 1963; 23: 1226–1243
- De Clercq E. Chemotherapeutic approaches to the treatment of the acquired immune deficiency syndrome (AIDS). J Med Chem 1986; 29: 1561–1569
- Baba M, Pauwels R, Herdewijn P, De Clercq E, Desmyter J, Vandeputte M. Both 2′,3′-dideoxythymidine and its 2′,3′-unsaturated derivatives (2′,3′-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem Biophys Res Commun 1987; 142: 128–134
- Kelley JL, Linn JA, Selway JWT. Synthesis and structure-activity relationship of 2-substituted-6-(dimethylamino)-9-(4-methybenzyl)-(4H)-purines with antirhinovirus activity. J Med Chem 1989; 32: 218–224
- Peet NP, Sunder S, Barbuch RJ. Mechanistic observations in Gewald syntheses of 2-aminothiophenes. J Heterocylic Chem 1986; 23: 129–134
- Siddiqui RA, English D, Harvey K, Cui Y, Martin MI, Wentland J, Akard L, Jansen J, Thompson J, Garcia JGN. Phorbol ester-induced priming of superoxide generation by phosphatidic acid-stimulated neutrophils and granule-free neutrophil cytoplasts. J Leukocytes Biol 1995; 58: 189–195
- Bagchi D, Bagchi M, Stohs SJ. In vitro effects of a smokeless tobacco extract in the production of reactive oxygen species by human oral epidermal cells and rat hepatic mitochondria and microsomes and peritoneal macrophages. Biosci Biotech Biochem 1995; 59: 822