Abstract
Glucose-6-phosphate dehydrogenase (G-6-PD) is the first enzyme in the pentose phosphate pathway. Cadmium is a toxic heavy metal that inhibits several enzymes. Zinc is an essential metal but overdoses of zinc have toxic effects on enzyme activities. In this study G-6-PD from lamb kidney cortex was competitively inhibited by zinc both with respect to glucose-6-phosphate (G-6-P) and NADP+ with Ki values of 1.066 ± 0.106 and 0.111 ± 0.007 mM respectively whereas cadmium was a non-competitive inhibitor with respect to both G-6-P and NADP+ Ki values of 2.028 ± 0.175 and 2.044 ± 0.289 mM respectively.
Introduction
Glucose-6-phosphate dehydrogenase (G-6-PD, D-glucose-6-phosphate: NADP+1-oxidoreductase EC 1.1.1.49) plays a key role in the generation of NADPH, which is essential for maintaining glutathione in the reduced state, and in the production of ribose 5-phosphate for the synthesis of nucleotides. G-6-PD in its active form is either a dimer or tetramer consisting of identical subunits and its gene is located on the X chromosome. Deficiency of G-6-PD is a most common metabolic disorder that affects an estimated 400 million people worldwide, and is associated with chronic and drug-or infection-induced hemolytic anemia [Citation1]. The roles of particular amino acids in substrate and coenzyme binding and catalysis of G-6-PD have been investigated; cysteine residues are responsible for activation of plastidic G-6-PD [Citation2] and are involved in binding NADP+ to bovine adrenal G-6-PD [Citation3]. In Leuconostoc mesenteroides G-6-PD, histidine was identified as the catalytic base and arginine was identified as important for NADP+ binding [Citation4]. The epsilon-amino group of lysine can favour a hydrogen bond within the active pocket essential for catalysis of human G-6-PD [Citation5].
Cadmium (Cd+2) is a toxic, mutagenic and carcinogenic metal (IARC Class 1 human carcinogen). It causes damage to eukaryotic cells both in acute and chronic modes of exposure via multiple biochemical mechanisms [Citation6]. After uptake to the organism via the digestive and respiratory pathways [Citation7], Cd2+ ions accumulated intracellularly due to its binding to cytoplasmic and nuclear proteins. It is taken up through calcium channels of the plasma membrane and then the Cd2+ binds to thiol groups in the cell [Citation8,Citation9]. Cd 2+ may induce oxidative damage in different tissues by enhancing peroxidation of membrane lipids and altering the antioxidant system of the cells [Citation10]. Cytotoxic level of Cd 2+ inhibits DNA, RNA and protein synthesis which cause apoptosis in the cells [Citation11,Citation12]. Cadmium is an essential material used in the battery, metal-coating, and alloy industries. In addition to these industrial uses, it is also a component of cigarette smoke. Therefore, exposure to cadmium is widespread and presents a considerable health concern [Citation13]. Environmental exposure to cadmium may cause kidney damage, tubular proteinuria [Citation14] and human lung cancer [Citation15].
For a variety of organisms, trace metals and their homeostatic control are essential for metabolism. Zinc is a divalent cation exhibiting an important role in health and disease as evidenced its functional capacity in more than 200 metallic enzymes including carbonic anhydrase, carboxypeptidases [Citation16], alcohol dehydrogenases [Citation17], RNA polymerases [Citation18] and matrix metalloproteinases [Citation19]. It is also exerts a vital role in various physiological functions, cell proliferation, apoptosis [Citation20], in mitotic cell division, DNA and RNA repair [Citation21]. Toxic doses of zinc inhibit intestinal alkaline phosphatase [Citation22], mitochondrial cytochrome c oxidase [Citation23], glyceraldehyde-3-phosphate dehydrogenase [Citation24], beta amylase [Citation25]. Furthermore Zn2+ decrease steady-state GTPase activity by inhibiting the binding of GTP to Galpha(s) and also, toxic doses modify excretion of nitrogen, phosphorus, and sulfur [Citation26]. Zinc ion has been found to be a powerful competitive inhibitor of G-6-PD from Aspergillus parasiticus. with Ki value about 2.5 mM with respect to G-6-P [Citation27].
Minerals are important for normal hematopoiesis and may play a role in acute hemolytic anemia induced by G-6-PD deficiency [Citation28]. G-6-PD deficiency are all hereditary disorders with higher potential for oxidative damage due to chronic redox imbalance in red cells which often results in clinical manifestation of mild to serve hemolysis in patients with these disorders [Citation29]. The consequences of cellular zinc overload may include increased cellular reactive oxygen species (ROS) production, loss of mitochondrial membrane potential and reduced cellular ATP levels. High intracellular free zinc promotes neuronal death by inhibiting cellular energy production [Citation30]. Zinc combines with other elements to form zinc compounds and these compounds are widely used in industry to make paint, rubber, dyes, wood preservatives, and ointments. Lipophilic zinc complexes easily penetrate the cell plasma membrane and have been found to be cytotoxic in direct relationship to their lipophilicity [Citation31].
G-6-PD activity, which regulates various physiological factors, is important in many cellular processes so that, investigation of inhibitors and activators of G-6-PD is important for explaining metabolic changes in the cell. The aim of the present study was to investigate the effects of zinc and cadmium on lamb kidney cortex G-6-PD.
Materials and methods
Materials
Cadmium chloride was obtained from BDH, England. Tris [Tris (hydroxymethyl) aminomethane], G-6-P, NADP+, and all other chemicals were analytical grade and obtained from Sigma, USA.
Measurement of G-6-PD activity
Enzyme activities were determined spectrophotometrically using a LKB Ultraspec Plus (4054 UV/visible) spectrophotometer, by monitoring NADPH production at 340 nm and at 37°C (ϵ340 = 6.22 mM− 1cm− 1) [Citation32]. The assay mixture contained 10 mM MgCl2, 0.2 mM NADP+ and 0.6 mM G-6-P in 100 mM Tris/HCl buffer, pH 8.0. Assays were carried out in duplicate and the activities were followed for 60 s. The reaction was linear during this time period.
One unit (U) of activity is the amount of enzyme required to reduce one μmol of NADP+ per min under the assay conditions. Specific activity is defined as units per mg of protein.
Enzyme preparations
In this study we used lamb kidney cortex enzyme, which had been purified about 4202 fold [Citation33]. The purification procedure consisted of two steps after ultracentrifugation: 2′, 5′-ADP Sepharose 4B affinity and DEAE Sepharose Fast Flow anion exchange chromatography. In our prior study a detailed kinetic mechanism of the reaction catalyzed by G-6-PD from lamb kidney cortex was reported [Citation34].
Zinc inhibition studies
The natural substrates, G-6-P and NADP+, were used for inhibitory studies. Activities were measured adding different concentrations of Zn 2+ or Cd2+ to the assay mixture given above for G-6-PD measurement. Enzyme was added to the incubation mixture last.
Protein assay
Protein concentration were determined by a micro method [Citation35] using bovine serum albumin as standard.
All kinetic experiments, including protein measurements were done with a LKB UV/visible spectrophotometer.
Statistical analysis of kinetic data
The data were analyzed and the kinetic constants were calculated by means of a nonlinear curve-fitting program of the Statistica Program.
Results
Kinetics of zinc inhibition of lamb kidney cortex G-6-PD
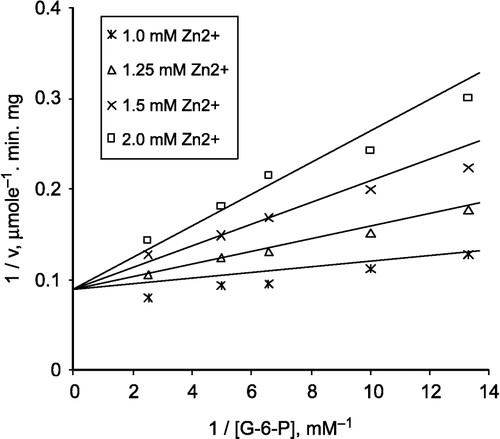
In this study, purified lamb kidney cortex G-6-PD [Citation36] was used to find out the effects of zinc on G-6-PD at different concentrations as inhibitor. G-6-PD was completely inhibited by zinc at 3.0 mM concentration ().
Figure 1 Inhibition of lamb kidney cortex glucose-6-phosphate dehydrogenase by zinc. The velocities were determined in 100 mM Tris/HCl buffer pH 8.0.
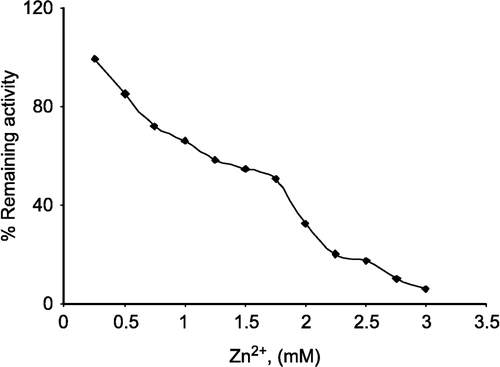
The effects of Zn2+ on G-6-PD from lamb kidney cortex were investigated by using a Lineweaver-Burk double reciprocal plot and analysed is of initial velocity data.
a) When G-6-P was varied substrate at constant and unsaturating NADP+ concentration (0.1 mM), different fixed concentrations of Zn2+(1.0–2.0mM) were added to the assay mixture. It was seen that Zn2+ acts as a competitive inhibitor with respect to G-6-P () and from the ‘Statistica Program’, Ki was calculated as 1.066 ± 0.106 mM.
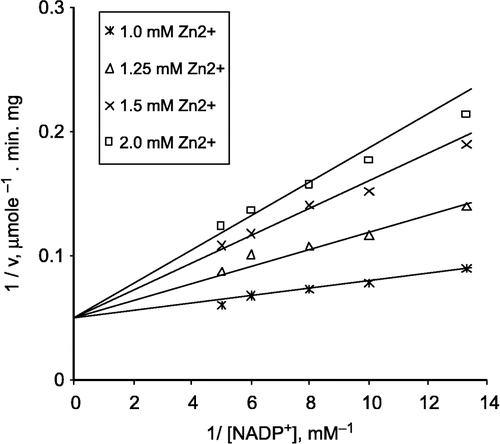
b) When NADP+ was varied substrate, at constant and unsaturating G-6-P concentration (0,4 mM), different fixed concentrations of Zn2+(1.0–2.0 mM) were added to the assay mixture. It was shown that Zn2+ acts also as a competitive inhibitor with respect to NADP+, Ki = 0.111 ± 0.007 mM ().
Kinetics of cadmium inhibition of lamb kidney cortex G-6-PD
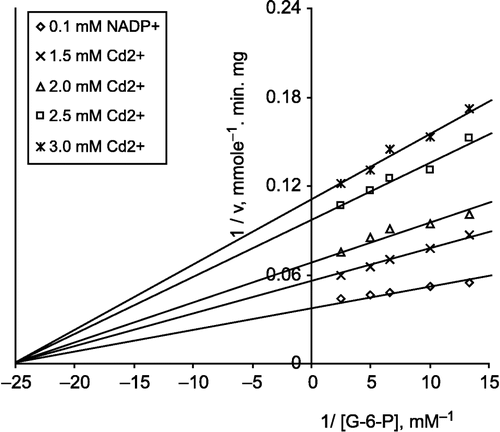
In the inhibition of lamb kidney cortex G-6-PD by cadmium, Cd 2+ was used at different concentrations as an inhibitor and it was found that G-6-PD was completely inhibited by cadmium at 3.5 mM (Figure.).
Figure 4 Inhibition of lamb kidney cortex glucose-6-phosphate dehydrogenase by cadmium. The velocities were determined in 100 mM Tris/HCl buffer pH 8.0.
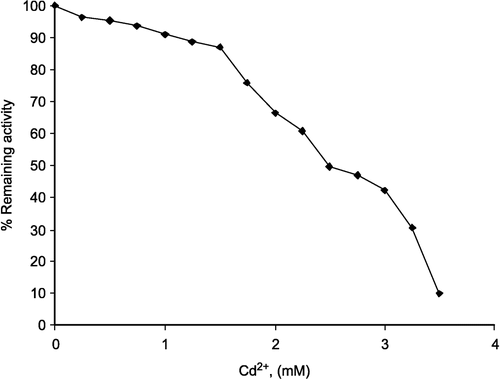
Kinetic studies on the inhibition of lamb kidney cortex G-6-PD by Cd2+ were investigated using a Linewear-Burk double reciprocal plot analysis of initial velocity data.
a) When G-6-P was varied substrate, at constant and unsaturating NADP+ concentration (0.1 mM), different fixed concentrations of Cd2+(1.5–3.0 mM) were added to the assay mixture. It was seen that () Cd2+ acts as a non-competitive inhibitor with respect to G-6-P and from the ‘Statistica Program’ Ki was calculated as 2.028 ± 0.175 mM.
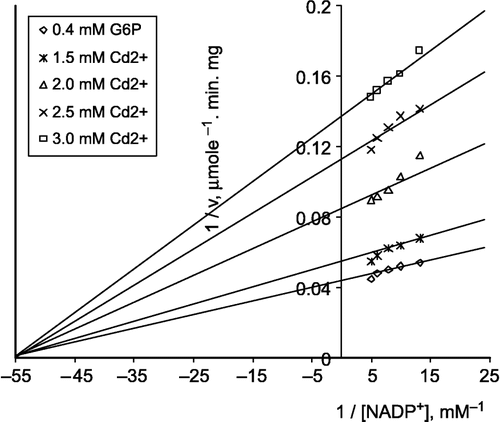
b) When NADP+ was varied substrate, at constant and unsaturating G-6-P concentration (0.4 mM), different fixed concentrations of Cd2+(1.5–3.0 mM) were added to the assay mixture. It was shown that Cd2+ acts as a non-competitive inhibitor with respect to NADP+, and Ki was calculated as 2.044 ± 0.289 mM ().
Discussion
G-6-PD is the first and rate-limiting step of the pentose phosphate metabolic pathway, which has the physiological role of providing NADPH for reductive biosynthesis and detoxifying reactions [Citation36]. In this study, we have investigated the inhibition effects of some divalent cations like zinc and cadmium on G-6-PD activity.
A number of eukaryotic, cytosolic enzymes not known to require zinc for activity are inhibited by this metal. Among these are glycolytic enzymes, a protease involved in apoptosis, and a tyrosine phosphatase. Some of these enzymes were found to be inhibited by much lower concentrations of zinc than had been reported previously [Citation37]. Excess body zinc interacts with free thiol groups on macromolecules, so blocking the active sites of enzymes, co-enzymes and membrane receptors [Citation38]. It has also been found that zinc ion was a powerful inhibitor of Aspergillus parasiticus G-6-PD, inhibition being competitive with respect to G-6-P, with Ki about 2.5 μM. Other divalent metal ions which also serve as inhibitors are nickel, cadmium, and cobalt [Citation27]. We have found that lamb kidney cortex G-6-PD was inhibited by zinc and the inhibition type was competitive with respect to both G-6-P and NADP+. This probably involves free thiol groups on the active site of the enzyme. Zinc contributes to normal immunological function and excess zinc (300 mg daily for six weeks to 11 volunteers) has been associated with impaired immune and inflammatory responses [Citation39]. In the ovary high-molecular-weight proteins, like G-6-PD, should be able to bind Zn 2+, leading to oxidative stress responsible for observed increase in endogenous glutathione content. Inhibition of the pentose phosphate pathway in the ovary by Zn 2+ can be responsible for the reproductive failure [Citation41]. Anaemia resulting from excess zinc uptake is usually due to zinc-induced copper deficiency via impaired gastrointestinal copper absorption [Citation40,Citation41].
In a various mammalian cells, cadmium is a potent inhibitor. Human placental G-6-PD, blood G-6-PD activity and cholinesterase activity was inhibited completely by Cd 2+ as CdCl2 in vitro [Citation42,Citation43]. In addition, the activities of G-6-PD were also significantly inhibited in different brain regions by intraperitoneal administration of Cd 2+[Citation44]. Cadmium had also a consistent inhibitory effect on pulmonary GSH peroxidase, and glutathione reductase Citation45-47. Cd 2+ increased lipid peroxidation and inhibited superoxide dismutase and Na+/K+ ATPase in the rat liver, intestine and kidney [Citation48]. Mammalian 8-oxoguanine-DNA glycosylase is inhibited by cadmium that implicates cadmium genotoxicity [Citation6].
Cadmium (up to 1.0 mM) showed an inhibition effect on G-6-PD, which was consistent with a mixed-type inhibition pattern. When NADP+ changes over an extensive range of concentrations, the inhibition effect shows an uncompetitive character. Furthermore, when G-6-P is the varied substrate, the character of the mixed-type inhibition is competitive [Citation49]. Because Cd 2+ ions possess a high affinity for sulfhydryl groups and thiolate anions, the cellular and molecular mechanisms involved in the handling and toxicity of Cd 2+ in target organs can be defined largely by the molecular interactions that occur between Cd 2+ ions and various sulfhydryl-containing molecules present in both the intracellular and extracellular compartments [Citation50].
In order to find out the inhibitory effects of cadmium on purified lamb kidney cortex G-6-PD, we have investigated the kinetic properties of the inhibition. It was found that cadmium acted as a non-competitive inhibitor of the enzyme. This enzyme's activity is thought to depend on the intactness of enzymic SH-groups; inhibitory effect of cadmium may be due to binding thiol groups in the enzyme. In consideration of the aminoacid residue in the substrate and NADP+ binding site or catalytic site, cysteine residues were found that are essential role for the activation of plastidic G-6-PD [Citation2,Citation3].
Cadmium is an industrial and environmental pollutant that adversely affects a number of organs in humans, and other mammals, including the kidneys, liver, lungs, pancreas, testis, and placenta. The liver and kidneys, which are the primary organs involved in the elimination of systemic Cd 2+, are especially sensitive to the toxic effects of Cd 2+[Citation50]. Zinc is an essential metal but higher doses of this metal may have a harmful effect on human health. The role of the heavy metals is known in the generation of free radical-mediated oxidative stress in normal human red cells and alteration in the polypeptide pattern of membrane proteins, and significant increases in methemoglobin. Furthermore it may damage red cells by the binding of Cd 2+ to the thiol groups of G-6-PD.
This study may be a useful model for understanding the mechanisms for oxidative damage associated with hemolytic anemias. Because of G-6-PD is an important enzyme in many reductive biosynthesis and glutathione regeneration, its inhibition by cadmium and zinc may induce oxidative damage that causes hemolytic anemia in G-6-PD deficiency. Cadmium and zinc uptake in G-6-PD deficiency may be more harmful for enzyme-deficient individuals.
References
- Fujii H. Nippon Rinsho 1995; 53: 1221–1225
- Wenderoth I, Scheibe R, Schaewen A von. J Biol Chem 1997; 272: 26985–26990
- Criss W, McKerns. Biochim Biophys Acta 1969; 184: 486–494
- Vought V, Ciccone T, Davino MH, Fairbairn L, Lin Y, Cosgrove MS, Adams MJ, Levy HR. Biochemistry 2000; 39(49)15012–15021
- Bautista JM, Mason PJ, Luzzatto L. FEBS Lett 1995; 366(1)61–64
- Zharkov DO, Rosenquist TA. DNA Repair (Amst) 2002; 1(8)661–670
- Thevenod F. Nephron Physiol 2003; 93(4)87–93
- Blazka ME, Shaikh ZA. Toxicol Appl Pharmacol 1992; 113(1)118–125
- Shaikh ZA, Blazka ME, Endo T. Environ Health Perspect 1995; 103(Suppl 1)73–75
- Sarkar S, Yadav P, Trivedi R, Bansal AK, Bhatnagar D. J Trace Elem Med Biol 1995; 9(3)144–149
- Watjen W, Beyersmann D. Biometals 2004; 17(1)65–78
- Poliandri A, Cabilla JP, Velardez MO, Bodo CC, Duvilanski BH. Toxicol Appl Pharmacol 2003; 190(1)17–24
- Spruill MD, Song B, Whong WZ, Ong T. J Toxicol Environ Health A. 2002; 65(24)2131–2144
- Hellstrom L, Elinder CG, Dahlberg B, Lundberg M, Jarup L, Persson B, Axelson O. Am J Kidney Dis 2001; 38(5)1001–1008
- Sorahan T, Esmen NA. Occup Environ Med 2004; 61(2)108–116
- Hu J, Spencer TE. Biol Reprod 2005; 73(1)131–138, Epub 2005 Mar 23
- Ceccarelli C, Liang ZX, Strickler M, Prehna G, Goldstein BM, Klinman JP, Bahnson BJ. Biochemistry 2004; 43(18)5266–5277
- King RA, Markov D, Sen R, Severinov K, Weisberg RA. J Mol Biol 2004; 342(4)1143–1154
- Sounni NE, Noel A. Biochimie. 2005; 87(3–4)329–342
- Takeda A, Tamano H, Ibuki Y. Anticancer Res 2004; 24(6)3869–3874
- Tudor R, Zalewski PD, Ratnaike RN. J Nutr Health Aging 2005; 9(1)45–51
- Yora T, Sakagishi Y. Comp Biochem Physiol B 1986; 85(3)649–658
- Kuznetsova SS, Azarkina NV, Vygodina TV, Siletsky SA, Konstantinov AA. Biochemistry (Mosc) 2005; 70(2)128–136
- Sheline CT, Behrens MM, Choi DW. J Neurosci 2000; 20(9)3139–3146
- Dahot MU, Saboury AA, Moosavi-Movahedi AA. J Enz Inhib Med Chem 2004; 19(2)157–160
- Gao X, Du Z, Patel TB. J Biol Chem 2005; 280(4)2579–2586
- Niehaus WG, Jr, Dilts RP, Jr. Arch Biochem Biophys 1984; 228(1)113–119
- Chen BH, Tsai JL, Tsai LY, Chao MC. Kaohsiung J Med Sci 1999; 15(11)646–650
- Chan AC, Chow CK, Chiu D. Proc Soc Exp Biol Med 1999; 222(3)274–282
- Dineley KE, Votyakova TV, Reynolds IJ. J Neurochem 2003; 85(3)563–570
- Merluzzi VJ, Cipriano D, McNeil D, Fuchs V, Supeau C, Rosenthal AS, Skiles JW. Res Commun Chem Path Pharmacol 1989; 66(3)425–440
- Betke K, Brewer GJ, Kirkman HN, Luzatto L, Motulsky AG. WHO Techn Rep Ser 1967; 366: 30–32
- Tandogan B, Ulusu NN. Turkish J Biochem 2005; 30(2)178–182
- Ulusu NN, Tandogan B, Tezcan EF. Biochimie 2005; 87(2)187–190
- Bradford MM. Anal Biochem 1976; 72: 248–254
- Ferri P, Biagiotti E, Ambrogini P, Santi S, Del Grande P, Ninfali P. Neurosci Res 2005; 51(2)185–197
- Wolfgang Maret, Claus Jacob, Bert L Valleet, Edmond H Fischer. Proc Natl Acad Sci U S A 1999; 96(5)1936–1940
- Naab F, Volcomirsky M, Burlon A, Caraballo ME, Debray M, Kesque JM, Kreiner AJ, Ozafran MJ, Schuff JA, Stoliar P, Vazquez ME, Davidson J, Davidson M, Fonovich de Schroeder TM. Arch Environ Contam Toxicol 2001; 41(2)201–207
- Chandra RK. J Amer Med Assoc 1984; 252: 1443–1446
- Patterson WP, Winkelmann M, Perry MC. Ann Intern Med 1985; 103: 385–386
- Simon SR, Branda RF, Tindle BH, Burns SL. Am J Hematol 1988; 28: 181–183
- Boadi WY, Urbach J, Barnea ER, Brandes JM, Yannai S. Pharmacol Toxicol 1992; 71(3 Pt 1)209–212
- Sheabar FZ, Yannai S. Vet Hum Toxicol 1989; 31(6)528–531
- Shukla GS, Srivastava RS, Chandra SV. Fundam Appl Toxicol 1988; 11(2)229–235
- Grose EC, Richards JH, Jaskot RH, Menache MG, Graham JA, Dauterman WC. J Toxicol Environ Health 1987; 21(1–2)219–232
- Ulusu NN, Açan NL, Turan B, Tezcan EF. Biol Trace Elem Res 2003; 91: 151–156
- Ulusu NN, Açan NL, Turan B, Tezcan EF. Turk J Biochem 2002; 27: 43–46
- Asagba SO, Eriyamremu GE, Adaıkpoh MA, Ezeoma A. Biol Trace Elem Res 2004; 100: 75–86
- Serafini MT, Romeu A, Arola L. Biochem Int 1989; 18(4)793–802
- Zalups RK, Ahmad S. Toxicol Appl Pharmacol 2003; 186(3)163–188