Abstract
The inhibition and activation effects of some drugs on the activities of superoxide dismutase enzymes (SOD) in human erythrocyte and leukocyte cells was investigated. Firstly, CuZnSOD enzyme was purified 837–fold and 12% efficiency from human erythrocytes by ethanol-chloroform treatment to remove hemoglobin and then ion exchange chromatography (DEAE-Sepharose) and copper chelate affinity chromatography techniques. Inhibition or activation effects of fourteen drugs on CuZnSOD was investigated. None of the studied drugs except for 5-fluorouracil showed any effects on the enzyme. 5-fluorouracil showed activation effects on CuZnSOD at 3.33 mg/ml and 4 mg/ml concentrations with 33% and 32% activation, respectively. Leukocytes were isolated from healthy human blood, lysed in liquid nitrogen and the effect of 5-fluorouracil on the lysate SOD activity investigated. 5-Fluorouracil showed inhibition effects on total SOD activity of human leukocytes at 2 mg/ml and 4 mg/ml concentrations with 42% and 62% inhibition, respectively.
Introduction
The superoxide radical () is generated within aerobic biological systems during both enzymatic and nonenzymatic oxidations [Citation1]. It can oxidize the [4Fe–4S] clusters of dehydratases, such as aconitase, reduce Fe+3 to Fe+2 which produces hydroxyl radical by the Fenton reaction, which inactivates catalase and peroxidases, attacks erythrocyte membranes and causes lysis Citation1Citation2-4. Therefore, the superoxide radical may result in many deleterious physiological effects. However, superoxide is eliminated by superoxide dismutases (SODs). SODs catalyze the dismutation reaction of the superoxide radical to O2 and H2O2 [Citation5].
Humans contains three SOD isoenzymes. These are copper, zinc superoxide dismutase (CuZnSOD, SOD1), manganese superoxide dismutase (MnSOD, SOD2), and extracellular superoxide dismutase (ECSOD, SOD3) [Citation6]. CuZnSOD has been found in the cytosol of all eukaryotic cells [Citation7]. MnSOD is also present in the mitochondrial matrix and peroxisomes of eukaryotes [Citation7]. ECSOD which is a glycoprotein is located in extracellular fluids e.g. blood plasma, lymph, and synovial fluid [Citation6,Citation7].
CuZnSOD is a very stable enzyme towards organic solvents, heat, proteases, urea (8 M), SDS (2%) but can be inhibited by CN− , H2O2 and diethyldithiocarbamate. Contrarily, MnSOD is not inhibited by CN− and diethyldithiocarbamate but is susceptible to many chemicals [Citation8].
Erythrocytes contain only CuZnSOD [Citation9] but leukocytes contain mitochondria additionally, and therefore they may contain MnSOD as well as CuZnSOD. Because of the important task of the SODs, inhibition or activation effects of chemicals (e.g. drugs) on the enzymes are important and determination of the effects of some drugs on the activities of SODs in human erythrocyte and leukocyte cells are the subject of this study. Hence, CuZnSOD enzyme was purified from human erythrocytes by ethanol–chloroform treatment to remove hemoglobin and then ion exchange (DEAE-Sepharose) and copper chelate affinity chromatography techniques. The inhibition or activation effects of fourteen drugs on CuZnSOD was investigated as well as the effect of 5-fluorouracil on the lysate SOD activity of human leukocytes.
Materials and methods
Materials
Diethylaminoethyl Sepharose (DEAE-Sepharose), imminodiacetic acid immobilized Sephadex 6B and 6-hydroxydopamine (6-OHDA) were obtained from Sigma. All other chemicals used were analytical grade and obtained from Sigma-Aldrich, Fluka or Merck.
Protein determination
After scanning at 280 nm, the fractions with significant absorbance were pooled. Quantitative protein determination was conducted by absorbance measurement at 595 nm according to Bradford's method, with bovine serum albumin as standard [Citation10].
SOD activity assay
Determination of SOD activities were based on the inhibitory effect of SOD on the spontaneous autoxidation of 6-hydroxydopamine (6-OHDA) [Citation11,Citation12]. 1 E.U. of SOD activity is the amount of superoxide dismutase required for 50% inhibition of the initial rate of 6-OHDA autoxidation. The autoxidation rate of 6-OHDA (4 × 10− 4 M) in 0.05 M phosphate buffer (pH 7.4) was determined by observing the absorbance change at 490 nm.
Stock 6-OHDA solution (0.01 M) was prepared daily. For this firstly, 0.01 M HCl solution containing KCl (final concentration 1 mM) was prepared and then dissolved O2 entirely removed by passing pure N2 through the solution, as confirmed by monitoring the dissolved oxygen concentration with MultiLab P4 device (WTW, Germany). Subsequently, the required 6-OHDA was dissolved in this solution.
To determine the SOD activity of the samples, the volume of SOD solution was adjusted to 730 μl with 0.05 M phosphate buffer (pH 7.4) in a cuvette. Then 30 μl 6-OHDA stock solution (0.01 M) was added to the SOD solution and the absorbance change at 490 nm immediately recorded for 1 min. To determine the effects of the drugs on SOD activity, the drug solution was mixed with the SOD solution and then the volume adjusted to 730 μl with the same buffer in a cuvette. In all cases, blanks containing the test sample without 6-OHDA were run.
Isolation of human erythrocytes and leukocytes
Fresh human blood (400 ml) containing citrate was centrifuged at 3,000 rpm for 15 min and the plasma was then removed with a Pasteur pipette. The leukocyte layer containing plasma and erythrocyte contamination was transferred to a tube to obtain packed leukocytes. The packed red cells (180 ml) were washed with 100 ml isotonic NaCl solution and then haemolysed by the addition of one volume of cold water at 4°C.
The leukocytes suspension was centrifuged at 3,000 rpm for 10 min and the plasma removed. To remove erythrocyte contamination from the packed leukocytes, 2 ml cold water (4°C) and, 20–25 seconds later, 2 ml 1.8% NaCl solution were added. Under conditions erythrocytes are lysed but leukocytes are not. This step was repeated until pure packed leukocytes were obtained. Finally, the packed leukocytes were lysed by addition of liquid nitrogen.
Ethanol-chloroform treatment
To precipitate the hemoglobin in the erythrocyte haemolysate pre-cooled ethanol (0.25 volumes) and then pre-cooled chloroform (0.31 volumes) were slowly added by mixing in an ice bath [Citation12]. Then 0.1 volume of water was added and the precipitate was removed by centrifugation. The supernatant was dialyzed against 2 L, 10 mM phosphate buffer (pH 6.4).
DEAE-Sepharose ion exchange chromatography
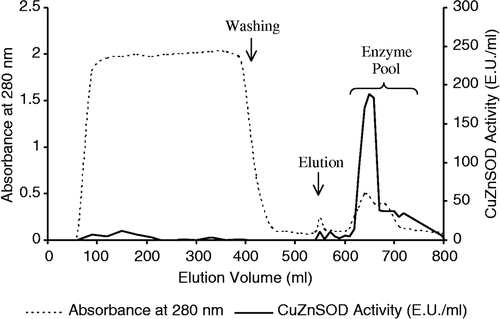
The dialyzed supernatant was applied to a DEAE-Sepharose (Sigma DFF-100) column (2.5 × 16 cm) equilibrated with 10 mM pH 6.4 phosphate buffer [Citation13]. After washing with the same buffer, elution of the CuZnSOD was achieved using a 0–0.5 M NaCl ionic range gradient at a flow rate 60 ml/h (). The enzyme in the eluate was then subjected to copper chelate affinity chromatography.
Copper chelate affinity chromatography
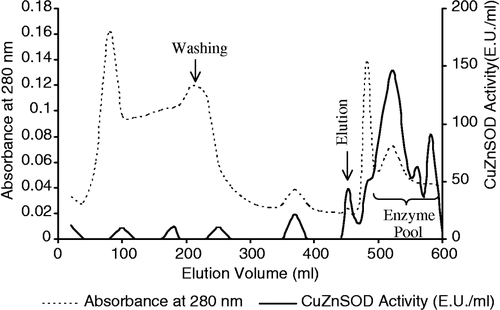
Copper chelate affinity chromatography technique was carried out as described by Weselake et al. with a slight modification [Citation13]. An affinity column (1.5 × 10 cm) was packed with imminodiacetic acid immobilized Sephadex 6B (Sigma I-4510) at a flow rate 42 ml/h and activated with 50 mM CuSO4 solution until the entire matrix became blue. A second column (1 × 10 cm) was packed with the same gel, which did not receive the copper solution, and connected to the bottom of the column containing the metal-activated gel. SOD activity can not be detected in solutions containing copper-II ions and the second column served as a guard to adsorb leakage of copper ions from the first column. The enzyme pool was applied to the first column equilibrated with 10 mM pH 6.4 phosphate buffer containing 0.1 M NaCl. After washing with the same buffer containing 1 M NaCl, CuZnSOD was eluted with 20 mM pH 5.0 citrate buffer at a flow rate of 42 ml/h (). The activity of CuZnSOD was determined in all the eluted fractions and activity-containing fractions were collected and the enzyme was dialyzed against distilled water and stored at − 80°C until use.
SDS-Polyacrylamide gel electrophoresis
Enzyme purity and molecular weight of the subunit of CuZnSOD was determined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) performed according to the Laemmli method using a vertical slab gel apparatus [Citation14]. The following proteins were used as SDS-PAGE molecular weight standards: rabbit myosin (205,000 Da), E. coli β-galactosidase (116,000 Da), rabbit phosphorylase B (97,400 Da), bovine albumin (66,000 Da), chicken ovalbumin (45,000 Da) and bovine carbonic anhydrase (29,000 Da). Molecular weight markers and enzyme samples were dissolved in phosphate buffer (0.01 M pH 7.2) containing 2% SDS, 5% β-mercaptoethanol, 10% glycerol or sucrose, 0.002% bromophenol blue and denaturated by incubation in boiling water for 3 min.
Electrophoresis was performed according to the discontinuous method with 3.75% stacking gel and 15% separation gel at 100 V. After electrophoresis, the gel was incubated in fixation solution (50% isopropyl alcohol, 10% trichloracetic acid and 40% water) for 35–40 min, and then stained with Coomassie brilliant blue R-250 dye solution during 2.5 h. Dyed gel was then destained with washing solution (50% methanol, 10% acetic acid and 40% water) thus clear protein bands were obtained.
Effects of drugs
Inhibition or activation effects of fourteen drugs on erythrocyte CuZnSOD and 5-fluorouracil on total leukocyte SOD activity were investigated at 4–8 different concentrations depending on the tested drug. The drugs tested and their maximum concentration range are as follows: 5-fluorouracil (6600 mg l− 1), theophylline ethylenediamine (1600), pheniramine hydrogen maleate (760), irinotecan hydrochloride (1330), tramadol HCl (3330), digoxin (16.7), dalteparin Na (166 × 104 IU ml–1), chlorpromazine (160 mg l–1), haloperidol (330), prednisolone (1660), methylprednisolone (1330), tranexamic acid (6660), lornoxicam (660) and ranitidine (1660).
Results and discussion
Hemoglobin has a strong affinity to affinity chromatography gel activated with Cu+2 and has to be removed from the erythrocyte haemolysate before applying affinity chromatography. To remove hemoglobin, cold ethanol and then cold chloroform were added to the erythrocyte haemolysate. Most of the hemoglobin was removed by this step (, lane 3). CuZnSOD can tolerate organic solvent effects [Citation15] and is stable under these conditions.
Figure 3 Gel electrophoresis of all purification steps of CuZnSOD. 1. Protein standards (myosin, rabbit: 205,000 Da; β-galactosidase, E. coli: 116,000 Da; phosphorylase B, rabbit: 97,400 Da; albumin, bovine: 66,000 Da; ovalbumin, chicken: 45,000 Da, carbonic anhydrase, bovine: 29,000 Da), 2. Haemolysate, 3. Ethanol-chloroform extraction, 4. Eluate of ion exchange chromatography, 5. Eluate of affinity chromatography.
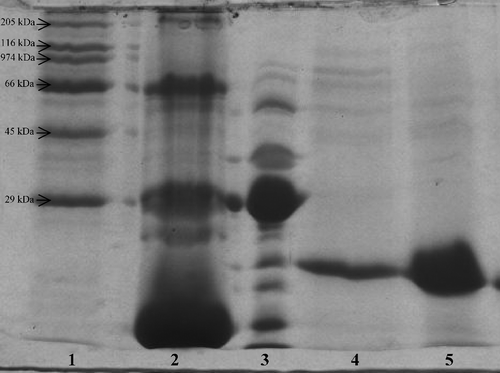
Previously it has been reported that the isoelectric point of human CuZnSOD is 4.8 [Citation16]. Therefore, CuZnSOD solution was dialyzed against 10 mM phosphate buffer (pH 6.4) before ion exchange chromatography (anion exchanger). Thus, CuZnSOD was negatively charged and also ions and citrate which are present in blood samples were removed by dialysis; otherwise, citrate can inactivate the affinity column by chelating and removing the copper. Elution of the CuZnSOD was achieved by establishing 0–0.5 M NaCl ionic range gradient. This is the first time that gradient elution has been used to purify CuZnSOD from human erythrocytes. It can be seen from and that the gradient elution step is sufficient to purify CuZnSOD with high purification fold. Most of the proteins were removed (lane 4) and CuZnSOD was purified 972- fold with a yield of 35.5% by gradient elution ().
Table I. Purification scheme for CuZnSOD.
To remove the remaining protein impurities from the CuZnSOD solution obtained from ion exchange step, copper chelate affinity chromatography was applied. Elution was performed with citrate buffer which is a chelating agent. CuZnSOD and the other proteins containing surface histidine residues are strongly bonded to the copper chelate affinity gel due to coordination between the copper of the affinity gel and surface histidine and cysteine residues on the proteins [Citation17,Citation18]. Citrate breaks the coordination bonds and the enzymes can be eluted. It can be seen in (lane 5) that CuZnSOD was completely purified by the affinity chromatography step. However the purification fold decreased () which may be due to loss of enzyme activity during to the purification process.
According to SDS-PAGE, isolated enzyme had a subunit with Mr 18,000–19,000 Da (18,900 Da) which was in agreement with Weselake et al [Citation13].
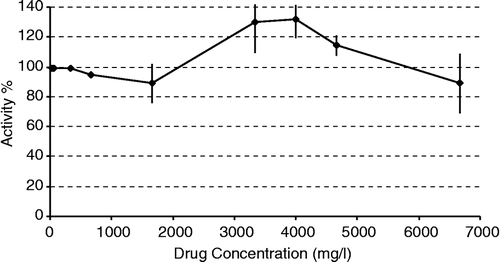
The effects of fourteen drugs on the enzyme were investigated. None of the drugs, except for 5-fluorouracil showed any effects on CuZnSOD. 5-Fluorouracil showed activation effects on CuZnSOD in the 3.33–5 mg/ml concentration range (). KCN used as a positive control entirely inhibited CuZnSOD at 0.4 mg/ml concentration.
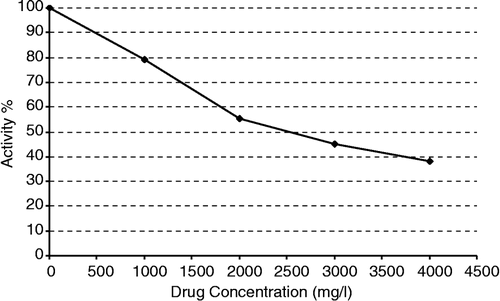
To purify MnSOD, leukocytes were isolated from healthy human blood and then lysed in liquid nitrogen. Since there was very low SOD activity in the homogenate, only the effect of 5-fluorouracil on the lysate SOD activity of the human leukocytes was investigated without purification of MnSOD. There has so far no report about the presence of MnSOD in human leucocytes. Although 5-fluorouracil does not has any inhibitory effect on CuZnSOD, it showed inhibition effects on the total SOD activity of leukocytes at the studied concentrations range (). This indicates that MnSOD is present in human leucocytes and that the inhibition of SOD activity may be caused by the inhibitory effect of 5-fluorouracil on the MnSOD.
In summary, 5-fluorouracil can activate CuZnSOD and inhibit MnSOD. However, the activation and inhibition concentrations of 5-fluorouracil are higher than its plasma concentration (about 3000-fold) which is about 1.1 μg/ml [Citation19]. Therefore 5-fluorouracil cannot activate or inhibit CuZnSOD or MnSOD, respectively, at the used concentration in cancer patients.
References
- Fridovich I. Arch. Biochem. Biophys. 1986; 247(1)1–11
- Lynch RE, Fridovich I. J. Biol. Chem. 1978; 253(6)1838–1845
- Fridovich I. Annu. Rev. Biochem. 1995; 64: 97–112
- Halliwell B, Clement MV, Long LH. FEBS Lett. 2000; 486: 10–13
- McCord JM, Fridovich I. J. Biol. Chem. 1969; 244: 6049–6055
- Mates JM, Perez-Gomez C, De Castro IN. Clin. Biochem. 1999; 32(8)595–603
- Donnelly JK, McLellan KM, Walker JL, Robinson DS. Food Chem. 1989; 33: 243–270
- Michalski WP. J. Chromatogr. B. 1996; 684: 59–75
- Arai K, Iizuka S, Makita A, Oikawa K, Taniguchi N. J. Immunol. Methods. 1986; 91: 139–143
- Bradford MM. Anal. Biochem. 1976; 72: 248–254
- Heikkila RE, Cabbat F. Anal. Biochem 1976; 75: 356–362
- Tarhan L, Tüzmen MN. Turk. J. Chem. 2000; 24: 109–116
- Weselake RJ, Chesney SL, Petkau A, Friesen AD. Anal. Biochem. 1986; 155: 193–197
- Laemmli UK. Nature. 1970; 227: 680–685
- Fridovich I. Adv. Enzymol. 1984; 58: 61–97
- Asayama K, Burr IM. Anal. Biochem. 1984; 136: 336–339
- Porath J, Carlsson J, Olsson I, Belfrage G. Nature. 1975; 258: 598–599
- Miyataasano M, Ito K, Ikeda H, Sekiguchi S, Arai K, Taniguchi N. J. Chromatogr. B. 1986; 370: 501–507
- Zheng JF, Wang HD. World J. Gastroenterol. 2005; 11(25)3944–3947