Abstract
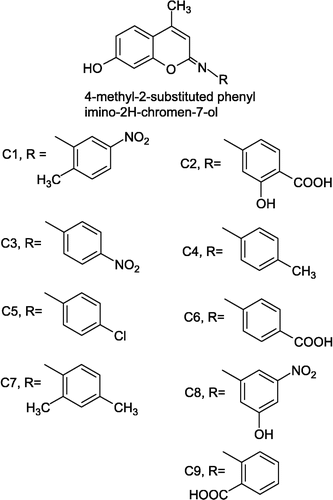
Coumarin Schiff-bases (CSB) possessing different substituents on the 4-methyl-2-substituted phenyl imino-2H-chromene-7-ol molecule were evaluated for their in-vitro antioxidant and plausible anti-inflammatory potential. The antioxidant studies of selected CSB were carried out by determining their reducing power, OH√ radical scavenging activity, scavenging of stable 2,2-diphenyl-1-picrylhydrazine (DPPH√) radical and inhibition of the polyphenol oxidase (PPO) enzyme. The assessment of possible anti-inflammatory potential was performed by trypsin inhibition assay and inhibition of β-glucuronidase. All the CSBs under study showed significant reducing effects. The majority of the tested CSB were found to be effective scavengers of DPPH√ radical with moderate to low OH√ scavenging ability and significantly inhibited the activity of PPO. With few exceptions, results from the inhibition assay of trypsin and β-glucuronidase were not encouraging, however they may be helpful in defining structure-activity relationships in further optimization of the lead molecules.
Keywords::
Introduction
In recent years antioxidants, a highly heterogeneous group, is emerging as a new approach of therapy called ‘antioxidant therapy’ [Citation1,Citation2]. The highly reactive, electrically charged free radicals usually generated during cellular respiration and normal metabolism have been implicated in the causation of various ailments in humans such as cancer, liver cirrhosis, atherosclerosis, diabetes, cardiovascular disease, immune system decline, brain dysfunction and cataracts [Citation3,Citation4]. Antioxidants allow aerobic organism to withstand daily episodes of oxidative stress by counteracting the adverse effect of free radicals generated in the body and helps to ameliorate the aforesaid ailments [Citation5]. In addition, antioxidants delay or inhibit oxidation process of important biological macromolecules such as carbohydrates, fats, proteins and DNA [Citation6].
Coumarin is a flavonoid which is a natural constituents of many plants and essential oils [Citation7]. Coumarin and its derivatives are reputed for their use in the treatment of diverse diseases such as cancer, burns, brucellosis, and rheumatic disorders [Citation8] and have been reported to possess considerable anti-inflammatory, antiallergic and antihaemorrhagic activities [Citation9]. Some of the coumarin analogs have been described as inhibitors of cyclooxygenase [Citation10] a key enzyme involved in inflammation process. Interestingly, coumarins also have an inhibitory effect on DNA gyrase which may be linked to their anti-human immunodeficiency virus (HIV) activity [Citation11]. Several novel 4-styryl coumarin derivatives are reported to be effective against HIV and cancer [Citation12]. Recently several linear and angular coumarins have been designed as anti-inflammatory and antioxidant agents [Citation13].
The circumstantial literature concerning coumarin derivatives cited above inspired us to conduct in-vitro antioxidant/possible anti-inflammatory evaluation of CSBs. Assessment of the in-vitro antioxidant activity of CSB was carried out by performing tests/assays of their reduction potential, interaction with DPPH√ radical, scavenging of OH√ radical and effect on PPO activity, while their plausible anti-inflammatory potential was described by performing trypsin and β-glucuronidase inhibition assay. An attempt has been made to correlate the obtained results with the structural features of the tested CSBs.
Materials and Methods
Materials
The compounds to be tested were selected from the ongoing research conducted in the synthesis of coumarin derivatives in the post graduate chemistry research laboratory, Yeshwant College Nanded (MS) India and were synthesized by the reported method [Citation14]. 2,2-Dphenyl-1-picrylhydrazine (DPPH√), β-glucuronidase (EC 3.2.1.31, 25,000 units, source: E. Coli), were obtained from Sigma-Aldrich Co. (St. Louis MO, USA), p-nitrophenyl-β-D-glucopyranosiduric acid was purchased from CALBIOCHEM (EMD Biosciences Inc. La Jolla CA) and trypsin form SISCO Research Lab. Ltd. Mumbai. L-DOPA (3,4 dihydroxyphenyl L-alanine), glutathione (reduced form) were purchased from s. d. Fine Chemicals Ltd. Mumbai.
All other reagents and solvents used were obtained from commercial sources and were of analytical grade.
Determination of the reducing power of CSBs [Citation15]
Applying the method of Sasaki et al (1991), which utilizes the ability of antioxidants to reduce Fe3+ of K3Fe(CN)e to Fe2+, the reducing power of CSB was determined by the decrease in absorption of K3Fe(CN)6 at 420 nm. The reaction mixture contained 500μl solution of individual CSBs (1mM in 0.5% v/v dimethyl formamide) in 3 ml of 1mM potassium ferricynide solution and the absorbence was measured at 420 nm after 10 min incubation time. Glutathione was used as a reference compound.
Determination of OH√ radical scavenging activity of CSBs [Citation16]
Hydroxyl radicals were generated by a Fe3+/ascorbic acid system. The detection of OH√ radicals was carried out by measuring the formaldehyde produced from the oxidation of dimethyl sulfoxide (DMSO). The reaction mixture contained 0.1mM EDTA, 167μM Fe3+, 33mM DMSO in phosphate buffer 50mM pH 7.4, 1mM of the test CSB and 150μl of ascorbic acid (10 mM in phosphate buffer) were added finally to start the reaction. The reaction was terminated after 30 min incubation by adding 17% (w/v) CCl3COOH. The formaldehyde produced was detected spectrophotomertically at 412 nm. Coumarin (1mM) was used as a standard compound.
Determination of DPPH√ radical scavenging activity of CSBs [Citation17]
The DPPH√ radical scavenging assay was carried out according to the reported method [Citation17]. The DPPH√ radical scavenging activity of the sample CSB (1mM, in absolute ethanol) was determined by mixing equal volumes of CSB and DPPH√ radical (10− 4M in absolute ethanol) solutions. After 20 min the absorbance at 517 nm was recorded on a UV-Visible spectrophotometer. Glutathione (1mM) was used as a reference compound.
Effect of CSBs on polyphenol oxidase (PPO) activity
A semi-pure preparation of PPO was used to study the effect of CSBs on PPO activity. Extraction of PPO was by the reported method [Citation18]. The determination of protein and the PPO assay were carried out by the methods described by Lowry, et al [Citation19] and Pathak, et al [Citation20], respectively. The reaction mixture contained L-DOPA (1 ml, 2mM), 0.5ml enzyme (0.618 mg protein), 1 ml test CSBs (1mM in 0.5% v/v, dimethyl formamide) and citrate buffer (0.5 ml, pH 4.8, 0.1 M). L-cysteine (1mM) was used as a reference compound. One unit of enzyme activity was defined as the amount of enzyme which caused an increase in absorbance of 0.001 min− 1 at 470 nm at 25°C and pH 4.8, which corresponds to the formation of 0.01μM of product (dopachrome).
Anti-proteolytic activity of CSBs [Citation21]
A trypsin inhibition assay was conducted to evaluate the anti-proteolytic potential of CSBs. The test CSB (1mM in 0.5% v/v, dimethyl formamide) was added to trypsin (0.075 mg/ml) for 20 min. After this time the substrate, bovine serum albumin (6g/100ml, in 0.1M phosphate buffer, pH 7.6) was added and after 20 min incubation at 37°C, the reaction was terminated by the addition of 3ml trichloroacetic acid (5%, w/v). The mixture were centrifuged at 5000 RPM for 10 min and the acid soluble fraction obtained in the supernatant was subjected to protein determination by the Folin-Lowry method [Citation19]. Salicylic acid (1mM) was used as a standard compound for comparison.
Effect of CSBs on β-glucuronidase activity [Citation22]
The β-glucuronidase inhibition assay was performed by preincubating the test CSB (1 mM), dissolved by adding 0.5% v/v, dimethyl formamide, in 0.1M acetate buffer pH 7.4 for 5 min at 37°C with 0.8 ml of 2.5 mM p-nitrophenyl-β-D-glucopyranosiduronic acid followed then by 0.1 ml of β-glucuronidase. After 30 min incubation the reaction was terminated by addition of 2ml of 0.5N NaOH and the mixture measured spectrophotometrically at 410 nm. Salicylic acid (1mM) was used as a standard compound ().
The % reducing activity, OH√ radical scavenging activity, DPPH√ radical scavenging activity, antiproteolytic activity and β-glucuronidase activity was calculated using the equation [Citation23]:
Results and Discussion
The results summarized in , shows that all the CSBs under study possess fair reducing activity within a range of 13.16 to 40.75%. Compound C2 showed a maximum of 40.75% reducing activity followed by compound C6 (32.51%) and C9 (34.33%). The reducing ability of CSBs seems to be related to the number of hydroxyl and /or proton donating groups present. In general, compounds possessing reducing activity and superoxide anion scavenging activity can be potential candidates for inhibition of cyclooxygenase, a key enzyme in recruiting inflammation [Citation24].
Table I. Profile of % reducing activity (% RA), OH√ radical scavenging activity (%OH√) and DPPH√ radical scavenging activity (%DPPH√) of CSBsa.
OH√ radicals have been reported to play a critical role in the physiological control of cell function [Citation25]. They react with extremely high rate constants with almost every type of molecules found in living cells: sugars, amino acids, phospholipids, DNA bases, organic acids and may change the normal physiological functions of the cells [Citation26]. Moreover in rheumatoid arthritis and related disorders, the reaction of nitric oxide with superoxide generates peroxynitrite which, under the acidic conditions often found in regions of inflammation and ischemia, yields hydroxyl radicals [Citation27]. The hydroxyl radicals thus generated in the above reaction have been blamed for the membrane damage of the cells in regions of inflammation [Citation28]. In the present study OH√ radicals were generated by the Fe3+/ascorbic acid system. The inhibition of formaldehyde production from the oxidation of DMSO was used for the evaluation of the OH√ radical scavenging ability of the CSBs. The results in indicate that compound C3 (46.15%) inhibition has shown the highest OH√ scavenging activity followed by compound C6(26.928). Compounds C2, C4 and C7 did not inhibit the oxidation of DMSO. Compounds C1 (17.94%), C5(14.10%) and C8(19.25%) exhibited considerable inhibition of formaldehyde formation. It is indeed difficult to explain the OH√ scavenging activity of the CSBs with respect to their structural features.
The assay for scavenging free radicals is based on measurement of the scavenging ability of antioxidants towards the DPPH√ radical, which reduced to the corresponding hydrazine when it reacts with hydrogen donors [Citation29]. This assay is considered valid and easy for the evaluation of the free radical scavenging activity of antioxidants, since the radical compound is stable and does not have to be generated as for other radical scavenging assays [Citation30]. In the present investigations it is evident from , that all CSBs have shown DPPH√ radical scavenging activity within a range of 10.89-47.74%. Compounds C2 (47.74%) and C6 (42.26%) were found to be the most reactive towards the DPPH√ radical, compounds C1, C3-C5, and C7 were found to possess a similar but average DPPH√ scavenging activity, whereas C8 (35.59%) and C9 (38.70%) showed significant interaction with the DPPH√ radical. As stated in the basis of this assay, antioxidants possessing hydrogen donors have more preference for scavenging DPPH√ radical and this applies to some of the CSBs showing maximum effect.
In the present studies, the enzyme polypenol oxidase (PPO, EC: 1.14.18.1) has been studied as a model oxidizing enzyme. PPO catalyzes the oxygen-dependent oxidation of diphenols to quinines [Citation31]. The results summarized in show that except for compound C2 all the CSBs showed PPO inhibitory activity. Compounds C8 (activity reduced to 2402 U/mg) and C9 (2496 U/mg) were found to be more effective as compared to other CSBs ().
Table II. Effect of CSBs on the activity of PPO, trypsin-induced protein hydrolysis and β-glucuronidase activity.a
It has been reported that the functional unit of PPO contains a pair of Cu ions and the interaction of the inhibitor with copper affect the site for binding the phenolic substrate [Citation32]. The tested CSBs may interference with the copper site thereby inhibiting the PPO activity. It has been also suggested that coumarin and related derivatives may chelate divalent metal ions [Citation13].
In all of the inflammatory cascades found in the body there are enzyme-mediated parts that involve serine proteases e.g. the pancreatic enzyme trypsin, elastase and enzymes of the complement system [Citation33]. Serine protease inhibition has been considered as one of the targets for designing anti-inflammatory agents [Citation34]. In the present work the inhibition of trypsin-induced hydrolysis of bovine serum albumin by CSBs was investigated. The results shown in indicate that compounds C3 (47.4%), C2 (34.2%) and C6 (31.12%) can be graded as the most effective anti-proteolytic agents, C5, C7 and C8 have no effect on the activity of trypsin and C1 (23.4%) and C9 (20.23%) can be considered as potential anti-proteolytic agents. Presence of electron donating groups and/or electronegative environment may be the possible reasons for the inhibition of trypsin by some compounds, however the same cannot be said for all the CSBs showing trypsin inhibitory activity.
Granulocytes, especially polymorphonuclear neutrophils have been implicated as mediators for inducing inflammations [Citation35]. The enzyme β-glucuronidase is present in the lysosomes of neutrophils and has been reported as one of the mediators for recruiting the inflammation process [Citation35,Citation36]. The results in show that, amongst the tested CSBs, compounds C1, C4, C5, C7 and C9 were not inhibitors of β-glucuronidase, while compounds C3 (1.1%), C2 (0.8), C6 (0.65%) and C8 (0.48%) were found to have negligible effect on its activity. It can be assumed that the presence of a nitro group and/or the electronegative influence and the overall steric effect of CSBs might be the contributing factors for inhibition of β-glucuronidase
Conclusion
It can be concluded from the results of the present work that CSBs containing variously substituted 4-methyl-2-substituted phenyl imino-2H-chromene-7-ol as a basic nucleus can be considered as a potential agent for designing antioxidant/anti-inflammatory agents. Further studies such as in-vivo anti-inflammatory activity and effect on cellular toxicity are underway and such information may give a better understanding for designing a lead molecule.
Acknowledgements
The Authors are thankful to UGC India for financial assistance and HOD, School of Life Sciences, S. R. T. M. University Nanded (MS) for providing the necessary facilities during this work.
References
- Salvatore C, et al. Pharmacol Rev 2001; 53: 135–159
- Yossi G-S, et al. Pharmacol Rev 2002; 54: 271–284
- Govindaragan R, et al. Indian J Exp Biol 2003; 41: 875–879
- Bruce NA, Mark KS, Tory MH. Proc Natl Acad Sci USA 1993; 90: 7915–7922
- Maria P, et al. Leukemia Res 2005; 29: 11–16
- Kerr ME, Bender CM, Monti EJ. Heart Lung 1996; 25: 200–209
- Egan D, et al. Drug Metab Rev 1990; 22: 503–529
- Murray RDH, Mendez I, Brawn SA. The Natural Coumarins. Wiley, New York 1983
- Yoshimoto T, et al. Biochem Biophys Res Commun 1983; 116: 612–618
- Kimura Y, et al. Biochim Biophys Acta 1985; 834: 224–229
- Matern U, Luer P, Kreusch D, et al. Comprehensive Natural Products Chemistry, D Barton. Elsevier Science Ltd, Oxford 1999; Vol. I: 623–637
- Maddi V, Raghu KS, Rao MNA. J Pharm Sci 1992; 81: 964–966
- Kontogiorgis C, Hadjipavlou-Litina D. J Enz Inhib Med Chem 2003; 18(1)63–69
- Tyagi M, et al. Ind J Chem 2002; 41B: 2367–2370
- Sasaki K, Matsumoto I, Beppu M. Affinity Chromatography. Tokyo Kagaku Dojin, Tokyo 1991; 117–130
- Nash T. J Biochem 1953; 55: 416–421
- Contreas-Guzman ES, Strong FC. J Assoc Off Anal Chem 1982; 65: 1215–1222
- Gacche RN, Waragkar SC, Ghole VS. J Enz Inhib Med Chem 2004; 19(2)175–179
- Lowry OH, et al. J Biol Chem 1951; 193: 265–275
- Pathak SU, et al. Phytochem 1992; 31(5)1481–1483
- Dimitra Hadjipavlou-Litina. Res Comm Mol Path Pharm 1997; 95(3)319–329
- Hadjipavlou-Litina D, Geronikaki AA. Arzneim Forsch Drug Res 1993; 46: 805–808
- Gulgun AK, et al. J Enz Inhib Med Chem 2004; 19(2)129–135
- Nicolaides D, et al. Eur J Med Chem 1998; 33: 715–724
- Drouge W. Physiol Rev 2002; 82: 47–95
- Barry H, John MCG. Biochem J 1984; 219: 1–14
- Brown GCH, Hall ND. Br J Rheumatol 1992; 31: 599–603
- Darligton LG, Stone TW. Br J Nutr 2001; 85: 251–269
- Contreas-Guzman ES, Strong FC. J Assoc Off Anal Chem 1982; 65: 1215–1222
- Sanchez-Moreno C. Food Sci Tech Int 2002; 8(3)121–137
- Giner J, et al. J Food Sci 2002; 67(4)1467–1472
- Mayer AM, Harel E. Phytochem 1979; 18: 193–215
- Trapnell JE, et al. Br J Surg 1974; 61: 177–182
- Bilfinger TV, George BS. Curr Pharm Design 2002; 8: 125–133
- Ito K, et al. Drug Res 1982; 32(I)117–122
- Savill J, Hasel C. Semin Cell Biol 1995; 6: 385–393