Abstract
A series of antibacterial and antifungal sulfonamide (sulfanilamide, sulfaguanidine, sulfamethaxozole, 4-aminoethylbenzenesulfonamide and 4-amino-6-trifluoromethyl-benzene-1,3-disulfonamide) derived chromones, previously reported as inhibitors of carbonic anhydrase, have been screened for in-vitro antibacterial activity against four Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi and Shigella flexeneri) and two Gram-positive (Bacillus subtilis and Staphylococcus aureus) bacterial strains, and for in-vitro antifungal activity against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani, Candida glaberata. All compounds (1)–(5) showed significant antibacterial activity against all four Gram-negative species and both Gram-positive species. However, three of them, (1), (4) and (5), were found to be comparatively much more active compared to (2) and (3). Of these, (5) was found to be the most active one. For antifungal activity, generally compounds (1) and (2) showed significant activity against more than three strains whereas (3)–(5) also showed significant activity against varied fungal strains. In the brine shrimp bioassay for in-vitro cytotoxic properties, only two compounds, (4) and (5) displayed potent cytotoxic activity, LD50 = 2.732 × 10− 4 M) and LD50 = 2.290 × 10− 4 M) respectively, against Artemia salina.
Introduction
In our previous studies Schiff bases of aromatic/heterocyclic sulfonamide derived chromones (Scheme 1) have been investigated Citation1-7 as inhibitors of the zinc enzyme carbonic anhydrase. The 15 CA isozymes presently known in humans are involved in many physiological and pathological processes and their inhibition may thus be exploited clinically for the treatment of glaucoma in which CA II and CA XII are targeted by sulfonamide or sulfamate inhibitors Citation8-11. Since these compounds have shown good carbonic anhydrase activity, it was considered worthwhile to study other potential aspects of these compounds such as antibacterial and antifungal activity. To the best of our knowledge, this class of compounds have been scarcely investigated previously for its activity against bacterial and fungal species. Here, compounds (1–5) were tested for in-vitro antibacterial activity against four Gram-negative; E. coli, P. aeruginosa, S. typhi and S. flexeneri, and two Gram-positive; B. subtilis and S. aureus bacterial strains, and for in-vitro antifungal activity against T. longifusus, C. albicans, A. flavus, M. canis, F. Solani and C. glaberata. These observations have strengthened our previous studies of sulfonamide-derived chromones [Citation12] to act as excellent sources of interaction with a variety of biomolecules that result in inhibition of biochemical and biophysical processes.
Material and methods
Reagents and solvents
All reagents and solvents were used as obtained from the supplier or recrystallized/ redistilled as necessary. Thin-layer chromatography was performed using aluminum sheets (Merck) coated with silica gel 60 F254. Infrared spectra (KBr discs) were recorded with a Hitachi Model 200-50 IR spectrophotometer. 1NMR spectra were recorded with Bruker AM 300 and AM 400 spectrometers (Rheinstetten–Forchheim, Germany) in d6-DMSO operating at 300 MHz. Tetramethylsilane was used as an internal standard. Microanalytical data were determined using an Elemental Analyzer Flash EA 1112. Melting points were taken on a Gallenkamp apparatus and are uncorrected. In-vitro antibacterial and antifungal properties were studied at HEJ Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Pakistan.
Chemistry
General procedure for the preparation of sulfonamide-derived compounds
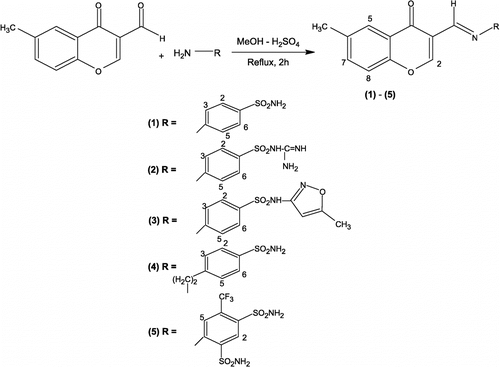
To a hot stirred solution of 3-formyl-6-methylchromone (0.01 mole) in methanol (40 mL) was added the respective sulfonamide (0.01 mole) in the presence of a catalytic amount of conc. H2SO4 (see Scheme ). The reaction mixture was refluxed for 2 h and the reaction was monitored by TLC. After completion of the reaction, the precipitates which appeared on cooling in an ice-bath were filtered and recrystallized from aqueous-ethanol (1:3) affording TLC-pure products in good yield.
4-{[(6-methyl-4-oxo-4H-chromen-3-yl)methylidene]amino}benzenesulfonamide (1)
Yield 80%; m.p. 164°C; IR (KBr, cm− 1): 3455 (NH2), 1715 (C = O), 1635 (HC = N), 1443 (S = O), 1143 (C–O); 1H NMR (DMSO-d6, δ, ppm): 1.54 (s, 3H, methyl), 7.35 (d, 1H, chromene C8-H), 7.58 (d, 2H, benzene C2,6-H), 7.62 (d, 1H, chromene C7-H), 7.65 (br s, 2H, SO2NH2), 7.85 (s, chromene C5-H), 7.88 (d, benzene C3,5-H), 8.13 (s, 1H, CH = N), 8.65 (s, 1H, chromene C2-H); Anal. Calcd. for C17H14N2O4S (342.37): C, 59.64; H, 4.12; N, 8.18. Found: C, 59.82; H, 4.62; N, 8.01%.
3-({[4-({[Amino(imino)methyl]amino)}sulfonyl)phenyl]imino}methyl)-6-methyl-4-oxo-4H-chromene (2)
Yield 90%; m.p. 240°C; IR (KBr, cm− 1): 3445 (NH2), 3160 (NH), 1720 (C = O), 1630 (HC = N), 1430 (S = O), 1140 (C–O); 1H NMR (DMSO-d6, δ, ppm): 1.56 (s, 3H, methyl), 7.37 (d, 1H, chromene C8-H), 7.60 (d, 2H, phenyl C2,6-H), 7.63 (d, 1H, chromene C7-H), 7.70 (s, 2H, NH2), 7.75 (s,1H, substituted NH), 7.83 (s, chromene C5-H), 7.91 (d, phenyl C3,5-H), 8.10 (s, 1H, C = NH),8.18 (s, 1H, CH = N), 8.66 (s, 1H, chromene C2-H); Anal. Calcd. for C18H16N4O4S (384.41): C, 56.24; H, 4.20; N, 14.57. Found: C, 56.52; H, 4.62; N, 14.18%.
N-(5-Methyl-3-isoxazolyl)-4-{[-(6-methyl-4-oxo-4H-chromen-3-yl)methylidene]amino}-benzenesulfonamide (3)
Yield 88%; m.p. 182°C; IR (KBr, cm− 1): 3150 (NH), 1710 (C = O), 1632 (HC = N), 1435 (S = O), 1148 (C–O); 1H NMR (DMSO-d6, δ, ppm): 1.57 (s, 3H, chromene methyl), 1.88 (s, 3H, isoxazolylmethyl), 5.83 (s, 1H, isoxazolyl),7.38 (d, 1H, chromene C8-H), 7.62 (d, 1H, chromene C7-H), 7.65 (d, 2H, benzene C2,6-H), 7.73 (s, 1H, substituted NH), 7.87 (s, chromene C5-H), 7.94 (d, benzene C3,5-H), 8.22 (s, 1H, CH = N), 8.64 (s, 1H, chromene C2-H); Anal. Calcd. for C21H17N3O5S (423.44): C, 59.57; H, 4.05; N, 9.92. Found: C, 59.71; H, 4.44; N, 9.57%.
4-(2-{[-(6-Methyl-4-oxo-4H-chromen-3-yl)methylidene]amino}ethyl)benzenesulfonamide (4)
Yield 80%; m.p. 158°C; IR (KBr, cm− 1): 3448 (NH2), 1712 (C = O), 1640 (HC = N), 1430 (S = O), 1145 (C–O); 1H NMR (DMSO-d6, δ, ppm): 1.53 (s, 3H, methyl), 3.6 (t, 2H, –CH2–N = C), 2.8 (t, 2H, –CH2–Ph),7.34 (d, 1H, chromene C8-H), 7.57 (d, 2H, benzene C2,6-H), 7.64 (d, 1H, chromene C7-H), 7.67 (s, 2H, SO2NH2), 7.90 (s, chromene C5-H), 7.87 (d, 2H, benzene C3,5-H), 8.16 (s, 1H, CH = N), 8.62 (s, 1H, chromene C2-H); Anal. Calcd. for C19H18N2O4S (370.42): C, 61.61; H, 4.90; N, 7.56. Found: C, 61.82; H, 4.66; N, 7.75%.
4-{[(-Methyl-4-oxo-4H-chromene-3-yl)methylidene]amino}-6-(trifluoromethyl)-1,3-benzenesulfonamide (5)
Yield 68%; m.p. 348°C; IR (KBr, cm− 1): 3435 (NH2), 1710 (C = O), 1625 (HC = N), 1420 (S = O), 1142 (C–O); 1H NMR (DMSO-d6, δ, ppm): 1.60 (s, 3H, methyl), 7.40 (d, 1H, chromene C8-H), 7.67 (d, 1H, chromene C7-H), 7.72 (s, 2H, SO2NH2), 7.84 (s, 2H, SO2NH2), 7.90 (s, chromene C5-H), 8.41 (s, 1H, benzene C2-H), 8.45 (s, 1H, CH = N), 8.73 (s, 1H, chromene C2-H), 9.45 (s,1H, benzene C5-H); Anal. Calcd. for C18H14F3N3O6S2 (489.44): C, 44.17; H, 2.88; N, 8.59. Found: C, 44.38; H, 2.62; N, 8.18%.
Antibacterial bioassay (in-vitro)
The synthesized compounds (1) - (5) were screened in vitro for their antibacterial activity against four Gram-negative (E. coli, P. aeruginosa, S. typhi and S. flexeneri) and two Gram-positive (B. subtilis and S. aureus) bacterial strains by the agar-well diffusion method [Citation13]. The wells (6 mm in diameter) were dug in the media with the help of a sterile metallic borer with centers at least 24 mm apart. Two to eight hours old bacterial inocula containing approximately 104–106 colony-forming units (CFU/ml) were spread on the surface of the nutrient agar with the help of a sterile cotton swab. The recommended concentration of the test sample (1 mg/ml in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, imipenum, served as negative and positive controls, respectively. The plates were incubated immediately at 37°C for 24 h. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). In order to clarify any participating role of DMSO in the biological screening, separate studies were carried out with the solutions alone of DMSO and they showed no activity against any bacterial strains.
Minimum inhibitory concentration (MIC)
Compounds containing high antibacterial activity (over 80%) were selected for minimum inhibitory concentration (MIC) studies. The minimum inhibitory concentration was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 μg/ml of the compounds and applying the described protocol [Citation15].
Antifungal activity (in-vitro)
Antifungal activities of all compounds were studied against six fungal cultures. Sabouraud dextrose agar (oxoid, Hampshire, England) was seeded with 105 (cfu) ml− 1 fungal spore suspensions and transferred to petri plates. Discs soaked in 20 ml (200 μg/ml in DMSO) of all compounds were placed at different positions on the agar surface. The plates were incubated at 32°C for seven days. The results were recorded [Citation14] as zone of inhibition (mm) and compared with standard drugs miconazole and amphotericin B.
Cytotoxicity (in-vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22x32 cm), filled with artificial seawater, which was prepared with a commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the smaller compartment was open to ordinary light. After two days nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 ml of DMF. From this stock solutions 500, 50 and 5 μg/ml were transferred to 9 vials (three for each dilution were used for each test sample and the LD50 is the mean of the three values) and one vial was kept as control having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After two days, when shrimp larvae were ready, 1 mL of sea water and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with sea water to 5 mL per vial. After 24 h the number of survivors was counted. Data were analyzed by a Finney computer program to determine the LD50 values [Citation16,Citation17].
Result and discussion
Compounds (1) - (5), reported herein have already been characterized and reported elsewhere [Citation12] for their carbonic anhydrase activity. These compounds have not previously been investigated for their antibacterial/antifungal activities and these activities are reported here.
Antibacterial bioassay
All compounds were tested against four Gram-negative (E. coli, S. flexenari, P. aeruginosa and S. typhi) and two Gram-positive (B. subtili and S. aureus) bacterial strains according to literature protocol [Citation13]. The results were compared with those of the standard drug imipenum. The synthesized compounds exhibited varying degrees of inhibitory effects on the growth of differently tested strains (). All the synthesized compounds (1) - (5) showed significant inhibitory action against all the four Gram-negative species, E. coli, P. aeruginosa S. typhi and S. flexneri, and the two Gram-positive species, B. Subtilis and S. aureus; however, three of them ((1), (4) and (5)) were found to be comparatively much more active, of which (5) was the most active. Compound (2) displayed moderate inhibitory effect on the growth of the two Gram-negative (E. coli and P. aeruginosa) and one Gram-positive bacteria (S. aureus), respectively.
Table I. Primary screening of the Schiff bases (1) - (5) for antibacterial activity (zone of inhibition in mm).
Minimum inhibitory concentration (MIC)
The preliminary screening showed that compounds (1), (4) and (5) were the most active ones against both Gram-negative and Gram-positive organisms. These three compounds were selected for minimum inhibitory concentration (MIC) studies (). The MIC of all the three active compounds varies from 2.2908 × 10− 8 – 2.043 × 10− 7 M. Compound (5) again proved to be the most active one; it inhibited the growth of S. typhi, S. flexneri and B. subtilis at 2.043 × 10− 8 M concentration.
Table II. Minimum Inhibitory Concentration (M) of Compounds (1), (4) and (5) against Selected Bacteria.
Table III. In-vitro Antifungal Activity data for Schiff's bases (1) - (5) (zone of inhibition in mm).
Antifungal bioassay
The antifungal screening of all compounds was carried out against T. longifusus, C. albicans, A. flavus, M. canis, F. solani and C. glaberata fungal strains according to the literature protocol [Citation14]. The results were compared with those from the standard drugs miconazole and amphotericin B. These results illustrated in indicate that compound (1) exhibited a significant activity against T. longifusus, C. albican, M. canis and F. solani and a moderate activity against C. glaberata and A. flavus. Compound (2) showed a significant activity against all fungal species except a moderate activity against C. albicans. Compound (3) showed a significant activity against all species. Compound (4) showed a significant activity against T. longifusus, A. flavus, M. canis, F. solani and C. glaberata and a weak activity against C. albican. Compound (5) showed a significant activity against T. longifusus, A. flavus, M. canis, and C. glaberata; a moderate activity against C. albicans and a weak activity against F. solani.
Cytotoxic bioassay
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyer et al. [Citation18]. From the data recorded in , it is evident that only two compounds, 4 and 5, displayed potent cytotoxic activity against Artemia salina, while the other compounds were inactive in this assay. Compound 4 showed maximum activity (LD50 = 2.732 × 10− 4 M) in the present series of compounds, whereas the other active compound 5 of the series had slightly less activity (LD50 = 2.290 × 10− 4 M) than compound 4.
Table IV. Brine shrimp bioassay data for Schiff's bases (1) - (5).
Acknowledgements
One of the authors (ZHC), is thankful to Higher Education Commission (HEC), Government of Pakistan for the financial support to carry out this research Project No. 20–16/Acad(R&D)/2nd Phase/03/211 that made this research possible.
References
- Supuran CT, Nicolae A, Popescu A. Eur. J Med Chem 1996; 31: 431–438
- Supuran CT, Popescu A, Ilisiu M, Costandache A, Banciu MD. Eur. J Med Chem 1996; 31: 439–447
- Spuran CT, Scozzafava A, Popescu A, Bobes-Tureac R, Banciu A, Creanga A, Bobes-Tureac G, Banciu MD. Eur J Med Chem 1997; 32: 445–452
- Popescu A, Simion A, Scozzafava A, Briganti F, Supuran CT. J Enz Inhib 1999; 14: 407–423
- Scozzafava A, Banciu MD, Popescu A, Supuran CT. J Enz Inhib 2000; 15: 533
- Ul-Hassan M, Scozzafava A, Chohan ZH, Supuran CT. J Enz Inhib 2001; 16: 499–505
- Mahmood UlH, Chohan ZH, Scozzafava A, Supuran CT. J Enz Inhib Med Chem 2004; 19: 263–267
- Winum JY, Casini A, Mincione F, Starnotti M, Montero JL, Scozzafava A, Supuran CT. Bioorg Med Chem Lett 2004; 14: 225–229
- Vullo D, Innocenti A, Nishimori I, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT. Bioorg Med Chem Lett 2005; 15: 963–969
- Whitson JT, Henry C, Hughes B, Lee DA, Terry S, Fechtner RD. J Glaucoma 2004; 13: 168–173
- Renard JP, Giraud JM, Oubaaz A. J Fr Ophthalmol 2004; 27: 701–705
- Puccetti L, Fosolis G, Vullo D, Chohan ZH, Scozzafava A, Supuran CT. Bioorg Med Chem Lett 2004; 15: 3096
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. Harwood Academic Publishers, The Netherlands 2001; 22
- McLaughlin JL, Chang C-J, Smith DL. Studies in natural products chemistry, “Bench-Top” bioassays for the discovery of bioactive natural products: an update, structure and chemistry (part-B), Atta-ur-Rahman. Elsevier Science Publishers B.V., The Netherlands 1991; 9: 383
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. Harwood Academic Publishers, The Netherlands 2001; 16
- Bauer AW, Kirby WM, Sherris JC, Turck M. Am J Clin Pathol 1966; 45: 493
- Finney DJ. Probit analysis3rd ed. Cambridge University Press, Cambridge 1971
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Planta Medica 1982; 45: 31