Abstract
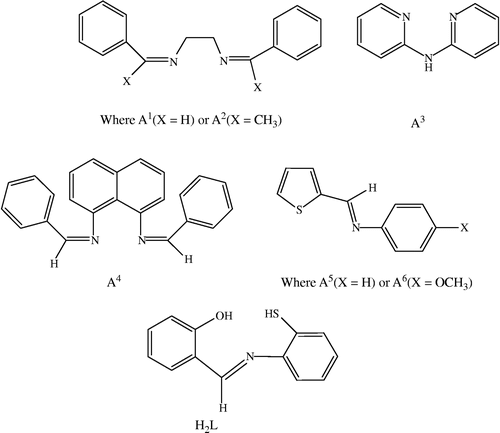
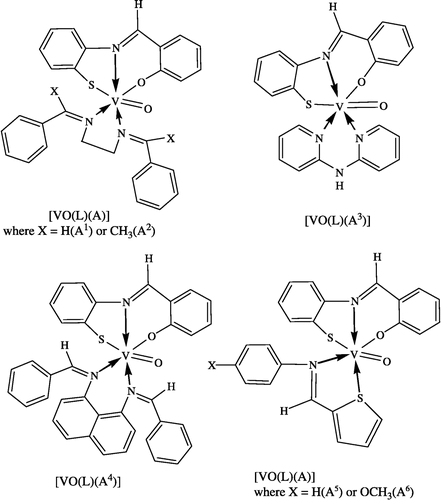
Oxovanadium(IV) complexes have been synthesized and characterized the general composition [VOL(A)], where H2L = salicylidene-o-aminothiophenol A1 = bis(benzylidene)ethylenediamine, A2 = bis(acetophenone)ethylenediamine, A3 = 2,2′-bipyridylamine, A4 = bis(benzylidene) − 1,8-diaminonaphthalene, A5 = thiophene-o-carboxaldeneaniline and A6 = thiophene-o-carboxaldene-p-anisidine. Spectral studies indicate that the oxovanadium(IV) complexes assume a six-coordinate octahedral geometry. The antibacterial activities of the complexes against Salmonella typhi, Escherichia coli and Serratia mercescens are higher as compared to the free ligands, vanadyl sulphate, and the control (DMSO) but of moderate activity as compared to the standard drug (tetracycline).
Introduction
The coordination chemistry of vanadium with sulfur-containing ligands is an emerging field of interest with relevance to several disparate biological system [Citation1,Citation2]. Interaction between transition metals and proteins are ubiquitous in biochemistry [Citation3]. The fundamental intrinsic nature of these interactions can be studied in metal complexes with amino acid Schiff bases [Citation4] which has led to the suggestion that Schiff bases act as a tridentate ligand containing an azomethine nitrogen atom and the terminal two oxygen atoms of the carboxylate group as well as the hydroxyl group [Citation5]. Several vanadium complexes of the tetradentate Schiff bases Sal2en-type ligands have been proposed for treatment of obesity and hypertension [Citation6,Citation7]. Some transition metal complexes of heterocyclic sulfonamides and aminosulfonamide derivatives possess powerful carbonic anhydrase inhibitory properties [Citation8]. Several quinolone derivatives like ciprofloxacin have excellent coordinating property and possess remarkable antibacterial activity [Citation9]. A major advance in the use of vanadium has been the development of organic vanadium complexes [Citation10] and the increase in interest in the bio-inorganic chemistry of vanadium has been documented by the appearance of two monographs [Citation11,Citation12] during the 1995s. Recently it has been proved that several coumarins and sulfonamides-derived Schiff bases and their metal complexes are less cytotoxic and the decrease in cytotoxicity is due to coordination with the metal ion [Citation13,Citation14].The present communication describes the preparation, characterization and bactericidal studies of some oxovanadium(IV) complexes with tridentate and bidentate ligands. Structures of the Schiff bases are shown in .
Materials and methods
Chemistry
All the chemicals used were of analytical grade. o-Aminothiophenol, 2,2′-bipyridylamine, 1,8-diaminonaphthalene and thiophene-o-carboxaldehyde were purchased from Lancaster, Morecambe, UK. Salicylaldehyde, benzaldehyde, acetophenone, ethylenediamine, aniline, p-anisidine and vanadyl sulphate were purchased from E. Merck (India) Limited. Mumbai. The organic solvents were purified by standard methods [Citation15].
Preparation of Schiff bases
Salicylidene-o-aminothiophenol (H2L): Salicylidene-o-aminothiophenol (H2L) was prepared by a condensation reaction between salicylaldehyde (10 mmol, 1.22 g) and o-aminothiophenol (10 mmol, 1.25 g) in ethanol (100 mL). The obtained compound was filtered and recrystallized from dilute acetic acid. The structure was confirmed by elemental analyses and IR spectra. Yield: 1.73 g (70%), M.p.: 128 °C.
Bis(benzylidene)ethylenediamine (A1): The bis(benzylidene)ethylenediamine(benen) was synthesized by a published procedure [Citation16].
Bis(acetophenone)ethylenediamine (A2): The bis(acetophenone)ethylenediamine was synthesized by a published procedure [Citation16].
Bis(benzylidene)–1,8-diaminonaphthalene (A4): An ethanolic solution (100 mL) of 1,8-diaminonaphthalene (10 mmol, 1.58 g) and an ethanolic solution (100 mL) of benzaldehyde (20 mmol, 2.12 g) in a molar ratio 1:2 were mixed with constant stirring, refluxed for 5 h and then cooled to 0-2 °C overnight to give a fine crystalline yellow product. The product was filtered and dried in air. Yield: 2.51 g (68%), M.p.: 235 °C.
Thiophene-o-carboxaldeneaniline (A5): An ethanolic solution (100 mL) of thiophene-o-carboxaldehyde (10 mmol, 1.12 g) and an ethanolic solution (100 mL) of aniline (10 mmol, 0.09 g) in the molar ratio of 1:1 were mixed with constant stirring, refluxed for 6 h and then cooled for overnight at room temperature. The yellow crystals formed were collected and dried in air. Yield: 0.73 g (60%), M.p.: >360 °C.
Thiophene-o-carboxaldene-p-anisidine (A6): An ethanolic solution (100 mL) of thiophene-o-carboxaldehyde (10 mmol, 1.12 g) and an ethanolic solution (100 mL) of p-anisidine (10 mmol, 1.07 g) in the molar ratio of 1:1 were mixed with constant stirring, refluxed for 6 h and then cooled overnight at room temperature. The yellow crystals formed were collected and dried in air. Yield: 1.31 g (60%), M.p.: >360 °C.
Synthesis of the complexes
The complexes were prepared by mixing an aqueous solution (100 mL) of vanadyl sulphate (10 mmol) and a hot methanolic solution (100 mL) of salicylidene-o-aminothiophenol (10 mmol, 2.29 g) and either A1 (10 mmol, 2.36 g) or A2 (10 mmol, 2.64 g) or A3 (10 mmol, 1.71 g) or A4 (10 mmol, 3.34 g) or A5 (10 mmol, 1.87 g) or A6 (10 mmol, 2.01 g) in a 1:1:1 molar ratio and heating the mixture in a water bath for 1-2 h at 50 °C. The mixture when kept overnight at room temperature gave a fine coloured product. The obtained crystals were collected by filtration, washed with water, ethanol and dried in air.
Physical measurements
Magnetic moments were obtained using a model 7304, vibrating sample magnetometer, Lake Shore, U.S.A. The vibrating sample magnetometer reports the total magnetic moment, m, of a sample in emu. However, the final aim of the magnetic measurement is not the moment in emu, but to get the effective magnetic moment. Therefore, the magnetic moment can be converted to susceptibility units by realizing that 1 emu = 1 gauss·cm3. The susceptibility of a sample has units of volume and is defined for paramagnetic material by the equation:
χ (cm3) = m(emu)/ H(oersted)
Gram susceptibility: χg = χ(cm3)/mass.
where T = absolute temperature (°K)
Infrared spectra were recorded on a FT-IR Nicolet 400D spectrophotometer in a KBr pellets. The reflectance spectra of the complexes were recorded in the range 1700-350 nm (as MgO discs) on a Beckman DK-2A spectrophotometer. C, H, N and S were analyzed with a model 240 Perkin-Elmer elemental analyzer.
Bactericidal activity
Preparation of discs
The compound (20 μL) in DMSO was applied to a paper disc, (filter paper Whatmann No. 4 discs 6 mm diameter) with the help of a micropipette. The discs were left in an incubator for 48 h at 37 °C and then applied to the bacteria grown on agar plates.
Preparation of agar plates: Nutrient agar (37 g) for the preparation of the agar plates was suspended and soaked in freshly distilled water (1 L) for 15 min and then boiled on a water bath until the agar was completely dissolved. The mixture was autoclaved for 15 min at 120 °C and then poured into previously washed and sterilized petri discs and stored at 40 °C ready for inoculation using a sterile platinum wire loop for application of bacterial strains.
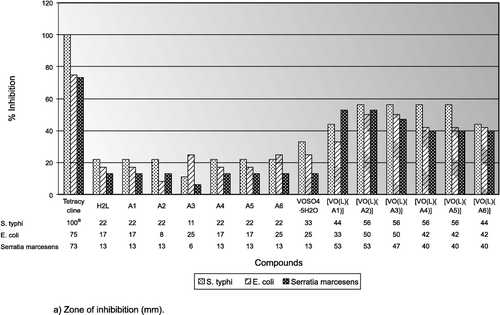
Application of discs: Sterilized forceps were used for the application of the paper disc on the earlier inoculated agar plates which were then incubated at 37 °C for 24 h. The zone of inhibition was then measured (in mm) around the disc. All experiments were performed in triplicate and tetracycline was used as a standard drug. The complexes were soluble in DMSO so the growth was compared with DMSO as the control and is expressed as zone of inhibition (mm) and percentage inhibition versus control. The results are represented in .
Results and discussion
All the synthesized complexes were green, crystalline solids and the Schiff bases were yellow crystalline solids which appear to be stable to atmospheric oxygen. All the complexes are insoluble in water, methanol and dimethyl formamide, but are soluble in dimethyl sulfoxide. Schiff bases are insoluble in water, and haxane, but soluble in ethanol, methanol, dimethyl formamide, and dimethyle sulfoxide. The elemental analyses of the ligands and complexes are in agreement with theoretical expectations (see ). They possess high melting points, so the analytical data show that the complexes are stable in air. Other data are presented in . The general equation for the formation of the oxovanadium(IV) complexes is given below.
Table I. Analytical data for the ligands and their VO(IV) complexesa.
IR spectra
The oxovanadium(IV) complexes exhibit a strong band near 1000 cm− 1, which has been assigned to ν(V = O)[Citation17]. The ν(SH)[Citation18] band of H2L appears at 2600 cm− 1. The absence of a band around ∼2600 cm− 1 in the spectra of all the complexes indicates that the –SH group loses the thiol proton to form a covalent bond between the sulphur and metal in all the complexes. This fact is further supported by the appearance of new bands in all the complexes around ∼410-425 cm− 1, which may be assigned to the ν(M-S) mode [Citation19]. The participation of the SH group in chelation is ascertained from the shift of the ν(C-S)[Citation20] band to lower frequencies from ∼765 cm− 1 in the free ligand to ∼750 cm− 1 in the spectra of the complexes. The ν(O-H)[Citation21] band originally found in the Schiff base disappeared on complexation indicating deprotonation of the phenolic hydroxyl group and coordination of phenolic oxygen to the metal. This is further supported by the shift in the stretching frequency of the phenolic ν(C-O) [Citation22] at 1530 cm− 1 to higher frequency by ∼10–20 cm− 1. The ν(C = N) band of 2,2′-bipyridylamine [Citation23] at 1580 cm− 1 shifts to higher frequency at ∼1610 cm− 1 in the complex indicating the bidentate N-N coordination of the ligand. The ν(C = N) band of A1, A2, A4, A5 and A6 found at ∼1625 cm− 1 shifts to lower frequency at ∼1610 cm− 1, indicating the participation of the azomethine nitrogen [Citation24] in coordination (M ← N). The coordination of the phenolic oxygen and azomethine nitrogen is further supported by the appearance of two non-ligand bands at ∼530 and ∼450 cm− 1 due to ν(M-O)[Citation25] and ν(M-N)[Citation26], respectively, in all of the complexes. The sharp bands in the range 750-780 cm− 1 and 1525 − 1535 cm− 1 are due to aromatic ν(C-H) and ν(C = C), respectively. The absorption in the 1160 − 1170 cm− 1 range is attributed to ν(C-N). The bands in the range 1470 − 1480 cm− 1 and 1370 − 1380 cm− 1 are due to ν(C-H) and ν(C-CH3), respectively.
Magnetic measurements
Oxovanadium(IV) complexes usually exhibit magnetic moments corresponding to the spin-only value of 1.73 B.M. at room temperature, the observed values of the magnetic moments for the present complexes are in the range 1.74 − 1.85 B.M. This data suggests that the mixed-ligand complexes prepared in this investigation are mononuclear [Citation27].
Electronic spectra
The electronic spectra of the complexes were recorded in the solid state. However, more evidences seem to favor the ordering proposed by Ballhausen and Gray [Citation28] with some modifications by Kuska and Yung [Citation29]. The complexes exhibit three-spin allowed transitions in the 12,500 − 13,500, 15,500 − 17,000 and 23,500-24,500 cm− 1 regions and have been assigned to 2B2 → 2E, 2B2 → 2B1 and 2B2 → 2A1 transitions, respectively [Citation27]. From the above discussion suggested octahedral structure of the complexes are shown in .
Bactericidal activity
Coordination compounds have been studied for their antitumour [Citation30], antiviral [Citation31], and antimalerial activity [Citation32], which has been related to the ability of metal ions to form stable complexes [Citation33]. The results have led to an understanding of the coordination sphere and the electronic properties of the metal ions and the factors such as chelate formation, ring size, number of aromatic rings, and the presence of amino groups, which modify the coordination sphere. Several workers have reported that heterocyclic rings containing sulfur, nitrogen, and/or oxygen are responsible for the biological activity of the Schiff bases and their metal complexes [Citation34]. Furthermore, it has been demonstrated that chelation in these compounds, to a large extent, is also responsible for such activity [Citation35].
The bactericidal activity of the ligands, vanadyl sulphate, tetracycline and its complexes were tested against various gram-negative bacterial cultures namely S.Typhi, E.Coli and Serratia marcescens using the Disc Diffusion Method [Citation36].
All the results show that the compounds are more bactericidal than their parent ligands and vanadyl sulphate against the same bacteria and under the same experimental conditions. The increase in bactericidal activity of the complexes may be due to the effect of the vanadium ion on the normal cell process. An acceptable reason for this increase in bactericidal activity may be considered in the light of Overtone's concept [Citation37] and Tweedy's chelation theory [Citation38]. According to Overtone's concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid-soluble materials so that liposolubility is an important factor which controls bactericidal activity. On chelation, the polarity of the vanadium ion will be reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the vanadium ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelate ring and enhances the lipophilicity of the complexes. This increased lipophilicity enhances the penetration of the complexes into lipid membranes and blocks the metal binding sites in bacterial enzymes. These complexes also disturb the respiratory processes of the cell and thus block the synthesis of proteins which restricts further growth of the organism. Furthermore, the mode of action of the compounds may involve the formation of a hydrogen bond through the azomethine group with the active centre of cell constituents, resulting in interference with normal cell processing [Citation39]. Although there is a sufficient increase in the bactericidal activity of the complexes as compared to the free ligands, metal salts and the control (DMSO), the complexes show moderate activities as compared to the standard drug (tetracycline).
Acknowledgements
We gratefully acknowledge Prof. R. M. Patel, Head, Department of Chemistry and Prof. M. Dutta, Head, Department of Biosciences, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India for providing the necessary laboratory facilities.
References
- Monga V, Thompson KH, Yuen VG, Sharma V, Patrick BO, McNeill JH, Orving C. Vanadium complexes with mixed O, S anionic ligands derived from maltol: Synthesis, characterization, and biological studies. Inorg Chem 2005; 44: 2678–2688
- Crans DC, Smee JJ, Gaidamauskas E, Yang L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 2004; 109: 849
- Buhl M. Molecular dynamics of a vanadate-dipeptide complex in aqueous solution. Inorg Chem 2005; 44: 6277–6283
- Panchal PK, Parekh HM, Patel MN. Synthesis and antifungal activity of oxovanadium(IV) complexes with Schiff bases. Pharm Chem J, [In Press]
- Panchal PK, Parekh HM, Patel MN. Preparation, characterization and toxic activity of some oxovanadium(IV) mixed-ligand complexes. Toxicol and Env Chem, [In Press]
- Durai N, Saminathan G. J Clin Biochem Nutr 1997; 2231
- Correia I, Costa Pessoa J, Duarte MT, Henriques RT, Piedade MFM, Veiros LF, Jakusch T, Dörnyrl Á, Kiss T, Castro MMCA, Geraldes CFGC, Avecilla F. Chem Eur J 2004; 10: 2301, N, N′-Ethylenebis (pyridoxylideneiminato) and N, N′-Ethylenebis(pyridoxylaminato) : Synthesis, Characterization, Potentiometric, Spectroscopic, and DFT Studies of Their Vanadium(IV) and Vanadium(V) Complexes.
- Hassan M, Chohan ZH, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Schiff's bases of aromatic and heterocyclic sulfonamides and their metal complexes. J Enz Inhib Med Chem 2004; 19: 263
- Chohan ZH, Supuran CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. J Enz Inhib Med Chem 2004; 20: 303
- Majithiya JB, Balaraman R, Giridhar R, Yadav MR. Effect of bis[curcumino]oxovanadium complex on non-diabetic and streptozotocin-induced diabetic rats. J Trace Element Med Biol 2005; 18: 211–217
- Vanadium and its role in life. Metal ions in biological systems, H Sigel, A Sigel. Marcel Dekker, New York 1995; 31, Chap. 1
- Vanadium compounds. Chemistry, biochemistry and therapeutic applications, AS Tracey, DC Crans. American Chemical Society, Washington, DC 1998
- Chohan ZH, Hassan M, Khan KM, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic properties of sulfonamide derived Schiff's bases and their metal complexes. J Enz Inhib Med Chem 2005; 20: 183
- Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enz Inhib Med Chem 2005; 20: 333
- Furniss B, Hannaford AJ, Smith PWG, Tatchell AR. Vogel's practical organic chemistry5th ed. Pearson Education Pte. Ltd, Singapore 2004; 395
- Dholakiya PP, Patel MN. Preparation, characterization and antibacterial activities of some mixed-ligand complexes of Mn(II), Co(II), Ni(II), Cu(II) and Cd(II) with monobasic bidentate (ON) Schiff base and neutral bidentate (NN) ligands. Synth React Inorg Met-Org Chem 2004; 34: 383–395
- Zamian JR, Dockal ER, Castellano G, Olive G. Synthesis and characterization of [N,N'-ethylenebis(3-ethoxysalideneaminato)]oxovanadium(IV). Polyhedron 1995; 14: 2411–2418
- Singh MS, Singh AK, Tawade K. N-benzoin-o-mercaptoaniline as an efficient synthon for organotin(IV) compounds. Synth React Inorg Met-Org Chem 2002; 32: 639–648
- Tarafder MTH, Miah MAJ, Bose RN, Ali AM. Metal complexes of some Schiff bases derived from s-benzyldithio-carbazate. J Inorg Nucl Chem 1981; 43: 1351
- Singh NK, Kushawaha SK, Srivastava A, Sodhi A. Synthesis, characterization and biological studies on some bivalent and trivalent transition metal complexes of N-benzony-N'-thiobenzhydrazide. Synth React Inorg Met-Org Chem 2002; 32: 1743–1758
- Felicio RC, Da Silva A, Ceridoroo LF, Dockal ER. Tetradentate Schiff base Cu(II) complexes. Synth React Inorg Met-Org Chem 1999; 29: 171–192
- Viswanathamurthi P, Dharmaraj N, Natarajan K. Rutherium(III) complexes containing monofunctional bidentate Schiff base. Synth React Inorg Met-Org Chem 2000; 30: 1273–1285
- Freedman HH. Interamolecular H-bands. I.A. Spectroscopic study of the hydrogen bond between hydroxyl and nitrogen. J Am Chem Soc 1961; 83: 2900–2905
- Çukurovali A, Yilmaz İ, Ahmetzade M. Synthesis and characterization of a new cyclobutane-substituted Schiff base ligand and its Co(II). Cu(II) and Ni(II) complexes. Synth React Inorg Met-Org Chem 2000; 30: 843–853
- Chohan ZH, Pervez HH. Studies on biologically active complexes of Ni(II), Cu(II) and Zn(II) with tridentate NNO, NNS and NNN donor pyrazine derived ligands. Synth React Inorg Met-Org Chem 1993; 23: 1061–1071
- Gaber M, Issia RM, Aly FA, Ayad MI. Studies of Ti(IV) and Zr(IV) chelates with N2O2 Schiff bases of 2-hydroxy − 1-naphthaldehyde with aromatic diamines. Thermochim Acta 1989; 155: 309–316
- Dutta SK, Edward, Tiekink AT, Chaudhury M. Mono- and dinuclear oxovanadium(IV) compounds containing VO(ONS) basic core: Synthesis, structure and spectroscopic properties. Polyhedron 1997; 16: 1863–1871
- Ballhausen CJ, Gray HB. The electronic structure of the vanadyl ion. Inorg Chem 1962; 1: 111–122
- Kuska HA, Yang P. Studies of bis(tetramethylurea)dichloro-oxovanadium(IV). Inorg Chem 1974; 13: 1090–1094
- Anthroline WE, Knight JM, Petering DH. Some properties of copper and zinc complexes of 2-formylpyridinethiosemicarbazone. Inorg Chem 1977; 16: 569–574
- Jones DH, Slack R, Squires S, Wooldrige KRH. Antiviral chemotherapy. I. Derivatives against neutrovaccinia in mice. J Med Chem 1965; 8: 676–680
- Klayman DL, Sconill JP, Bafosevich JF, Bruce J. 2-acetylpyridinethiosemicarbazones. 5. 1-[1-(2-pyridyl)ethyl]3-thio-semicarbazides as potential antimalarial agents. J Med Chem 1983; 26: 35–39
- Agrawal KC, Santorellli AC. Relationship between structure and antineoplastic activity of arylsulphonylhydrazones of 2-formylpyridine N-oxide. J Med Chem 1978; 21: 218–221
- Sharma RC, Trpathi SP, Sharma RS. Biologically active mixed-ligand complexes of rare earths. Curr Sci 1981; 50: 748–750
- Campbel MJM. Transition metal complexes of thiosemicarbazide and thiosemicarbazones. Coord Chem Rev 1975; 15: 279–320
- Stokes EJ, Ridgway GL. Clinical Bacteriology5th ed. Edward Arnold Publisher, Baltimore, Maryland; USA 1980; 205
- Anjaneyula Y, Rao RP. Preparation, characterization and antimicrobial activity study on some ternary complexes of Cu(II) with acetyl acetone and various salicylic acids. Synth React Inorg Met-Org Chem 1986; 16: 257
- Tweedy BG. Phytopathology 1964; 55: 910
- Dharmaraj N, Viswanathamurthi P, Natarajan K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Trans Met Chem 2001; 26: 105