Abstract
The in vitro antibacterial and antifungal activities of the compounds synthesised from some 1,2,3,5-tetrahalogeno benzenes in presence of sodium piperidide and sodium pyrrolidide (2,6-dipiperidino-1,4-dihalogenobenzenes; 2,6-dipyrrolidino-1,4-dibromobenzene; 2,4,6-tripyrrolidino chlorobenzene; and 1,3-dipyrrolidino benzene) were investigated. The in vitro antimicrobial activities were screened against the standard strains: Staphylococcus aureus ATCC 25923 and Bacillus subtilis ATCC 6633 as Gram positive, Yersinia enterocolitica ATCC 1501, Escherichia coli ATCC 11230 and Klebsiella pneumoniae as Gram negative, and Candida albicans as yeast-like fungus. Compounds (3, 5, 6, 7) inhibited the growth of all the test strains at MIC values of 32–512 μg/ml. None of the four compounds (1, 2, 4, 8) studied showed antimicrobial activity against any of the test strains within the MIC range 0.25–512 μg/ml.
Introduction
Piperidine, an alicyclic base is used as a raw material for pharmaceuticals, agrochemicals, rubber chemicals, surface active agents and other organic chemicals. Pyrrolidine, a similar base found in tobacco leaves with a strong piperidine-like odor provides derivatives used in pharmaceuticals, especially as modifiers of quinolone antibacterial agents [Citation1,Citation2]. Numerous biological compounds possess pyrrolidine and piperidine rings in their framework, and some of them are pharmaceutically important [Citation3,Citation4]. In the last several decades, halogenated aromatic hydrocarbons have been used extensively as intermediates in the chemical industry and agriculture. Some of these chemicals are carcinogenic and have deleterious effects on organisms [Citation5,Citation6].
Pharmaceuticals have a history of success in controlling morbidity and mortality caused by infectious diseases but as a consequence of frequent use, bacterial resistance to known classes of antibiotics has become a severe global problem in recent years [Citation7,Citation8]. There are serious concerns that untreatable pathogens may develop at an alarming rate in the near future. In this respect, we screened the antimicrobial activities of some piperidine and pyrrolidine substituted halogenobenzene derivatives.
Materials and methods
Synthesis
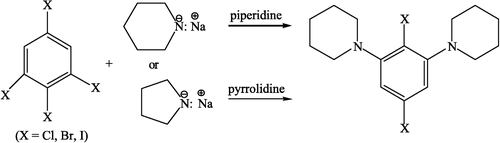
The tetrasubstituted benzene derivatives were prepared using well-known procedures [Citation9]. Tetrasubstituted benzene derivatives, 1-chloro (or bromo or iodo)-2,4,6-trichloro, 1-chloro (or bromo or iodo)-2,4,6-tribromo, and 1-chloro (or bromo or iodo)-2,4,6-triiodo benzenes were synthesized as reactants. 2,4,6-trichloro, tribromo and triiodo anilines were used as the starting material and the procedures were carried out according to the methods given in literature. In nucleophilic substitution experiments, the sodium salts of piperidine and pyrrolidine were prepared with sodium amide and the reactions of these derivatives were successful [Citation9].
Microbiological assays
Minimum inhibitory concentrations (MICs) were determined by broth microdilution following the procedures recommended by the National Committee for Clinical Laboratory Standards [Citation10,Citation11]. All the compounds were tested for their in vitro growth inhibitory activity against Staphylococcus aureus ATCC 25923 and Bacillus subtilis ATCC 6633 as Gram positive bacteria, Yersinia enterocolitica ATCC 1501, Escherichia coli ATCC 11230 and Klebsiella pneumoniae as Gram negative bacteria and Candida albicans as a yeast-like fungus. K. pneumoniae and C. albicans were obtained from the Department of Microbiology, Faculty of Medicine, Abant Izzet Baysal University, Turkey.
All tests were performed in Mueller-Hinton Broth (MHB). Bacterial strains were cultured overnight at 37°C in Brain Heart Infusion broth (BHI) and the yeast was cultured overnight at 30°C in Sabouraud Dextrose Broth (SDB). Test strains were suspended in MHB to give a final density of 5 × 105 cfu/ ml. The compounds under test were dissolved in 100% DMSO and the final twofold dilutions gave concentrations from 1–512 μg/ml. Ampicillin and fluconazole were used as reference antibiotics for the bacteria and fungus, respectively 0.25–512 μg/ml. Microtiter plates were incubated for 18–24 h at 37°C for the bacterial tests and for the yeast-like fungus, MICs were determined after incubation for 48 h at 30°C. The lowest concentration of the compounds that prevented visible growth was considered to be the minimal inhibitory concentration (MIC). MIC values for the compounds and the standards are presented in .
Results and discussion
Synthesis
The compounds synthesised were 2,6-Dipiperidino-4-chlorobromobenzene (1); 2,6-Dipiperidino-4-chloroiodobenzene (2); 2,6-Dipiperidino-4-bromochlorobenzene (3); 2,6-Dipiperidino-1,4-dibromobenzene (4); 2,6-Dipiperidino-4-bromoiodobenzene (5); 2,4,6-Tripyrrolidinochlorobenzene (6); 2,6-Dipyrrolidino-1,4-dibromobenzene (7); 1,3-Dipyrrolidinobenzene (8) [Citation9] (Scheme ). These compounds were prepared from sodium piperidide and sodium pyrrolidide and 2,4,6-trichloro-, tribromo- and triiodoanilines according to the literature methods [Citation9]. The structures of all the tetrasubstituted benzene derivatives are given in and were confirmed by IR, NMR (100 M Hz spectrometer) and elemental analysis ().
Table I. IR spectral data for the compounds.
Table II. NMR spectral data of the Dipiperidino- and Pyrrolidinobenzenes.
Table III. Physical and chemical data for the compounds.
Antimicrobial activity
The in vitro antibacterial screening of the compounds against S. aureus, B. subtilis, Y. enterocolitica, E. coli and K. pneumoniae and the antifungal screening against C. albicans was carried out by the minimum inhibitory concentration method [Citation10,Citation11]. The antimicrobial activities (MIC values) are given in , which also contains those for ampicillin and flucanozole used as reference compounds as comparism and a check on the reliability of the method used.
Table IV. The MICs (μg/ml) of the tested compounds.
In this study, eight derivatives were tested against standard strains of gram-positive bacteria, gram-negative bacteria and fungus. Compounds 3, 5, 6 and 7 inhibited the growth of all microorganisms, include fungus, with MICs 32–512 μg/ml (). Among the tested compounds, 5, 6 and 7 showed good antifungal activity against C. albicans with MICs 32–64 μg/ml, which was comparable with that for fluconazole (128 μg/ml). Four compounds (1, 2, 4, 8) did not show antimicrobial activity against any of the test strains within the range MICs 0.25–512 μg/ml. The inhibitory compounds had the highest antimicrobial activities against S. aureus and C. albicans with MICs of 32–128 μg/ml, but poor activity of the compounds was observed against gram-negative bacteria such as Y. enterocolitica ATCC 1501, E. coli ATCC 11230 and K. pneumoniae, which are highly pathogenic to humans. Indeed, the bacterium K. pneumoniae showed lower microbial susceptibility compared to the other tested microorganisms. The compounds tested here generally show lower antimicrobial activity against gram-negative bacteria. The antimicrobial actitivity against gram-positive bacteria and fungus may depend on the differences between the cell structures of these microorganisms.
The results () show that 1,3-dipyrrolidinobenzene (8) had no activity against any of the test microorganisms so we can say that the bioactivities of compounds 6 and 7 (pyrrolidine derivatives) are mainly correlated with the halogen substituents. Here the bromo, chloro and pyrrolidino substituents in combination showed inhibitory activity against the microorganisms (). However, the results of the antimicrobial tests with compounds 1–5 indicated that the type and the position of the halogen and piperidine tetrasubstituents had a significantly different effects on the growth of microorganisms. In conclusion, we report that some of the compounds (piperidine and pyrrolidine substituted halogenobenzene derivatives) had antimicrobial activity against some hazardous microbes.
References
- Babalola GO. Antibacterial activity of synthetic N-heterocyclic oxidizing compounds. Lett App Microbiol 1998; 26: 43–46
- Higashio Y, Shoji T. Heterocyclic compounds such as pyrrole, pyridines, pyrrolidine, piperidine, indole, imidazol and pyrazines. App Catalysis A: Gen 2004; 260: 251–259
- Huryn DM. Comprehensive Organic Synthesis, BM Trost, I Fleming. Pergamon. 1991; 64–71
- Shikai Z, Jeremiah PF, Bacon CL, Fox CB, O'Driscoll E, Foley AG, Kelly J, Farrell U, Regan C, Mizsak SA, Szmuszkovicz J. Syntheses of 1,2 diamino and 1,2 aminoalcohol derivatives in the piperidine and pyrrolidine series as anti-amnesic agents. Bioorgan Med Chem 1999; 7: 1647–1654
- Han SK, Jiang LQ, Wang LS, Zhang Z. Hydrolysis of henylthio, phenylsulfinyl and phenylsulfonyl acetates, and neighboring group effect. Chemosphere 1992; 25: 643–649
- He YB, Wang LS, Han SK, Zhao YH, Zhang Z, Zou GW. Determination and estimation of physicochemical properties for phenylsulfonyl acetates. Chemosphere 1995; 30: 117–125
- Gold HS, Moellering RC. Drug therapy: Antimicrobial-drug resistance. N Eng J Med 1996; 335: 1445–1453
- Finch R. Bacterial resistance-the clinical challenge. Clin Microbiol Inf 2002; 8(s3)21–32
- Tüzün C, Öktemer A. Nucleophilic substitution reactions of some tetrahalogeno benzenes, Part II. Tome 25 1979. p 61–71
- NCCLS (National Committee for Clinical Laboratory Standards). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard, M7- A4, Wayne, Pa 1997.
- NCCLS (National Committee for Clinical Laboratory Standards). Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard, M27- A, Wayne, Pa 1997.