Abstract
Fatty acid synthase (FAS) has been identified as a potential antitumor target. The extract from the leaves of Acer truncatum Bunge (Extr) was prepared to assay its inhibitory activity against FAS, which was isolated from duck liver, and the correlated antitumor bioactivity. Its inhibition of FAS is composed of reversible fast-binding inhibition, IC50 = 0.7 μg/ml, and irreversible slow-binding inhibition following saturation kinetics with a dissociation constant of 0.68 μg/ml and a limiting rate constant of 0.0288 min− 1. The Extr exhibited different type of inhibitions against the three substrates in the FAS overall reaction. Compared with EGCG in inhibition constant and IC50 value, the Extr appeared to be a more efficient inhibitor, and exhibited a considerable inhibition against the growth of four kinds of cancer cells (patent application number 200510068054.2). It was infered that the inhibitory activity is likely attributable to the co-operative effect of the components.
Introduction
Animal fatty acid synthase (EC 2.3.1.85, FAS) comprises two identical, 260–270 kDa subunits juxtaposed head to tail, each containing an acyl carrier protein and seven enzymatic active sites, including acetyl transacylase, malonyl transacylase, β-ketoacyl synthase, β-ketoacyl reductase, β-hydroxyacyl dehydratase, enoyl reductase, and thioesterase [Citation1]. Animal FAS catalyses the de novo synthesis of fatty acids from acetyl-CoA and malonyl-CoA in the presence of NADPH through the reaction which elongates the acetyl group by C2 units derived from malonyl-CoA in a stepwise and sequential manner [Citation2]. The overall reaction catalyzed by the animal FAS can be represented by the following equation:
The amino acid sequence of the human FAS has 79% and 63% identity with those of the rat and fowl enzymes, respectively [Citation3]. Most information about FAS is derived from non-human animal studies. Traditional fowl FAS is a good model for the kinetic study of animal FAS.
FAS is an important lipogenic enzyme participating in energy metabolism in vivo and is related to various human diseases such as cancer and obesity [Citation4]. Human cancer cells express high levels of FAS Citation5-10. It has been reported that FAS inhibitors are selectively cytotoxic to many kinds of human cancer cells Citation11-14. On the basis of much research, FAS has been suggested as a potential new therapeutic target for the treatment of cancer Citation15-17. Inhibitors of FAS such as cerulenin and synthesized C75 are known for their effects on some human cancers [Citation18]. However, efficient FAS inhibitors, especially prepared from natural plants, are still scarce and novel [Citation19]. So it is significant to identify new inhibitors and investigate their inhibition mechanism in vitro and in vivo.
Acer truncatum Bunge is a local species of Acer in China. It is a prominent species in the hardwood forests of north China. It is horticulturally important and widely planted for the brilliant autumn colours of its leaves. It has been used as a Chinese folk medicine for the treatment of coronary artery cirrhosis, cerebrovascular diseases and angina pectoris [Citation20]. The extract (Extr) of the leaves of Acer truncatum Bunge shows high oxidation resistance [Citation21]. It contains tannin [Citation22], chlorogenic acid and flavonoids [Citation23]. However, few enzyme-inhibitor investigations concerning this plant have been reported. In this paper, we report that the EtOAc extract from the fallen leaves collected from Beijing, China has FAS inhibitory activity and our investigation of its inhibition kinetics and antitumor activity.
Materials and methods
Preparation and activity assay of FAS
The FAS from duck liver was purified with an improved method [Citation24] described previously by Soulie et al. (1984). The preparation was homogeneous on polyacrylamide gel electrophoresis in the presence and absence of SDS. FAS with a specific activity of 150 U/mg was stored in 0.1 mol/L potassium phosphate buffer of pH7.0 containing 10 mmol/L DTT and 1.0 mmol/L EDTA, and then during all the following experiments, 1.0 mmol/L DTT and 1.0 mmol/L EDTA were added to the reaction solutions to protect the essential thiol groups of FAS and to remove metal ions which could inactivate FAS. Acetyl-CoA and malonyl-CoA were purchased from Sigma and NADPH was from Fluka. All other reagents were local products of analytical grade.
The assay for FAS activity which was described previously [Citation25] was performed with a Shimadzu UV2550 double wavelength/double beam spectrophotometer at 37°C by following the decrease of NADPH at 340 nm. The reaction system contained 0.1 mol/L potassium phosphate buffer, pH 7.0; 1.0 mmol/L EDTA; 1.0 mmol/L DTT; 3 μmol/L acetyl-CoA; 10 μmol/L malonyl-CoA; 32 μmol/L NADPH and duck liver FAS 10 μg in a total volume of 2.0 ml. Fast-binding inhibition was determined by adding the inhibitor (Extr) to the reaction system before FAS initiated the reaction. After the enzyme solutions were mixed with various concentrations of Extr, aliquots were taken to measure the remaining activity at the indicated time intervals to measure slow-binding inactivation.
Centrichromatography was carried out as follows. The enzyme solutions were mixed with the inhibitors and aliquots were added to Sephedex G25 column (0.7 × 8.0 cm) and centrifuged at the indicated time interval. The same enzyme solution without inhibitors was used to centrifuge as a control.
Preparation of the extracts from fallen leaves of Acer truncatum Bunge
The fallen leaves of Acer truncatum Bunge were collected from Baiwang mountain of Beijing in November 2004, and were identified by Professor Chen Yuting of Beijing University of Traditional Chinese Medicine. After appropriate treatment, the cleaned and dried leaves were cut into small pieces and mixed with 70% ethanol in the ratio of 1 to 20 (w/v) followed by magnetic stirring for 24 h at room temperature to afford a dark-red concentrated solution on removal of ethanol under reduced pressure which was then mixed with an equal volume of petroleum ether to extract and remove lipids. The extraction was repeated three times to give a residual solution free of lipid which was then extracted in turn with an equal volume of EtOAc three times. The pooled EtOAc extract was evaporated under reduced pressure to yield a brown residue which was further dried in a vacuum at < 50°C.
Measurement of inhibition of FAS
Inhibition of FAS by different concentrations of inhibitor solution was measured and repeated three times. IC50 values were obtained from the dose-response curves for the inhibitors. The time course of inactivation was determined by taking aliquots to measure the residual activity at the indicated time intervals after the enzyme solution was mixed with the inhibitors. The time-dependent inhibition course is often an irreversible process with formation of a covalent bond between the inhibitor and enzyme known as slow-binding inactivation. The system without inhibitor was used as a control in these experiments. FAS activity in the control remains unchanged in 4 h. The apparent first-order inactivation rate constant, kobs, was obtained from the semilogarithmic plot of the inactivation time course.
Experiments with human cancer cell lines
The effects of Acer truncatum Bunge extract on cell growth were investigated using the human liver cancer cell line (BEL-7402), human esophageal cancer cell (CAES-17), human breast cancer cell (MCF-7) and gastric cancer cell line (BGC-823) purchased from the Institute of Medicament Research of Chinese Academy of Medical Sciences.
The growth inhibition assay used various kinds of cancer cell line. The above various kinds of cancer cells were seeded in RPMI-1640 containing 10% fetal bovine serum, 100 μg/mL penicillin, and 100 μg/mL streptomysin on a 96-well plate with 2 × 105 cells in each well and incubated at 37°C for 24 h in a CO2 incubator. Then the extract dissolved in dimethylsulfoxide at a preset concentration was added to the cell culture and the mixture incubated for an additional 24 h. Cell viability was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetra-zolium bromide] assay [Citation26]. The inhibition rate at each Extr concentration is an average value of three independent experimental results.
Results
Fast-binding inhibition of FAS by Extr
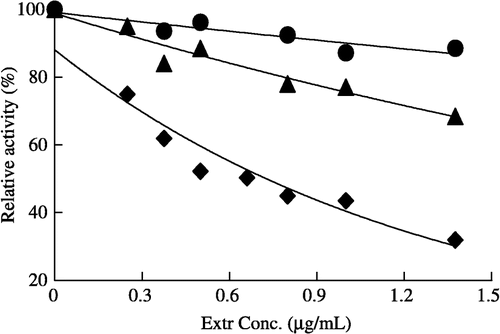
The catalyzed reaction and fast-binding inhibition occurred simultaneously during the assay of enzyme activity in the presence of Extr (). As is shown in , compared to the considerable fast-binding inhibition of the overall reaction, the inhibition against enoyl reduction reaction was not obvious, but the ketoacyl reduction reaction appeared to be inhibited with further increasing Extr. The IC50 values of Extr for the FAS overall reaction and the ketoacyl reduction reaction are 0.7, and 16 μg/ml, respectively.
Figure 1 Fast-binding inhibition of FAS by different Extr concentrations, the overall reaction (♦), ketoacyl reduction reaction (▴), and enoyl reduction reaction (•) in the presence of Extr. The reaction system contains 0.1 mol/L potassium phosphate buffer, pH7.0; 1.0 mmol/L EDTA; 1.0 mmol/L DTT; 3 μmol/L acetyl-CoA; 10 μmol/L malonyl-CoA; 32 μmol/L NADPH and FAS 20 μg in a total volume of 2.0 ml, 37°C, by following the decease of NADPH at 340 nm within 1.5 min.
Comparison of inhibitory effects of the Extr with catechins on FAS
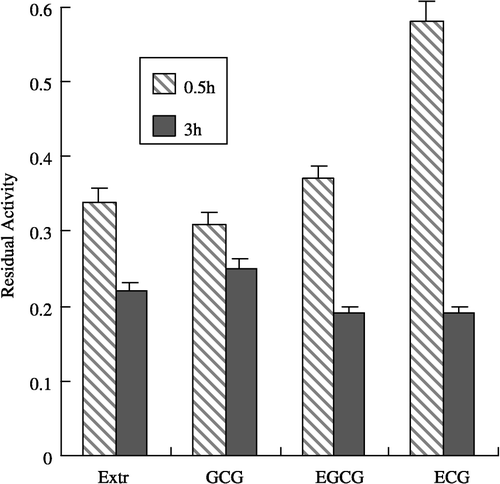
It is known that gallated catechins such as EGCG, ECG have obvious inhibitory effects on FAS and these have been studied extensively Citation27-29. The inhibition of FAS by EGCG was measured and compared with that by the extract of leaves at the same concentration in the reaction system (). FAS activity did not change in the control sample. After preincubation with FAS for 0.5 h, EGCG, ECG and GCG, as well as the extract of leaves showed noticeable inhibitory effects. Besides GCG, the extract of leaves exhibited more inhibition against FAS, which was indicated by the residual activity of FAS following incubation. The residual activity of FAS for the extract of leaves, GCG, EGCG, and ECG (structures shown in ) were 34, 31, 37, and 58%, respectively, while with the incubation prolonged for 3.0 h, the residual activity of FAS for the 4 species mentioned above were 22, 25, 19, and 19%, respectively ().
Figure 2 Comparison of the inhibitory effects of Extr and gallated catechins on FAS. The residual activity of FAS were measured after the enzyme was incubated with the inhibitors for 0.5 and 3.0 h, repectively. The concentrations of Extr, GCG, EGCG, and ECG in the incubation system are 1.3, 1.25,1.25, and 1.25 μg/mL, respectively. The FAS concentration was 1.9 μM. The experiment results are expressed as mean ± SD values.
Reversibility and the type of inhibition by Extr
The time courses of both the overall reaction and ketoacyl reduction reaction, as well as enoyl reduction at a concentration of 0.5 μg/ml of the extract of leaves as an inhibitor are shown in . The time course of the overall reaction of FAS underwent two processes; that in the first three minutes where FAS activity rapidly decreased by 40%, which is attributed to the fast and reversible binding of Extr to the enzyme, whereas the further decrease in FAS activity was an irreversible inactivation due to the slow-binding of Extr, and exhibited a time-dependency. The plot of logarithm of residual activity versus time (insert of ) exhibited the two parts more clearly. The plot appeared initially sharply concave, but later became linear versus time as indicated by data points in the insert of . The inhibited FAS recovered considerably after centrichromatography, whereas after a relatively long period the inactivated enzyme could not reactivate which is consistent with the definition of slow-binding inhibition [Citation30]. This indicates that the initial inhibition is reversible, probably due to a fast binding of Extr to the enzyme.
Figure 4 Kinetic time course of inhibition of the overall reaction (•) and ketoacyl reduction reaction (♦) in the presence of Extr. The insert is plot of ln R.A. (relative activity) versus time calculated from data. The FAS solution (0.6 μM) was mixed with Extr (0.5 μg/mL) and aliquots were taken to assay remaining activity at the indicated time intervals.
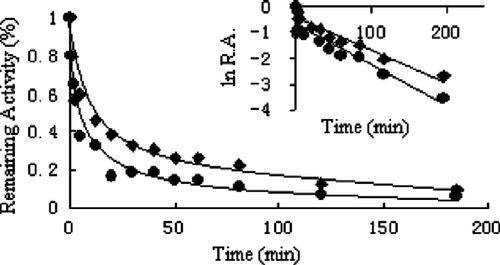
The semilogarithmic plot of relative activity (ln R.A.) versus time gave the irreversible inactivation rate constants, kobs, which was equal to the fitted slopes of the curves (see insert of ). kobs was 0.0132 min− 1 for inactivation of the overall reaction and 0.0129 min− 1 for inactivation of the ketoacyl reduction reaction. We have examined the dependence of the inactivation rate constant (kobs) on the Extr concentration as an inhibitor in the range 0.14–1.2 μg/ml. The plot of kobs value versus Extr concentration shown in is hyperbolic, demonstrating a two step reaction sequence for affinity labeling which may be explained as follows. Firstly, Extr as an inhibitor reversibly associated with the enzyme; the resulting an enzyme-inhibitor complex (E-I) then underwent an irreversible chemical modification,
where k is the first order rate constant at infinite inhibitor concentration, and Ks is the apparent half-saturation constant, the dissociation constant of E-I; when k is rate-limiting, as is likely in this case. EI is inactive. The reciprocal of l/kobs vs l/[Extr] shown in was linear and fitted to the equation,
Figure 5 Effect on the apparent inactivation rate constant, kobs, with increasing Extr concentration. The concentration of FAS in the inactivation system was 2.1 μM (A) dependence of the observed first order inactivation rate constant (kobs) on Extr concentration (B) plot of the reciprocal of kobs versus the reciprocal of Extr, as an inhibitor, concentration.
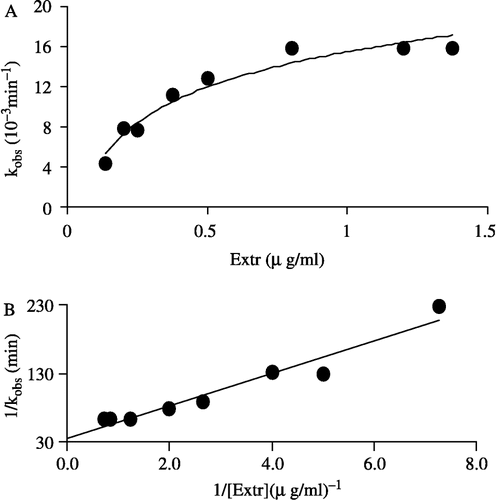
Ks value of 0.68 μg/ml and k of 0.0288 min− l were calculated from .
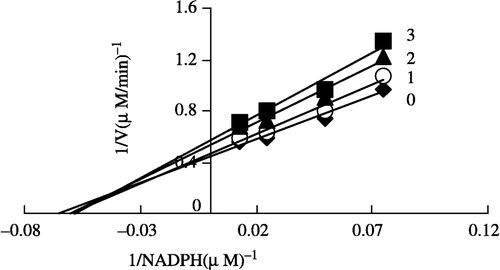
In order to explore the effects on the overall reaction and the ketoacyl reduction, the inhibition type for the Extr and the relevant kinetic parameters were estimated. FAS activities for the ketoacyl reduction and the overall reaction were studied in the presence of fixed but different Extr concentrations and NADPH, Malonyl-CoA, and Acetyl-CoA as the variable substrates, respectively (see ). In the overall reaction the Lineweaver-Burk plots show that Extr is a competitive inhibitor of malonyl-CoA (), whereas it exhibited an uncompetitive inhibition against NADPH (), but for acetyl-CoA Extr appeared to be a mixed type of inhibitor (), (). Extr may compete for the same binding site with malonyl-CoA. A Lineweaver-Burk plot of the inhibition of the β-ketoacyl reduction of FAS by Extr showed a mixed type of inhibition against NADPH as the variable substrate ().
Figure 6 Lineweaver-Burk plot of the inhibition of overall reaction of FAS by Extr. The concentration of Extr in the reaction system was 0(0), 0.25 μg/mL (1), 0.5 μg/mL (2) and 1.0 μg/mL (3). The FAS concentration was 0.012 μM, and the fixed concentrations of Malonyl-CoA and Acetyl-CoA were 10 and 2.5 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the overall reaction.
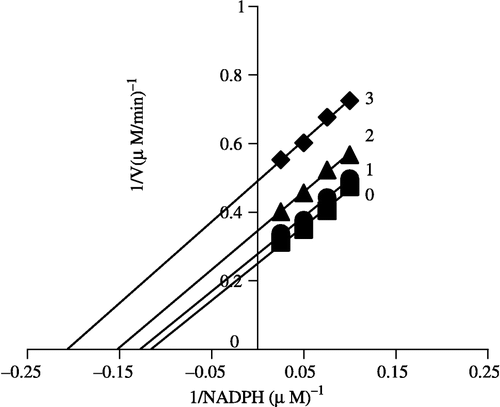
Figure 7 Lineweaver-Burk plot of the inhibition of overall reaction of FAS by Extr. The concentration of Extr in the reaction system was 0 (♦), 0.25 μg/mL (•), 0.5 μg/mL (▴), and 1.0 μg/mL (▪). The FAS concentration was 0.012 μM, and the fixed concentrations of NADPH and Acetyl-CoA were 32 and 2.5 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the overall reaction.
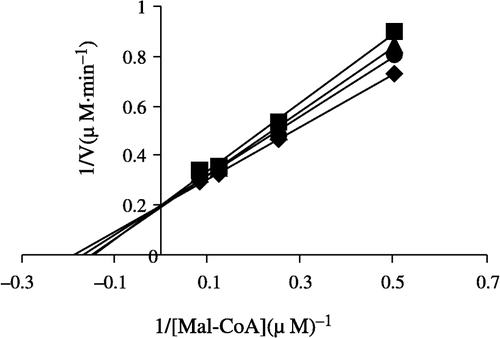
Figure 8 Lineweaver-Burk plot of the inhibition of the overall reaction of FAS by Extr. The concentration of Extr in the reaction system was 0(0), 0. 5 μg/mL (l), 1.0 μg/mL (2), and 1.25 μg/mL (3). The FAS concentration was 0.012 μM, and the fixed concentrations of NADPH and Malonyl-CoA were 32 and 10 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the overall reaction.
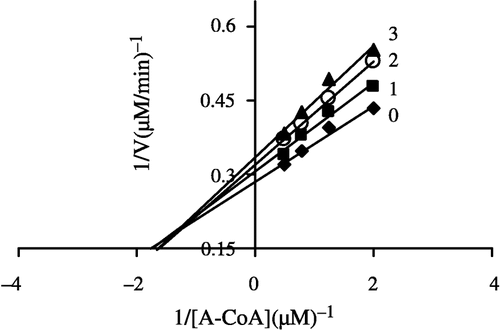
Table I. The inhibition type and inhibition constants for overall reaction of FAS with Extr and EGCG.
Figure 9 Lineweaver-Burk plot of the inhibition of β-ketoacyl reduction reaction of FAS by Extr. The fixed concentration of ethyl acetoacetate is 40 mM. The concentration of Extr in the reaction system was 0(0), 0. 25 μg/mL (1), 0.5 μg/mL (2), and 1.0 μg/mL (3). The FAS concentration was 0.012 μM, and the fixed concentrations of Acetyl-CoA and Malonyl-CoA were 2.5 and 10 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the ketoacyl reduction reaction.
Effects of Acer truncatum Bunge extract on growth of various cancer cell lines
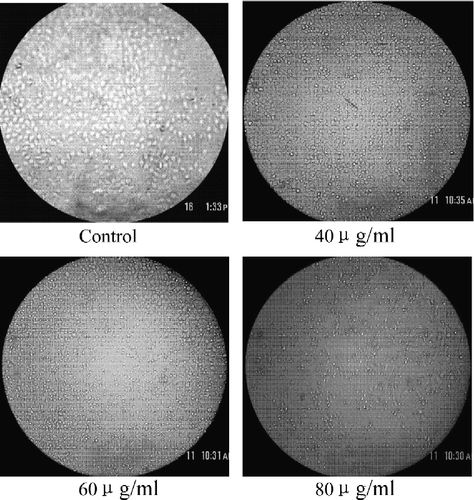
Effect of Acer truncatum Bunge extract on cancer cell growth were investigated using CAES-17, BGC-823, MCF-7, and BEL-7402 cells. Treatment with serial dilutions of Acer truncatum Bunge extract from 5 to 80 μg/ml exhibited a significant growth inhibition on the above four kinds of cells, and extent of inhibition exceeded 50% when the extract concentration reached 60 μg/ml (). shows the inhibition of growth of BEL-7402 liver cancer cells by Extr.
Table II. The extent of inhibition of various cancer cells by Extr.
Discussions
In the present work we have shown that an extract from the leaves of Acer truncatum Bunge can inhibit the overall reaction and ketoacyl reduction of FAS effectively and significantly inhibit the growth cancer cell. This inhibition involves both reversible and irreversible inhibition. For the fast-binding inhibition, the IC50 values of Extr for both the overall reactions and ketoacyl reduction reaction of the FAS clearly demonstrate that Extr's inhibitory effect remarkably exceeds that of a gallated catechin like EGCG.
The Extr rapidly associates with FAS for fast-binding inhibition then the irreversibly inactivates FAS until the enzyme loses its activity completely. The inhibition by Extr exhibits saturation kinetics behavior typical of affinity labeling, but the chemically modified sites are unknown.
As summarized in , for the overall reaction of FAS there are different types of inhibition between Extr and three substrates (C + N, C, U, ). Extr shows an inhibition, effect on many steps of the FAS overall reaction, including acetyl transacylation, malonyl transacylation and β-ketoacyl reduction. The IC50 values of EGCG and ECG for FAS overall reaction are 24 and 18 μg/mL while for the ketoacyl reduction reaction their IC50 values are 46 and 30 μg/mL [Citation31], respectively, obviously larger than those of Extr (0.7 and 16 μg/ml).
As compared with EGCG in the values of the inhibition constants (ki), which were calculated by the secondary plots on the basis of and listed , Extr was demonstrated to have much a more potent inhibitory effect on FAS. Also, for the β-ketoacyl reduction reaction of FAS the ki value of 2.18 μg/mL of Extr is noticeably less than that of 21 μg/mL for EGCG, implying that Extr binds to the β-ketoacyl reductase of FAS more tightly and inhibits more efficiently. For the slow-binding inhibition, Extr has a comparable inhibitory effect with that of EGCG, and ECG (). Overall, Extr is a more efficient inhibitor of FAS.
Wang and Tian reported that EGCG acts on the binding site of NADPH to β-ketoacyl reductase, and is a specific inhibitor of ketoacyl reductase [Citation32]. The IC50 of Extr for the ketoacyl reduction reaction is 16 μg/mL but for the FAS overall reaction is only 0.7 μg/mL. This remarkable difference in IC50 values indicates that in contrast to EGCG, the inhibition of ketoacyl reduction is not the main reason for FAS inactivation. Inactivation of FAS could rather be attributed to the co-operative effect of the components of Extr on the intermediate step-reactions of FAS.
Xing-Cong Li et al. [Citation33] reported thirteen compounds isolated from plants and their FAS inhibitory activity. These compounds represent five chemotypes, i.e isoflavones, flavones, biflavonoids, hydrolyzable tannin-related derivatives, and triterpenoids. Two flavonoid compounds from these were the most potent against FAS with IC50 values of 2.3 and 7.5 μg/mL, respectively. Thin layer chromatography detection suggested that Extr comprises flavonoids, hydrolyzable tannin-related derivatives and triterpenoids [Citation34]. Considering the reported inhibitory activities of flavonoids and polyphenols [Citation33,Citation35], the remarkable FAS inhibitory activity of Extr is likely due to a co-operative effect of these components rather than a single one.
Overexpression of FAS is a common molecular feature in subsets of sex-steroid-related tumors [Citation17,Citation36] including endometrium and breast carcinomas, ovarian and prostate cancers. Pharmacological inhibition of tumour-associated FAS hyperactivity is under investigation as a chemotherapeutic target. FAS hyperactivity also appears in squamous cell carcinoma of the lung and intestinal metaplasia of the stomach [Citation37]. The above three kinds of cancer cells other than those of breast cancer are non sex-steroid-related, but were similarly inhibited by Extr (), which may be related to the inhibition of lipogenesis in these cells.
EGCG as the main component of green tea polyphenols is safe at the drinking dosage used. Recently, its biological function has become more evident in view of an inhibition of FAS [Citation27]. Its cancer preventative activity has been studied and possible mechanisms were also inrvestigated [Citation38,Citation39]. Brusselmans [Citation38] et al. investigated EGCG inhibition against FAS in cultured prostate cancer cells and how this inhibition affected endogenous lipid synthesis, cell proliferation and cell viability. The high levels of FAS activity in LNCaP cells were dose-dependently inhibited by EGCG and this inhibition was paralleled by decreased endogenous lipid synthesis, inhibition of cell growth and induction of apoptosis. In contrast, epicatechin (EC), another closely related green tea polyphenolic compound, which does not inhibit FAS, had no effect on LNCaP cell growth or viability. Treatment of nonmalignant cells having low levels of FAS activity (fibroblasts) with EGCG led to a decrease in the growth rate but not to induction of apoptosis. These data indicate that EGCG inhibits FAS activity as efficiently as presently known synthetic inhibitors and selectively causes apoptosis in LNCaP cells but not in non-tumoral fibroblasts. These findings establish EGCG as a potent natural inhibitor of FAS in intact cells and strengthens the molecular basis for the use of EGCG as a chemopreventive and therapeutic antineoplastic agent. Yeh et al. [Citation39] studied the suppression of FAS in MCF-7 breast cancer cells by tea polyphenols such as EGCG and found that it lead to the inhibition of cell lipogenesis and proliferation and that tea polyphenols might induce antiproliferative effects by suppressing FAS.
Acer truncatum Bunge leaves have been used as a Chinese folk medicine and the beverage prepared from its spring leaves have been drunk like tea for a long time, and no toxicity has been found, showing that the flavonoids and hydrolyzable tannin-related derivatives as the main components of the beverage are safe under a certain dosage. As a new natural source of FAS inhibitors and more efficient than EGCG, it would consequentially find a wide application in the field of health care, and furnish useful ideas and new clues to the development of a cancer preventive medicine.
Acknowledgements
The present investigation was supported by the Scientific Research Common Program of Beijing Municipal Commission of Education No. KM200410025013, China.
References
- Wakil SJ, Stoops JK, Joshi VC. Ann Rev Biochem 1983; 52: 537–553
- Wakil SJ. Biochemistry 1989; 28(11)4523–4530
- Jayakumar A, Tai MH, Huang WY, al-Feel W, Hsu M, Abu-Elheiga L, et al. Proc Natl Acad Sci USA 1995; 92(19)8695–8699
- Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, et al. Science 2000; 288(5475)2379–2381
- Swinnen JV, Roskams T, Joniau S, Van Poppel H, Oyen R, Baert L, Heyns W. Int J Cancer 2002; 98(1)19–22
- Wang Y, Kuhajda FP, Li JN, Pizer ES, Han WF, Sokoll LJ, et al. Cancer Lett 2001; 167(1)99–104
- Vlad LD, Axiotis CA, Merino MJ. Mod Pathol 1999; 12: 70–75
- Piyathilake CJ, Frost AR, Manne U. Hum Pathol 2000; 31(9)23–28
- Innocenzi D, Alo PL, Balzani A, Balzani A, Sebastiani V, Silipo V, et al. J Cutan Pathol 2003; 30(1)23–28
- Visca P, Sebastiani V, Pizer ES, Botti C, De Carli P, Filippi S, et al. Anticancer Res 2003; 23(1A)335–339
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA, et al. Proc Natl Acad Sci USA 2000; 97(7)3450–3454
- Pizer ES, Wood FD, Heine HS, Romantsev FE, Pastemack GR, Kuhajda FP, et al. Cancer Res 1996; 56(12)1189–1193
- Pizer ES, Chrest FJ, Digiuseppe JA, Han WF. Cancer Res 1998; 58(20)4611–4615
- Kuhajda FP. Nutrition 2000; 16(3)202–208
- Pizer ES, Thupari J, Han WF. Cancer Res 2000; 60(2)213–218
- Kuhajda FP, Pizer ES, Li JN. Proc Natl Acad Sci USA 2000; 97(7)3450–3454
- Menendez JA, Oza BP, Atlas E, Verma VA, Mehmi I, Lupu R. Oncogene 2004; 23(28)4945–4958
- Gabrielson EW, Pinn ML, Testa JR, Kuhajda FP. Clin Cancer Res 2001; 7(1)153–157
- Tian WX, Li LC, Wu XD, Chen CC. Life Sci 2004; 74(19)2389–2399
- Shi WSh, Lei X. Chinese Trad Pat Med 2003; 25(11)948–950
- Wei Q, Ma XH. Food Sci 1998; 19(8)3–6
- Wei XY, Zhang XY, Lu JX, Li YL, Wang XY. J Xi'An Med Univ 1995; 290(16)415–419
- Liu XY. Yunnan Chem Technol 2003; 30(1)23–26
- Tian WX, Wang YS, Hsu RY. Biochim Biophys Acta 1989; 998: 310–316
- Tian WX, Hsu RY, Wang YS. J Biol Chem 1985; 260(20)11375–11387
- Mosmann T. J Immunol Meth 1983; 65(l–2)55–63
- Zhang YM, Rock CO. J Biol Chem 2004; 279(30)994–1001
- Wang X, Song KSH, Guo QX, Tian WX. Biochem Pharmacol 2003; 66(10)2039–2047
- Du YT, Wang X, Wu XD, Tian WX. J Enz Inhib Med Chem 2005; 20(4)349–356
- Morrison JF, Walsh CT. Adv Enzymol Relat Areas Mol Biol 1988; 61: 201–301
- Zhang R, Xiao WP, Wang X, Wu XD, Tian WX. Biotech and Appl Biochem 2006; 43(1)1–7
- Wang X, Tian WX. Biochem Biophys Res Comm 2001; 288: 1200–1206
- Li XC, Joshi AS, Elsohly HN, Khan SI, Jacob MR. Zhang Zh. J Nat Prod 2002; 65(12)1909–1914
- Li YZh, Zeng FJ, Zeng L. Food and Ferment Ind 2004; 30(6)90–95
- Yeh CW, Chen WJ, Chiang CT, Lin-Shiau SY, Lin JK. Pharmacogenomics J 2003; 3(5)267–276
- Pizer ES, Pflug BR, Bova GS. Prostate 2001; 47(2)102–110
- Kusakabe T, Nashimoto A, Honma K, Suzuki T. Histopath 2002; 40(1)71–79
- Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Int J Cancer 2003; 106(6)856–862
- Yeh CW, Chen WJ, Chiang CT, Lin Shiau SY, Lin JK. Pharmacogenomics J 2003; 3(5)267–276