Abstract
The crude methanolic extract and various fractions of Andrachne cardifolia Muell, including chloroform, ethyl acetate and n-butanol fractions were subjected to in vitro enzyme inhibition activity against acetylcholinesterase, butyrylcholinesterase, lipoxygenase and urease enzymes. A significant enzyme inhibition activity (40–89%) was shown by the crude methanolic extract and its fractions against lipoxygenase, while low to significant activity (40–71%) against butyrylcholinesterase. The crude methanolic extract and its various fractions demonstrated poor to significant activity (25–73%) against acetylcholinesterase and no activity against urease.
Introduction
Acetylcholine (ACh) is a neurotransmitter, which is responsible for transporting signals in nerve cells. Acetylcholinesterase (AChE) hydrolyzes acetylcholine, and at a greater velocity than choline esters with acyl groups larger than acetate or propionate [Citation1]. Alzheimer's disease and cerebrovascular ischemia (vascular dementia) are the two most common causes of dementia. Sixty to 70% of individuals with dementia have Alzheimer's disease. Dementia causes a high burden of suffering for patients and their families. For patients, it increases dependency and complicates other medical conditions, and the annual economic cost of dementia is estimated to be $100 billion [Citation2]. The best clinical choice is a cholinesterase inhibitor because deficiency in cholinergic activity is responsible for memory impairment in patients with senile dementia of the Alzheimer's type [Citation3]. Additionally, it has been found that cholinesterase inhibitors stabilize cognitive symptoms of Alzheimer's disease, slow cognitive deterioration and improve behavioral and daily living conditions Citation4-7. Butyrylcholinesterase (BChE) is produced in the liver and enriched in the circulation. The exact physiological role of this enzyme is still elusive, but it is generally viewed as a back-up for the homologous AChE [Citation8], as it may be an effective tool for the treatment of AD and related dementias.
The unique role of the enzyme 5-lipoxygenase (5-LO) in the production of leukotrienes (LTs) makes it a likely target for biochemical manipulation. The arachidonic cascade involves three types of metabolic pathways; cyclo-oxygenase, lipoxygenase (LO), and cytochrome P450. The rationale for using 5-LO inhibitors for the treatment of inflammatory bowel disease (IBD) is based on the increased generation of LTs in the inflamed mucosa, LTB4 being the most potent chemotactic and chemokinetic metabolite of arachidonic acid [Citation9]. The products of the LO pathway participate in the pathogenesis of a variety of diseases such as allergy and hypertension [Citation10].
In herbal medicine, the genus Andrachne is used for the treatment of eye sores and to improve eyesight [Citation11], it also has a stimulating action on respiration and the blood pressure of the dog and cat and as well as spasmolytic activity on the tracheal muscle of the cat and anti-histaminic activity on guineapig illium [Citation12]. Interest in the phytochemical exploration of Andrachne cardifolia Muell began in 1983 when Ikram et al., isolated two bisbenzylisoquinoline alkaloids, cocsulin and penduline, from the roots of Andrachne cardifolia Muell [Citation13]. This achievement was followed by the isolation of a new triterpene alcohol, glut-5 (10)-en-1β-ol [Citation14] and a pentacyclic triterpenic ketone, Glut-5 (10)-en-one [Citation15] from the whole plant of Andrachne cardifolia Muell. Since then, though thoroughly deserved, this plant has not been extensively subjected to isolation and characterization, but is still needed to be thoroughly investigated for its beneficial uses in the light of present scientific advancement in the field of natural products.
Methods and materials
Plant material
Andrachne cardifolia Muell. (Euphorbiaceae), as a whole plant was collected from the Dir district, NWFP (Pakistan) in 2004, and identified by Professor Dr Jahander Shah, Plant Taxonomist, Vice Chancellor University of Malakand, Chakdara Dir. A voucher specimen (CA-012) has been deposited in the herbarium of the University of Malakand.
Extraction
The shade dried plant material was chopped into small pieces and then pulverized into a fine powder. The plant material (15 kg) was soaked in methanol with occasional shaking, at room temperature. After 15 days, the methanol soluble materials were filtered off. The filtrate was concentrated under vacuum at low temperature (40°C) using a rotary evaporator. A blackish crude extract (295 gm) was obtained.
Fractionation
The crude methanolic extract (295 gm) was suspended in distilled water (500 mL) and partitioned with n-hexane (3 × 500 mL), chloroform (3 × 500 mL), ethyl acetate (3 × 500 mL) and n-butanol (3 × 500 mL) to yield the n-hexane (33 gm), chloroform (69 gm), ethyl acetate (36 gm), n-butanol (41 gm) and aqueous (75 gm) fractions, respectively.
Cholinesterase inhibition assay
Acetylcholinesterase and butyrylcholinesterase inhibiting activities were measured by slightly modifying the spectrophotometric method previously developed [Citation16]. Electric-eel AChE (type VI-S, Sigma) and horse-serum BChE (Sigma) were used as sources of both cholinesterases. Acetylthiocholine iodide and butyrylthiocholine chloride (Sigma) respectively were used as substrates of the reaction. 5,5-Dithiobis (2-nitrobenzoic acid) (DTNB, Sigma) for the measurement of cholinesterase activity. 140 μL of 100 mM sodium phosphate buffer (pH 8.0), 10 μL of DTNB, 20 μL of the test samples solutions and 20 μL of acetylcholinesterase/butyrylcholinesterase solution were mixed and incubated for 15 min at 25°C. The reaction was then initiated by the addition of l0 μL acetylthiocholine or butyrylthiocholine, respectively. The hydrolysis of acetylthiocholine and butyrylthiocholine was monitored at a wavelength of 412 nm by the formation of the yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine and butyrylthiocholine, respectively. The sample was dissolved in 50% ethanol which was used for the control. All the reactions were performed in triplicate.
Lipoxygenase inhibition assay
Lipoxygenase inhibiting activity was conveniently measured by slightly modifying the spectrometric method previously developed [Citation17]. Lipoxygenase (1.13.11.12) type I-B and linoleic acid were purchased from sigma (St. Loius, MO) and were used without further purification. All other chemicals were of analytical grade. 160 μL of 0.1 mM sodium phosphate buffer (pH 7.0), 10 μL of the sample solution and 20 μL of lipoxygenase solution were mixed and incubated for 5 min at 25°C. Subsequently, the reaction was initiated by the addition of 10 μL linoleic acid solution (substrate), with the formation of (9Z, 11E)-13S)-13-hydroperoxyoctadeca-9, 11-dienoate, and the change of reaction was followed for 10 min. The test sample was dissolved in 50% ethanol which was used for the control. All the reactions were performed in triplicate. The IC50 values (concentrations of sample causing 50% reduction in activity relative to the control) were calculated using the EZ-Fit Enzymes Kinetics programme.
Urease inhibition assay
Reaction mixture comprising 25 μL of enzyme (Jack bean urease) solution and 55 μL of buffer pH 8.2 (0.01 M K2HPO4·3H2O, 1 mM EDTA and 0.01 M LiC12) containing 100 mM urea were incubated with 5 mL of test compounds at 30°C for 15 min in 96-well plates. Urease activity was determined by measuring ammonia production using the indophenol method as previously described [Citation18]. Briefly 45 μL each of phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside) and 70 mL of alkali reagent (0.5% w/v NaOH and 0.1% active chloride NaOCl) were added to each well. The increasing absorbance at 630 nm was measured after 50 min using a micro plate reader (Molecular Device, USA). All reactions were performed in triplicate in a final volume of 200 mL. The results (change in absorbance per min), were processed using Soft Max Pro software (Molecular Device, USA). Percentage inhibitions were calculated from [100 − (ODtestwell/ODcontrol) × 100]. Thiourea was used as a standard urease inhibitor.
Results and discussion
Pakistan is endowed with a wealth of medicinal plants and has a valuable heritage of herbal remedies and, like most developing countries, its rural population still relies on the indigenous system of medicine to a great extent [Citation19]. Pakistan exports large quantities of crude plants to international market (worth US$ 6 million). So the current need is to convert this valuable heritage of herbal remedies into practice. Therefore, the current study was designed to screen, confirm and provide a scientific basis for the use of Andrachne cardifolia Muell in the transitional system of medicine, especially for its enzyme inhibitory activity. Furthermore, the present study will provide a base to meet the demand of the WHO for high quality products at affordable prices. In vitro enzyme inhibition activities of the crude methanolic extract and subsequent fractions of Andrachne cardifolia Muell were carried out against acetylcholinesterase, butyrylcholinesterase, lipoxygenase and urease enzymes.
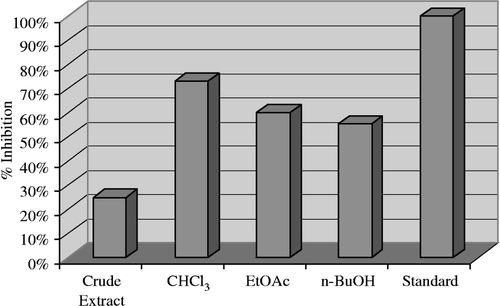
In continuation of our previous work on the indigenous medicinal plants of the hilly area of Swat, Pakistan Citation20-23, we have screened another rare species, Andrachne cardifolia of the Euphorbiaceae family for enzymes inhibition activities. The results obtained with the crude extract and subsequent fractions of Andrachne cardifolia for inhibitory activity against acetylcholinesterase are shown in . The crude methanolic extract exhibited low (25.30%) inhibition, while moderate activity was displayed by the n-butanol fraction (55.10%), good inhibitory activity (73.25%) and (60.10%) were indicated by chloroform and ethyl acetate fractions respectively.
Figure 1 Acetylcholinesterase inhibition by the crude extract and subsequent fractions of Andrachne cardifolia Muell 40 μg/200 μL.
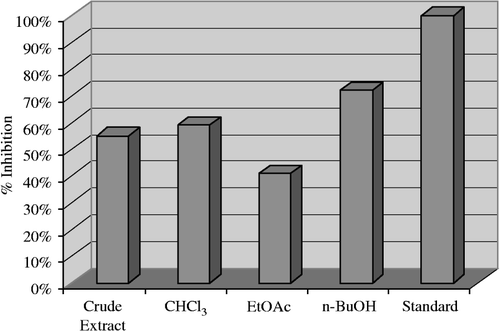
The enzyme inhibitory activities for butyrylcholinesterase obtained in the current study are shown in . An excellent result (71.50%) was expressed by the n-butanol fraction while (59.30%), (55.40%) and (41.10%), inhibition was shown by the chloroform, crude extract and ethyl acetate fractions respectively.
Figure 2 Butyrylcholinesterase inhibition by the crude extract and subsequent fractions of Andrachne cardifolia Meull 40 μg/200 μL.
shows the enzyme inhibition found in the current study against lipoxygenase. Overall outstanding enzyme inhibition was displayed by the crude extract and subsequent fractions of Andrachne cardifolia against lipoxygenase; the crude extract (75.10%), chloroform fraction (89.10%), ethyl acetate fraction (45.40%), and n-butanol fraction (55.25%). However, no enzyme inhibition activity was found against urease.
Figure 3 Lipoxgenase inhibition by the crude extract and subsequent fractions of Andrachne cardifolia Muell 240 μg/200 μL.
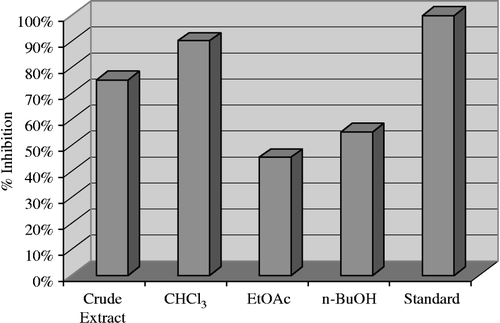
As shown in , it can be postulated that the crude extract and subsequent fractions of Andrachne cardifolia Muell have a strong potential to inhibit the lipoxygenase enzyme and can, therefore, offer a great opportunity for the isolation of active agents for the treatment of different diseases. Moreover, this effort has also provided a platform for further research on this plant species.
References
- The pharmacological basis of therapeutics8th ed., LS Goodman, AG Gilman, 1990; 96
- Ernst RL, Hay JW. The US economic and social costs of Alzheimer's disease revisited. Am J Public Health 1994; 84: 1261–1264
- Basic pathology4th ed., V Robbins, SL Kumar, 1987; 747
- Farlow M, Gracon SI, Hershey LA. Controlled trial of tacrine in Alzheimer's disease. J Am Med Assoc 1992; 268: 2523–2529
- Knapp MJ, Knopman DS, Solomon PRA. 30 week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. J Am Med Assoc 1994; 271: 985–991
- Canal I, Imbimbo BP. Clinical trials and therapeutics: Relationship between pharmacodynamic activity and cognitive effects of eptastigmine in patients with Alzheimer's disease. Clin Pharmacol Therap 1996; 15: 49–59
- Imbimbo BP, Martelli P, Troetel WM. Efficacy and safety of eptastigmine for the treatment of patients with Alzheimer's disease. Neurology 1999; 52: 700–708
- Schwaez M, Glick D, Loewensten Y, Soreq H. Pharmacology 1995; 67: 283
- Rask-Madsen J, Bukhave K, Laursen LS, Lauritsen K. 5-Lipoxygenase inhibitors for the treatment of inflammatory bowel disease. Agents Actions 1992; C37–C46, Spec No
- Hisaki R, Fujita H, Saito F, Kanmatsuse K. Lipoxygenase inhibition and protection of cardiovascular system. Nippon Rinsho 2004; 62: 187–192
- Gopalan G. Medicinal plants of India. Indian Council of Medical Research, New Delhi 1976; 1: 64
- Derasari HR, Khasa JH. Indian J Pharm 1966; 28(9)237
- Ikram M, Fazal Hussain S. Planta Med 1983; 47: 191
- Mukharjee KS, Bhattachcharjee P, Mukharjee RK, Ghosh PK. Phytochem. 1986; 25(11)2669–2670
- Mukharjee KS, Bhattachcharjee P. Phytochem. 1987; 26(5)1539–1540
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88–95
- Tappel AL. Methods in enzymology. Academic Press. 1962; 5: 539–542
- Weatherburn MW. Phenol-hypochlorite reaction for the determination of the ammonia. Anal Chem 1967; 39: 971–974
- Kattak SG, Gilani SN, Ikram M. J Ethnopharmacol 1985; 14: 45–51
- Ahmad Bashir, Jan Qasim, Nisar Muhammad, Bashir Shumaila, Iqbal Chaudhary M. Asian J Plant Sci 2003; 2(15–16)1107–1111
- Ahmad Bashir, Jan Qasim, Mansoor Ahmad. Int Chem Pharm Med J 2004; 1: 55–62
- Ahmad Bashir, Khan Haroon, Bashir Shumaila, Nisar M, Hassan M. J Enz Inhib Med Chem 2006; 21(4)449–452, August
- Ahmad Waqar, Ahmad Bashir, Iqbal Zafar, Nisar M. J Biol Sci 2003; 3(11)1046
