Abstract
A series of, anilino-5- (substituted) phenyl -3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione and 2-chloroanilino-5- (substituted) phenyl -3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione were synthesized by the reaction between hydrazine hydrate and the chalcones (3a–k) followed by condensation with the appropriate aryl isothiocyanate which yielded the N-substituted pyrazoline derivatives. These were tested for their in-vitro anti-mycobacterial activity against INH resistant Mycobacterium tuberculosis (INHR MTB) using the BACTEC 460 radiometric system. Compound 2-chloroanilino-5-(2,6-dichlorophenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6i) was found to be most active agent with a minimum inhibitory concentration of 0.96μg/mL.
Introduction
Among infectious diseases, tuberculosis (TB) is the leading killer with over two million casualties annually worldwide. The WHO considers tuberculosis to be the most dangerous chronic communicable disease in the world [Citation1]. The emergence of AIDS, decline of socioeconomic standards and a reduced emphasis on tuberculosis control programs contribute to the disease's resurgence in industrialized countries [Citation2]. Resistance of Mycobacterium tuberculosis strains to anti-mycobacterial agents is an increasing problem worldwide Citation3-5. In spite of severe toxicity on repeated dosing of isoniazid (INH), it is still considered to be a first line drug for the chemotherapy of tuberculosis. Literature survey reveals pyrazoline derivatives are active against many mycobacteria Citation6-9. The current work describes the synthesis of novel pyrazoline moieties with encouraging anti-mycobacterial activity against M. tuberculosis H37Rv.
Materials
Chemicals were supplied by E.Merck (Germany) and S.D fine chemicals (India). Melting points were determined by open tube capillary method and are uncorrected. Purity of the compounds was checked on thin layer chromatography (TLC) plates (silica gel G) in the solvent system toluene-ethyl formate- formic acid (5:4:1) and benzene (CARE-CARCINOGENIC) -methanol (8:2) and the spots were located under iodine vapors or UV light. IR spectra were obtained on a Perkin-Elmer 1720 FT-IR spectrometer (KBr Pellets). 1H NMR spectra were recorded or a Bruker AC 300 MHz spectrometer using TMS as internal standard in DMSO-d6.
Methods
Chemistry
General method for the preparation of 1-(4-hydroxy-3-methyl-phenyl)-3- (substituted) phenyl-2-propen-1-ones (3a–k)
4-Hydroxy-3-methyl acetophenone (1.5017 g, 0.01 mmol), appropriate aldehyde (0.01 mmol), were dissolved in ethanol and sodium hydroxide (30%, 5mL) with 10 ml of petroleum ether was stirred under room temperature for 4 h. The resulting solution was allowed to stand overnight then poured into ice-cold water then neutralized with HCl. The solid which separated was filtered off dried and purified from ethanol.
1-(4′-hydroxy-3′-methyl-phenyl)-3-(4″-methoxy phenyl)-2-propen-1-one (3a)
IR: (KBr) cm− 1 3200(OH), 3040(CH), 1680(C = O); 1H–NMR (DMSO-d6) ppm: 2.2(3H,s,CH3), 3.9 (3H,s, OCH3), 6.9–7.5(1H × 2,dd, –CH = CH), 7.7–8.2(7H,s, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methyl-phenyl)-3- (4″-choloro phenyl)-2-propen-1-one (3b)
IR: (KBr) cm− 1 3200(OH), 3040(CH), 1680(C = O), 772(C–Cl); 1H–NMR (DMSO-d6) ppm: 2.2(3H,s, CH3), 6.9–7.5(1H × 2,dd, –CH = CH), 7.7–8.2(7H,m, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methyl-phenyl-3- (4″-dimethyl amino phenyl)-2-propen-1-one (3c)
IR: (KBr) cm− 1 3200(OH), 3040(CH), 1680(C = O); 1H–NMR (DMSO-d6) ppm: 2.2(3H,s,CH3), 3.9(6H,s, N (CH3 × 2), 6.9–7.5(1H × 2,dd, –CH = CH), 7.7–8.2(7H,m, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methyl-phenyl)-3- phenyl-2-propen-1-one (3d)
IR: (KBr) cm− 1 3200(OH), 3040(CH), 1680(C = O); 1H – NMR (DMSO-d6) ppm: 2.2(3H,s, CH3), 6.9–7.5(1H × 2,dd, –CH = CH), 7.7–8.2(8H,m, aromatic), 9.2 (1H,s, OH).
1-(4-hydroxy-3-methyl-phenyl)-3- (3″,4″-dimethoxy phenyl)-2-propen-1-one (3e)
IR: (KBr) cm− 1 3200(OH), 3040(CH), 1680(C = O); 1H–NMR (DMSO-d6) ppm: 2.2(3H,s, CH3), 3.9(6H,s, OCH3 × 2), 6.9–7.5(1H × 2,dd, –CH = CH), 7.7–8.2(6H,m, aromatic), 9.2 (1H,s, OH).
General method for the preparation of
4-[5′-(substituted) phenyl -4,5-dihydro-1H-3-pyrazolyl]-2-methylphenols (4a–k)
Chalcone (3a–k) (0.01 mol) and ethanol (20mL) was mixed and hydrazine hydrate (99%) (0.02 mol, 0.1mL) was added dropwise. The reaction mixture was heated under reflux for 7 h, then cooled and poured onto crushed ice. The obtained solid was filtered and recrystalized from ethanol.
4-[5-(4′-methoxyphenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4a)
IR: (KBr, cm − 1) 3307(OH), 1590(C = N), 1320(C–N)); 1H–NMR (DMSO-d6, ppm): 2.3(2H,s, CH2), 3.4(3H,s,CH3), 3.9(3H,s,OCH3),4.24 (1H,s, CH), 5.52(1H,s, NH), 7.3–7.8(7H,m, aromatic),9.5(1H,s, OH).
4-[5-(4′-chlorophenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4b)
IR: (KBr, cm − 1) 3307(OH), 1590(C = N), 1320(C–N), 770(C–Cl); 1H–NMR (DMSO-d6, ppm): 2.3(2H,s, CH2), 3.4(3H,s,CH3), 4.24 (1H,s, CH), 5.50(1H,s, NH),7.0–7.6(7H,m, aromatic), 9.5(1H,s, OH).
4-[5-(4′-dimethylamonophenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4c)
IR: (KBr, cm − 1) 3307(OH), 1580(C = N), 1324(C–N); 1H–NMR (DMSO-d6, ppm): 2.3(2H,s, CH2), 2.9(3H × 2,s, N(CH3)2), 3.4(3H,s,CH3), 4.24 (1H,s, CH), 5.52(1H,s, NH), 7.4–8.0(7H,m, aromatic), 9.5(1H,s, OH).
2-methyl-4-(5′-phenyl-4,5-dihydro-1H-3-pyrazolyl)phenol (4d)
IR: (KBr, cm − 1) 3307(OH), 1590(C = N), 1320(C–N); 1H–NMR (DMSO-d6, ppm): 2.3(2H,s, CH2), 3.4(3H,s,CH3), 5.54(1H,s, NH), 4.24 (1H,s, CH), 7.3–7.8(8H,m, aromatic), 9.5(1H,s, OH).
4-[5-(3′,4′-dimethoxyphenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4e)
IR: (KBr, cm − 1) 3310(OH), 1590(C = N), 1320(C–N); 1H–NMR (DMSO-d6, ppm): 2.3(2H,s, CH2), 3.4(3H,s, CH3), 3.7(6H,s,2 × OCH3), 7.0–7.8(6H,m,aromatic), 5.50(1H,s,NH), 4.24 (1H, s,CH), 9.2(1H,s,OH).
4-[5-(3′,4′,5′-trimethoxyphenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4f)
IR: (KBr, cm − 1) 3307(OH), 1596(C = N), 1320(C–N)); 1H–NMR (DMSO-d6, ppm): 2.3(2H,s, CH2), 3.4(3H,s,CH3),3.6(9H,s,OCH3), 4.24 (1H,s, CH), 5.48(1H,s, NH), 7.3–7.8(5H,m,aromatic),9.5(1H,s,OH).
4-[5-(4′-fluorophenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4 g)
IR: (KBr, cm − 1) 3312(OH), 1590(C = N), 1320(C–N), 700(C–F); 1H–NMR (DMSO-d6, ppm): 9.4(1H,s, OH), 7.3–7.8(7H,m, aromatic), 5.42(1H,s,NH), 4.24 (1H,s, CH), 3.4(3H,s,CH3), 2.3(2H,s, CH2).
4-[5-(2′-chlorophenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4 h)
(KBr, cm − 1) 3306(OH), 1586(C = N), 1320(C–N), 774(C–Cl); 1H–NMR (DMSO-d6, ppm): 9.5(1H,s, OH), 7.6–8.2(7H,m, aromatic), 5.50(1H,s, NH), 4.24 (1H,s, CH), 3.4(3H,s,CH3), 2.3(2H,s, CH2).
4-[5-(2′,6′-dichlorophenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4i)
IR: (KBr, cm − 1) 3317(OH), 1594(C = N), 1320(C–N), 770(C–Cl); 1H–NMR (DMSO-d6, ppm): 9.5(1H,s, OH), 7.3–7.8(6H,m, aromatic), 5.54(1H,s, NH), 4.24 (1H,s, CH), 3.4(3H,s,CH3), 2.3(2H,s, CH2).
4-[5-(3′-nitrophenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4j)
IR: (KBr, cm − 1) 3307(OH), 1590(C = N), 1320(C–N); 1H–NMR (DMSO-d6, ppm): 9.4(1H,s, OH), 7.8–8.4(7H,m, aromatic), 5.56(1H,s, NH), 4.20 (1H,s, CH), 3.2(3H,s,CH3), 2.7(2H,s, CH2).
4-[5-(2′-furyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methylphenol (4k)
IR: (KBr, cm − 1) 3317(OH), 1590(C = N), 1320(C–N); 1H–NMR (DMSO-d6, ppm): 7.3–7.8(3H,m, aromatic), 7.8–8.2(3H,m, furan), 5.52(1H,s, NH), 4.20 (1H,s, CH), 3.42(3H,s,CH3), 2.3(2H,s, CH2), 9.2(1H,s, OH).
General method for the preparation of
anilino- (substituted) phenyl 3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethiones (5a–k)
To a solution of pyrazoline 4a–k (0.01 mol) in ethanol (20mL) was added phenyl isothiocyanate (1.50 mL, 0.01 mol) and the reaction mixture was refluxed for 4 h. The reaction mixture was cooled and then poured onto crushed ice. Then obtained solid was filtered, washed with water and purified from ethanol.
anilino-5-(4- methoxy phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5a)
IR: (KBr) cm − 1 3307(OH), 3224(NH),1596(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.4(2H,s, CH2), 2.7(3H,s, CH3), 3.9(3H,s, OCH3), 5.3 (1H,s, CH), 6.9–7.5(12H,m, aromatic), 9.4(1H,s, OH), 11.0(1H, s, NH); MS m/z: 418(M+1); Cal/Ana[C (69.04) 69.05,H (5.55) 5.57,N (10.06) 10.04%]
anilino-5-(4- chloro phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5b)
IR: (KBr) cm − 1 3317(OH), 3220(NH),1590(C = N), 1320(C–N), 1130 (C = S), 770(C–Cl); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2), 2.7(6H,s, 2 × CH3), 5.2 (1H,s, CH), 7.2–7.6(12H,m, aromatic), 9.5(1H,s, OH), 10.0(IH, s, NH); MS m/z 421(M+); Cal/Ana[C (65.47) 65.45,H (4.78) 4.77,N (9.96) 9.96%]
anilino-5-(4- dimethyl amino phenyl-3-(4-hydroxy-3-methylphenyl)-4, 5-dihydro-1H-1-pyrazolyl methanethione (5c)
IR: (KBr) cm − 1 3307(OH), 3220(NH),1590(C = N), 1320(C–N),1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2), 2.6(6H,s, CH3), 3.3(6H,s, –N(CH3)2), 4.9 (1H,s, CH), 7.2–8.0 (12H,m, aromatic), 9.0(1H,s, OH), 10.0(IH, s, NH); MS m/z: 431(M+1); Cal/Ana[C (69.74) 69.73,H (6.09) 6.03,N (13.01) 13.04%]
anilino-5-(phenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5d)
IR: (KBr) cm − 1 3307(OH), 3220(NH),1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.1(2H,s, CH2), 2.9(3H,s,CH3), 5.1 (1H,s, CH), 7.2–7.8 (13H,m, aromatic), 9.7(1H,s, OH), 10.0(IH, s, NH);MS m/z: 386(M− 1); Cal/Ana[C (71.29) 71.27,H (5.46) 5.47,N (10.84) 10.85%]
anilino-5-(3,4- dimethoxy phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5e)
IR: (KBr) cm − 1 3307(OH), 3220(NH),1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.1(2H,s, CH2), 2.5(3H,s, CH3), 3.3(6H,s, OCH3 × 2), 5.9 (1H,s, CH),7.2–7.4 (11H,m, aromatic), 9.8(1H,s, OH), 10.01(IH, s, NH); MS m/z: 447(M+); Cal/Ana[C (67.09) 67.06,H (5.63) 5.62,N (9.39) 9.39%]
anilino-5-(3, 4, 5- trimethoxy phenyl -3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5f)
IR: (KBr) cm − 1 3317(OH), 3220(NH),1596(C = N), 1320(C–N), 1132 (C = S); 1H–NMR (DMSO-d6) ppm: 2.0(2H,s, CH2), 2.8(3H,s, CH3), 3.5(9H,s, OCH3 × 3), 5.9 (1H,s, CH), 7.2–7.8 (10H,m, aromatic), 9.2(1H,s, OH), 10.4 (IH, s, NH); MS m/z: 478(M+1); Cal/Ana[C (65.39) 65.39,H (5.70) 5.71,N (8.80) 8.80%]
anilino-5-(4-fluoro phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5 g)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S), 824(C–F); 1H–NMR (DMSO-d6) ppm: 2.0(2H,s, CH2), 2.6(6H,s, CH3), 5.3 (1H,s, CH), 7.2–7.8(11H,m, aromatic), 9.9(1H,s, OH), 10.0(IH, s, NH); MS m/z: 405(M+); Cal/Ana[C (68.13) 68.10,H (4.97) 4.98,N (10.36) 10.36%]
anilino-5-(2-chloro phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5 h)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S), 770(C–Cl); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2), 2.8(3H,s, CH3), 5.2 (1H,s, CH), 7.2–7.4(11H,m, aromatic), 9.4(1H,s, OH), 10.0(IH, s, NH); MS m/z: 421(M+); Cal/Ana[C (65.47) 65.47,H (4.78) 4.77,N (9.96) 9.96%]
anilino-5-(2,6-dichloro phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5i)
IR: (KBr) cm − 1 3307(OH), 3220(NH),1590(C = N), 1320(C–N), 1130 (C = S),770(C–Cl); 1H–NMR (DMSO-d6) ppm: 1.2 (2H,s, CH2), 2.5(3H,s, CH3), 4.6 (1H,s, CH), 7.1–8.4(10H,m, aromatic), 9.8(1H,s, OH), 12.1(IH, s, NH); MS m/z: 457(M+1); Cal/Ana[C (60.53) 60.50,H (4.20) 4.21,N (9.21) 9.21%]
anilino-5-(3-nitro phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (5j)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.1(2H,s, CH2), 2.4(6H,s, 2 × CH3), 5.3 (1H,s, CH),7.2–7.4(11H,m, aromatic), 8.6(1H,s, OH), 13.5(IH, s, NH); MS m/z: 432(M+); Cal/Ana[C (63.87) 63.86,H (4.66) 4.65,N (12.95)12.94%]
anilino-5-(furfuryl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methane thione (5k)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N) 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.1(2H,s, CH2), 2.5(3H,s,CH3), 5.8 (1H,s, CH), 6.3–7.5(8H,m, aromatic), 7.6–7.7(3H,m,furan), 9.8(1H,s, OH), 9.9(IH, s, NH); MS m/z: 378(M+1); Cal/Ana[C (66.82) 66.81,H (5.07) 5.08,N (11.13) 11.12%]
General method for the preparation of compounds
2-chloroanilino-5- (sub) phenyl -3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethiones (6a–k)
To a solution of pyrazolines (0.01moL) (4a–k) in ethanol (20mL) was added 2-chloro aryl isothiocyanate (1.66 mL, 0.01 mol) and the reaction mixture was refluxed for 4 h. The reaction mixture was cooled and then poured onto crushed ice, the obtained solid filtered, washed with water and purified from ethanol.
2-chloroanilino-5- (4-methoxy phenyl -3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (6a)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1130 (C = S), 1320(C–N); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2), 2.5(3H,s, CH3), 3.3(3H,s, OCH3), 5.2 (1H,s, CH), 6.5–8.4(11H,m, aromatic), 9.7(1H,s, OH), 10.1(IH, s, NH); MS m/z: 451(M+); Cal/Ana [C (63.78) 63.77,H (4.91) 4.90,N (9.30) 9.32%]
2-chloroanilino-5- (4-chlorophenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (6b)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S), 770(C–Cl); 1H–NMR (DMSO-d6) ppm: 2.3(3H,s, CH3), 2.8(2H,s, CH2), 5.0 (1H,s, CH), 7.2–8.0(11H,m, aromatic), 9.9(1H,s, OH), 10.02(IH, s, NH); MS m/z: 456(M+); Cal/Ana [C (60.53) 60.53,H (4.20) 4.20,N (9.21) 9.22%]
2-chloroanilino-5- (4-dimethylaminophenyl -3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (6c)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.0(2H,s, CH2), 2.5(3H,s, CH3), 3.1(6H,s, –N (CH3)2), 5.4 (1H,s, CH), 7.6–8.4(11H,m, aromatic), 9.7(1H,s, OH), 10.0 (IH, s, NH); MS m/z: 466(M+1); Cal/Ana[C (64.51) 64.50,H (5.42) 5.41,N (12.05) 12.0%]
2-chloroanilino-5- phenyl -3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (6d)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.3(2H,s, CH2), 2.7(3H,s, CH3), 5.24 (1H,s, CH), 6.7–8.1(12H,m, aromatic), 8.7(1H,s, OH), 9.9 (IH, s, NH);MS m/z:421(M+); Cal/Ana [C (65.47) 65.48,H (4.78) 4.77,N (9.96) 9.97%]
2-chloroanilino-5- (3,4-dimethoxy phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl methanethione (6e)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2),2.5(3H,s, CH3), 3.3(3H,s, OCH3 × 2), 4.40 (1H,s, CH), 6.0–8.0(11H,m, aromatic), 9.8(1H,s, OH), 9.9 (IH, s, NH); MS m/z: 482(M+1); Cal/Ana[C (62.30) 62.32,H (5.02) 5.02,N (8.72) 8.74%]
2-chloroanilino-5- (3,4,5-tri methoxy phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6f )
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2), 2.5(3H,s, CH3), 3.6(12H,s, OCH3 × 3), 5.3 (1H,s, CH), 6.5–7.5(11H,m, aromatic), 11.3(1H,s, OH), 12.90 (IH, s, NH); MS m/z: 511(M− 1); Cal/Ana[C (60.99) 60.98,H (5.12) 5.13,N (8.21) 8.22%]
2-chloroanilino-5-(4-fluorophenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6 g)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S), 820(C–F); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2),2.8(6H,s,CH3),5.3(1H,s,CH),7.2–7.7 (11H, m, aromatic), 9.5 (1H,s, OH), 10.0 (IH, s, NH): MS m/z:439(M+); Cal/Ana[C (62.75) 62.76,H (4.35) 4.36,N (9.55) 9.53%]
2-chloroanilino-5-(2-chlorophenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6 h)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S), 770(C–Cl); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2), 2.8(3H,s, CH3), 5.6(1H,s,CH), 7.0–8.01(11H,m, aromatic), 10.0(1H,s, OH), 11.10 (1H, s, NH); MS m/z: 457(M+1); Cal/Ana[C (60.53) 60.52,H (4.20) 4.19,N (9.21) 9.20%]
2-chloroanilino-5-(2,6dichloro phenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6i)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1130 (C = S), 1590(C = N),1320(C–N), 770(C–Cl); 1H–NMR (DMSO-d6) ppm: 1.3(3H,s,CH3), 2.5(3H,s, CH3), 5.7(1H,s,CH), 7.1–7.7 (10H,m, aromatic), 9.7(1H,s, OH), 11.10 (1H, s, NH); MS m/z: 491(M+); Cal/Ana[C (56.28) 56.28,H (3.70) 3.71,N (8.56) 8.57%]
2-chloroanilino-5-(3-nitrophenyl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6j)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH2), 2.8(6H,s, CH3), 5.7(1H,s,CH), 7.2–7.9(11H,m, aromatic), 9.7(1H,s, OH), 11. 00 (IH, s, NH); MS m/z:466(M+); Cal/Ana[C (59.16) 59.15,H (4.10) 4.13,N (12.00) 12.02]
2-chloroanilino-5-furfuryl-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6k)
IR: (KBr) cm − 1 3307(OH), 3220(NH), 1590(C = N), 1320(C–N), 1130 (C = S); 1H–NMR (DMSO-d6) ppm: 2.2(2H,s, CH3), 2.5(6H,s, CH3), 6.9–7.6(7H,m, aromatic), 7.8–8.2(3H,s,furan), 9.2(1H,s, OH), 12.0 (IH, s, NH); MS m/z: 412(M+1);Cal/Ana[C (61.23) 61.12,H (4.40) 4.41,N (10.20) 10.18%]
Biology
The primary screen was conducted using 6.25μg/mL (or molar equivalent of highest molecular weight compound in a series of congeners) against Mycobacterium tuberculosis H37RV (ATCC27294) in BACTEC 12B medium using the BACTEC 460 radiometric system [Citation10].
Cytotoxicity
All the compounds were tested for cytotoxicity (IC50) in VERO cells at concentrations of 62.5μg/mL or 10-fold. After 72 h exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega Cell Titer 96 Non-radioactive Cell proliferation method [Citation11].
Result and discussion
Chemistry
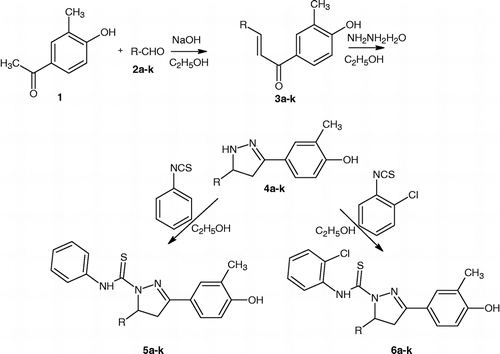
N1-substituted thiocarbamoyl 3-(4′-hydroxy-3′-methyl phenyl)-5-[(substituted) phenyl]–2- pyrazolines (5a–k) and (6a–k) described in this study are shown in Tables and , and a reaction sequence for their preparation is outlined in . The chalcones were prepared by reacting 3-methyl-4-hydroxy acetophenone with the appropriate aldehyde in presence of base by a conventional Claisen-Schmidt condensation. Reaction between the synthesized chalcones with hydrazine hydrate in ethanol led to the novel pyrazolines (4a–k), which on treatment with various aryl isothiocyanates afforded the respective 1,3,5-trisubstituted pyrazolines (5a–k) & (6a–k) in 65–92% yield. The purity of the compounds was confirmed by TLC and elemental analyses. Spectral data (1H-NMR and IR) for all the synthesized compounds were in full agreement with the proposed structures.
Table I. Physical constants and anti-mycobacterial activity of the synthesized compounds. .
.
Table II. Physical constants and antimycobacterial activity of the synthesized compounds.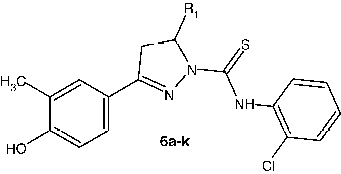 .
.
Antimycobacterial activity
The ring-substituted pyrazoline derivatives (5a–k) and (6a–k) were tested for their anti-mycobacterial activity in-vitro against INH resistant Mycobacterium tuberculosis (INHR-MTB) using the BACTEC 460-radiometric system. The results are summarized in Tables and with INH, a standard used for comparison. Among the twenty-two newly synthesized compounds, 2-chloroanilino-5-(2,6-dichlorophenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolylmethanethione (6i) had the highest potency and exhibited >90% inhibition at MIC 0.96 μg/mL. followed by (6 g) and (6b) which showed moderate inhibitory activity with MIC 1.00 μg/mL and 1.40 μg/mL, respectively. The 2,6-dichloro group substituted derivative, (6i), displayed relatively higher inhibitory activity in general. However the electron withdrawing groups such as, 4-flurophenyl, 2-chlorophenyl, 2,6-dichlorophenyl and 3-nitrophenyl present in the substituted analogue 5 g, 5 h, 5i, 5j, 6 h and 6j produced moderate inhibitory activity against (INHR-MTB). On the other hand the analogues with an electron donating group (OCH3) substituted at the 4′-phenyl (6a), 3′,4′- phenyl (6e) and 3′,4′,5′- phenyl (6f ) position showed significantly decreased inhibitory activity. But among the (5a–k) derivatives, compounds with 4′-methoxy (5a), 3′,4′-dimethoxy-(5e) and 3′,4′,5′-trimethoxy phenyl substitution (5f) exhibited relatively low inhibitory activity against (INHR-MTB). Replacement of phenyl substitution at C-5 with a 2-chlorophenyl group in the pyrazoline analogue improves antitubercular activity. These results clearly showed that the presence of a N-1 2-chlorophenyl substutuent with a dichloro substitution at the C-5 of the pyrazoline (6a–k) derivatives, as in 6i, caused a remarkable improvement in anti-mycobacterial activity.
All the compounds were tested for cytotoxicity (IC50) in a mammalian VERO cells at a concentration of 62.5μg/mL. After 72 hours exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega Cell Titer 96 Non-radioactive Cell proliferation method. Most of the active compounds were found to be non-toxic at this concentration (62.5μg/mL).
Among the newer derivatives, it is conceivable that derivatives showing anti-mycobacterial activity can be further modified to exhibit better potency than the standard drugs. Further studies to acquire more information about the Structure-Activity Relationships (SAR) within the series are in progress in our laboratory. The pyrazoline derivatives discovered in this study may provide valuable therapeutic intervention for the treatment of tubercular diseases.
Acknowledgements
The author (M.Shahar Yar) wishes to express his thanks to the University Grant Commission – New Delhi, India for the research award and we thank the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF), National Institute of Allergy and Infections Diseases Southern Research Institute/GW Long Hansen's Disease Center, Colorado State University Birmingham, Alamba, USA, for the in vitro anti-mycobacterial screening and Dr Kiran Smith, National Cancer Institute – USA, for valuable suggestions.
References
- Bloom BR, Murray CJL. Tuberculosis: commentary on a re-emergent killer. Science 1992; 257: 1055
- Barnes PF, Blotch AB, Davidson PT, Snider DE, Jr. Tuberculosis in patients with immuno-deficiency virus infection. N Engl J Med 1991; 324: 1644
- Sbarbaro JA. Multidrug-resistant tuberculosis. It is time to focus on the private sector of medicine. Chest 1997; 111: 1149
- Fujiwara PI, Cook SV, Rutherford CM, Crawford JT, Glickman SE, Kreiswirth BN, Sachdev PS, Osahan SS, Ebrahimzadeh A, Frieden TR. A continuing survey of drug-resistant tuberculosis. Arch Intern Med 1997; 157: 531
- Schaberg T, Gloger G, Reichert B, Mauch H, Lode H. Resistant lung tuberculosis in Berlin 1987–1993. Pneumologie 1996; 50: 21
- Kucukguzel SG, Rollas S. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Farmaco 2002; 57: 583
- Shaharyar M, Siddiqui AA, Ali MA, Sriram D, Yogeeswari P. Synthesis and in vitro antimycobacterial activity of N(1)-nicotinoyl-3-(4′-hydroxy-3′-methyl phenyl)-5-[(sub)phenyl]-2-pyrazolines. Bioorg Med Chem Lett 2006; 16: 3947–3949
- Nauduri D, Reddy GB. Antibacterials and antimycotics: Part 1: Synthesis and activity of 2-pyrazoline derivatives. Chem Pharm Bull (Tokyo) 1998; 46(8)1254–1260
- Santilli AA, Kim DH, Gregory FJ. Antitubercular activity of substituted 5-oxo-1-thiocarbamoyl-3-pyrazoline-4-alkanoic acid derivatives. J Pharm Sci. 1975; 64(6)1057–1058
- Collins L, Frazblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium Antimicrob. Agents Chemother 1997; 41: 1004
- Gundersen LL, Nissen-Meyer J, Spilsberg B. Synthesis and antimycobacterial activity of 6-arylpurines: The requirements for the N-9 substituent in active antimycobacterial purines. J Med Chem 2002; 45: 1383