Abstract
The whitening effects of the flavonoid constituents of Alpinia officinarum Hance were investigated on melanin biosynthesis in B16 mouse melanoma cells, tyrosinase inhibition and UV absorption. The melanin content was reduced to 1.276 μg /105cell for flavonoid mixture and 1.161 μg /105cell for galangin while the melanin control was 1.632 μg /105cell. Both flavonoid mixture and galangin reduced melanin production with an inhibition of 21.81% and 28.86% at a concentration of 26.5 μ g/mL and 29 μg /mL (107.4 μM), respectively. Tyrosinase inhibition by the flavonoid mixture and galangin were higher at lower concentrations and galangin showed competitive inhibition at a concentration less than 21.23 μg/mL which was soluble. In addition, the flavonoid mixture and galangin showed a broad absorption band at 270 ∼ 290 nm related to the UV-B area. These observations suggest that galangin may be a whitening agent and a promising candidate for prevention of skin cancer. This is the first full scale report on the evaluation of the whitening effect of galangin.
Introduction
Recently, much attention has been drawn to the application of natural products as whitening agent in cosmetics. The main characteristic of whitening agent is to influence melanin biosynthesis. Melanin biosynthesis commences with two conversions catalyzed by tyrosinase, the hydroxylation of L-tyrosine to 3,4-dihydroxyphenyl-L-alanine (L-Dopa) followed by the oxidation of L-Dopa to dopaquinone [Citation1]; further polymerization reactions yield melanin pigments [Citation2]. Tyrosinase is the key enzyme in melanogenesis and there are many factors related to tyrosinase activity and melanin biosynthesis. For instance, ultraviolet radiation (UV) not only acts directly to stimulate melanin biosynthesis and the proliferation of melanocytes [Citation3], but also induces the tyrosinase level in melanocytes [Citation4].
Many natural products in plant extracts have been investigated for inhibition of melanin biosynthesis, including some plant extracts and natural standards such as licorice roots [Citation5], grape-seed extracts [Citation6], and arbutin [Citation7] which are used to inhibit melanogenesis as cosmetic additives.
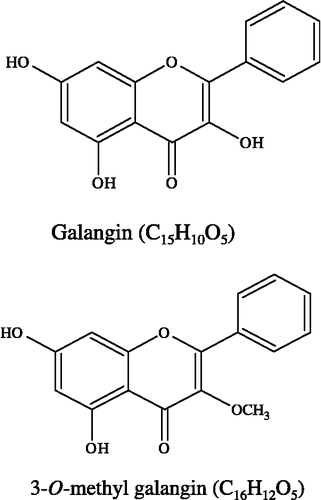
Alpinia officinarum Hance (AO), a traditional herbal plant, has been used as an aromatic stomachic, analgesic, and antiemetic in Asia [Citation8]. AO contains many types of flavonoids, such as galangin and 3-O-methyl galangin (). Galangin has been identified as a potent tyrosinase inhibitor and inhibited tyrosinase activity using L-Dopa as substrate [Citation9]. Prior to the current study studies have not reported on the flavonoid constituents of Alpinia officinarum Hance on melanogenesis in B16 mouse melanoma cells.
In this study, the whitening effects of the flavonoid constituents of AO were tested on melanin biosynthesis, tyrosinase inhibition and their propensity for UV absorption. Furthermore, we also investigated the mechanism of the tyrosinase inhibition by galangin.
Materials and methods
Materials
Tyrosinase from mushroom (EC 1.14.18.1, T3824, tyrosinase activity 3900 unit/mg solid) was purchased from Sigma-Aldrich (St Louis, Mo). Dulbecco's modified Eagle's medium (DMEM) was from GIBCO BRL, Grand Island, (New York), synthetic melanin was from Sigma-Aldrich Chemie GmbH (Germany) and the standard compounds (kojic, arbutin) and L-3,4-dihydroxyphenylalanine (L-DOPA) from Qiude Biotech Ltd. (Shanghai, China). The standard galangin was isolated and purified in our laboratory with individual purity not less than 98% (HPLC-DAD array). Alpinia officinarum Hance (AO) was collected in Guangdong Province and voucher specimens were identified and deposited at the Institute of Biochemistry, East China University of Science and Technology, Shanghai, 200237, China.
Preparation of flavonoid mixture and standard
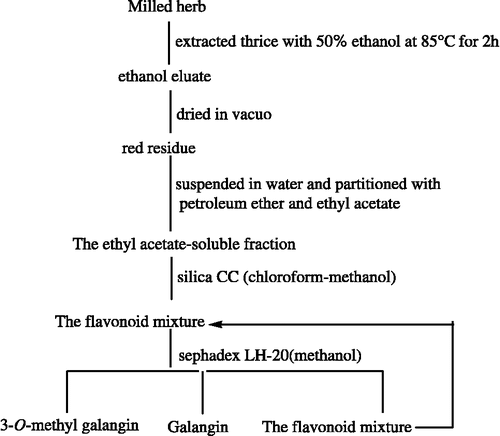
Respective dried and pulverized medicinal materials were thrice extracted with 50% ethanol at 85°C for 2 h and the ethanol eluate was concentrated to dryness in vacuum. The red residue was then suspended in water and partitioned with petroleum ether and ethyl acetate to afford an ethyl acetate-soluble fraction. This fraction was applied to a silica chromatography column and the flavonoid mixture was collected according to TLC monitoring. Finally, the flavonoid mixture was passed through a Sephadex LH-20 column ().
Hplc-dad Analysis
The constituents of the flavonoid mixture were analyzed by an Agilent 1100 series system, equipped with a photodiode array detector working in the range of 190 ∼ 400 nm, a quatemary pump, and an autosampler. Separation was achieved with a reverse phase column (ZORBAX, Eclipse SB-C18, 5 μm, 4.6 × 250 mm, Agilent, USA) provided with a C18 guard column and methanol- water- phosphoric acid (60-38-2,v/v/v, isocratically) was employed as the mobile phase. The flow rate was kept constant at 0.8 mL·/min and the peaks were identified using UV absorbance at 254 nm. The temperature of the column during analysis was maintained at 40°C. The injection volume was 10 μL on each occasion.
Cell lines and cultures
The B16 mouse melanoma cells were purchased from the Chinese Type Culture Collection (Shanghai Institute of Cell Biology, Chinese Academy of Science, Shanghai, China). The cells were maintained in 10% FBS-DMEM, containing penicillin (100 U/mL) and streptomycin (100 U/mL) in a humidified atmosphere with 5% CO2 at 37°C.
MTT assay
Effects of test samples on cell viability were measured by the MTT method. Subcultures of B16 cells were seeded in 96-well plates at a density of 2 × 105 cells/mL and cultured for 24 h. The medium was then replaced with 200 μL fresh 10% FBS-DMEM containing 1% sample-DMSO solution. After 3 days in culture, the medium was replaced with 90 μL fresh medium and 10 μL 5.0 mg/mL MTT-PBS solution. After culturing for 4 h, the medium and non-metabolized MTT were carefully removed and 100 μL of DMSO was added to each well. After shaking for 30 min at room temperature, the plates were read at 570 nm with an automated Bio-Rad 550 microtiter plate reader (Bio-Rad Laboratories, CA). Controls containing DMEM instead of test samples and blanks containing DMEM instead of DMSO were also made.
Determination of melanin content
This assay followed Ando's method [Citation10] with slight modification. Briefly, subcultures of B16 cells were seeded in 24-well plates at a density of 2 × 105 cells/mL and cultured for 24 h. The medium was then replaced with 1.0 mL fresh 10% FBS-DMEM containing 1% DMSO (v/v) to dissolve each test sample. After culturing for 3 days, the cells were harvested and suspended in 0.5 mL 1N NaOH-10% DMSO solution (v/v), maintaining at 80°C for 30 min. The solution was then sonicated for 60 min and the melanin content determined by reading the absorbance at 405 nm. Controls containing 1% DMSO without test samples were also made. The melanin content was determined by calculation from a synthetic melanin standard curve.
Measurement of tyrosinase inhibition
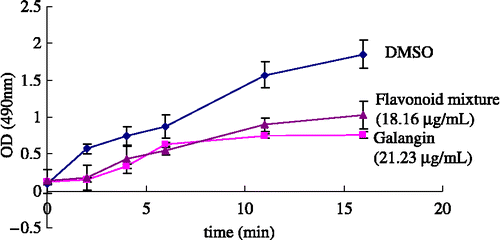
Tyrosinase inhibitory activity was measured according to the method of Mason and Peterson [Citation11]. Phosphate buffer (70 μL, 0.1M) at pH 6.8, 30 μL of mushroom tyrosinase (167 units/mL) and 2 μL of the tested samples (galangin, 21.23 μg/mL; flavonoid mixture, 18.16 μg/mL) dissolved in DMSO were inserted into 96-well plates. After 5 min of incubation at 30°C, 100 μL L-DOPA (12 mmol/L) was added. Optical density (OD) at 492 nm was measured on a Technician Sunrise microplate reader.
Results and discussion
Determination of the constituents and UV absorption
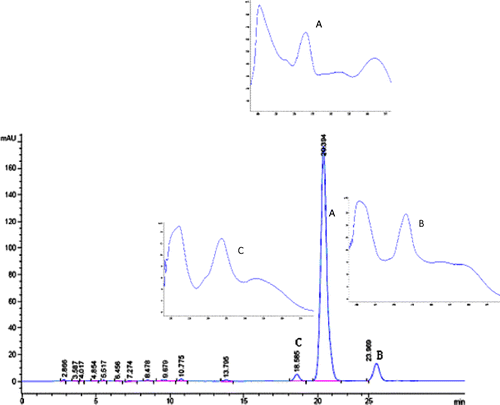
As shown in , there were three constituents in the flavonoid mixture. Compound A was identified as galangin and compound B as 3-O-methyl galangin [Citation12]. In the chosen mixture the content of galangin was 10.8 mg/g while that of 3-O-methyl galangin was 1.02 mg/g. The UV absorption of the flavonoid mixture was the total absorption of compounds A, B and C.
Both flavonoid mixture and galangin showed a broad absorption band at 270 ∼ 290 nm related to the UV-B area and another band, in spite of a weak absorption at 350 ∼ 370 nm, corresponding to the UV-A area (see ).
Effects on the viability of B16 mouse melanoma cells
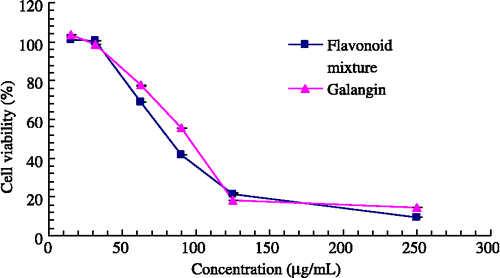
The cell viability test was aimed at finding a relatively safe dosage for further intracellular determination of the melanin content in B16 cells. As shown in , both test samples gave significant antiproliferation effect in B16 mouse melanoma cells with the concentration ranging from 15 to 250 μg/mL after 16 hours in culture. The estimated IC50 values of the flavonoid mixture and galangin were 79.52 μg/mL and 91.65 μg/mL, respectively. For the range of concentration without inhibition of cell growth, the flavonoid mixture was less than 32.56 μg/mL and galangin less than 31.25 μg/mL.
Effects on melanogenesis in B16 mouse melanoma cells
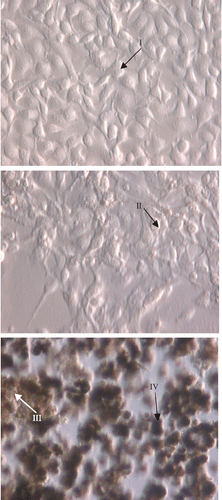
In a previous review, melanin synthesis is confined in tyrosinase-rich specialized organelles, the melanosomes. These can be found in the cytosol of melanocytes in four different maturation stages, defined according to their degree of melanin deposition Citation13-14. Stage I premelanosomes are spherical vacuoles lacking an organized internal structure, whereas in stage II, the organelle becomes elliptical and a well-organized matrix is usually evident [Citation15]. Stage III melanosomes are fully functional organelles, where melanogenesis is active, with progressive deposition of the pigment. Finally, stage IV melanosomes are fully electron-opaque granules, where pigment deposition has completely obliterated the underlying structure, and with little melanin synthetic activity [Citation16].
Based on the results of the MTT test, we chose 26.5 μg/mL for the flavonoid mixture and 29 μg/mL for galanglin for determination of melanin content. As a result, the test samples were used in the concentration range where inhibition of cell growth does not occur. We observed four stages of melanosomes production with the treatments () and there were no differences between treatments and controls (photos not shown).
As shown in , both flavonoid mixture and galangin reduced melanin production with an inhibition of 21.81% and 28.86%. The melanin content was 1.276 μg /105cell for the flavonoid mixture and 1.161 μg /105 cell for galangin.
Table I. The inhibitory effect of flavonoid mixture and galangin on production of melanin.
Inhibition of tyrosinase activity
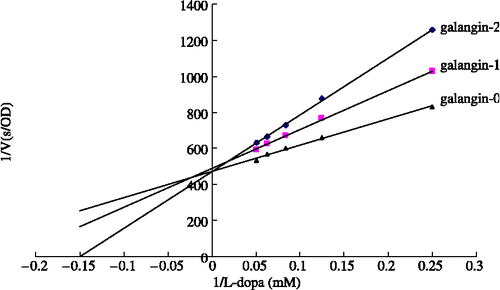
The effects of increasing concentrations of both samples on the diphenolase activated forms of tyrosinase are shown in . Both tested samples are hardly soluble in the water-based test media so their IC50 values could not be measured unequivocally for precise comparison. Nevertheless, the flavonoid mixture and galangin exhibited higher inhibitions at lower concentrations. In addition, galangin seemed to show almost competitive inhibitory activity at the concentration (less than 21.23 μg/mL) which it was soluble ().
Conclusion
By using different assay methods, we have demonstrated that the flavonoid constituents of AO not only inhibited the diphenolase activity of mushroom tyrosinase and the biosynthesis of melanin in melanocytes, but also had the propensity for UV absorption. In addition, kinetic analysis of galangin suggests that it acts as a competitive inhibitor. Based on our results, galangin may be a whitening agent and a promising candidate for prevention of skin cancer. Furthermore, while it is important for cosmetic additives to be safe, the application of galangin and flavonoid mixture should be confined to a relatively safe range of concentrations. Although the whitening effect of flavonoid constituents of AO has been established, further research is needed on the inhibitory mechanism of melanogenesis.
References
- Ito S. A chemist's view of melanogenesis. Pigment Cell Res 2003; 16: 230–236
- Toshihiko S, Masumoto S, Moriichi N, Kobori M, Kanda T, Shinnito H, Tsushida TT. Procyanidin trimers to pentamers fractionated from apple inhibit melanogenesis in B16 mouse melanoma cells. J Agric Food Chem 2005; 53: 6105–6111
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem 1992; 267: 24675–24680
- Karg E, Odh G, Wittbjer A, Rosengren E, Rorsman H. Hydrogen peroxide as an inducer of elevated tyrosinase level in melanoma cells. J Invest Dermatol 1993; 100: 209S–213S
- Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, Tamir S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J Agric Food Chem 2003; 51: 1201–1207
- Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidins-rich extract from grape seeds. Pigment Cell Res 2003; 16: 629–638
- Akiu S, Suzuki Y, Fujinuma Y, Asahara T, Fukuda M. Inhibitory effect of arbutin on melanogenesis: biochemical study in cultured B16 melanoma cells and effect on the UV-induced pigmentation in human skin. Proc Jpn Soc Invest Dermatol 1988; 12: 138–139
- Ching Su New College. The encyclopedia of Chinese Materia Medica. Shanghai Science&Technical Publishers, Shanghai 1987
- Li PX, Qing XC, Huang H, Hong ZW, Rong QZ. Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry(Moscow) 2003; 68: 487–491
- Ando H, Itoh A, Mishima Y, Ichihashi M. Correlation between the number of melanosomes, tyrosinase mRNA levels, and tyrosinase activity in cultured murine melanoma cells in response to various melanogenesis regulatory agents. J Cell Physiol 1995; 163: 608–614
- Mason HS, Peterson EW, Melanoproteins I. Reactions between enzyme-generated quinones and amino acids. Biochim Biophys Acta 1965; 111(1)134–146
- Lin Tao, Zheng-tao Wang, En-Yuan Zhu, Yan-hua Lu, Dong-zhi Wei. HPLC analysis of bioactive flavonoids from the rhizome of alpinia officinarum. S Afric J Botany 2006; 72: 163–166
- Seiji M, Shimao K, Birbeck MSC, Fitzpatrick TB. Subcellular localization of melanin biosynthesis. Ann NY Acad Sci 1963; 100: 497–533
- Moellmann G, Solminski A, Kuklinska E, Lerner AB. Regulation of melanogenesis in melanocytes. Pigment Cell Res 1988; 1: 79–87
- Orlow SJ. The biogenesis of melanosomes. The Pigmentary System. Physiology and Pathophysiology, J Nordlund, R Boissy, V Hearing, R King, JP Ortonne. Oxford University Press, New York 1998; 6: 97–106
- María Martínez-Esparza, Concepción Ferrer, María-Teresa Castells, José Carlos García_Borrón, Adelina Zuasti. Transforming growth factor β1 mediates hypopigmentation of B16 mouse melanoma cells by inhibition of melanin formation and melanosome maturation. Intern J Biochem Cell Biol 2001; 33: 971–983