Abstract
Flavonoids, polyphenolic phytochemicals, are ubiquitous in plants and are commonly present in the human diet. They may exert diverse beneficial effects, including antioxidant and anticarcinogenic activities. The present study was designed to evaluate three biomolecules that play important roles in the apoptotic process: mitogen-activated protein kinases, protein phosphatases and NFκB, using HL60 cells treated with fisetin as an experimental model. Our results demonstrated that cells treated with fisetin presented high expression of NFκB, activation of MAPK p38 and an increase of phosphoprotein levels; inhibition of enzymes involved in redox status maintenance were also observed. Our findings reinforce the hypothesis that fisetin is likely to exert beneficial and/or toxic actions on cells not through its potential as antioxidant but rather through its modulation of protein kinase and phosphatase signaling cascades. Additionally, our results also indicate that the cellular effects of fisetin will ultimately depend on the cell type and on the extent to which they associate with the cells, either by interactions at the membrane or by uptake into the cytosol.
Introduction
Flavonoids are polyphenolic compounds widely found in plants [Citation1]. Components of fruits, vegetables and beverages, such as wine and tea, many flavonoids are present in a regular diet [Citation2]. Flavonoids exhibit a variety of effects such as inhibition of malignant cell growth [Citation1], regulation of lymphocyte activation, cell proliferation and differentiation Citation2-4. These biological effects of flavonoids on cells can be due to the inhibition of different key enzymes. For these reasons, the flavonoids can be considered potential compounds in the selective blocking of signal transduction pathways and in the design of more potent analogues for use in proliferative disease therapies.
Several studies have demonstrated that, depending on their structures, flavonoids can be potent inhibitors of several kinases involved in signal transduction, mainly protein kinase C (PKC) [Citation5] and tyrosine kinases [Citation6]. On the other hand, some flavonoids can activate cell differentiation through activation of the Ras-ERK cascade [Citation7].
Fisetin is a common dietary component found in several fruits and vegetables [Citation8]. Some authors have demonstrated different biological activities for this flavonoid: inhibition of topoisomerase II, an essential nuclear enzyme for DNA replication [Citation9,Citation10], neuroprotective, cardioprotective and anti-carcinogenic activities, which have been attributed to its antioxidant properties Citation7Citation11-13, inhibition of cellular proliferation and in vitro angiogenesis [Citation14], induction of apoptosis in leukemic cells [Citation13]. Recently, Haddad et al. [Citation15] have demonstrated that fisetin caused cell cycle arrest (G2/M) in a prostate cancer human cell line (PC3). In addition, fisetin inhibited glucose uptake in a competitive manner in a myeloid cell (U937), which indicated that this flavonoid could be used as an alternative blocker of glucose uptake in vitro [Citation16].
The present study was designed to evaluate three biomolecules that play important roles in the apoptotic process: mitogen-activated protein kinases (MAPKs), protein phosphatases and NFκB, using HL60 cells treated with fisetin as an experimental model. Our results demonstrated that cells treated with fisetin presented high expression of NFκB, activation of MAPK p38 and an increase of phosphoprotein levels; inhibition of enzymes involved in redox status maintenance was also observed.
Materials and methods
Materials
HL60 cells was from ATCC (Rockville, MD) and fisetin () was from Sigma Chemical Co. (St Louis, MO). The polyclonal antibodies against antiphospho-p38 mitogen-activated protein kinase (p38), antiphospho-p42/44 (ERK 1/2), antiphospho-c-jun NH2-terminal protein kinase (JNK), antiphospho-MAPK/ERK kinase 1 (MEK1), antirabbit and antimouse peroxidase conjugated antibodies were obtained from Cell Signaling Technology (Beverly, MA).
Cell culture
HL60 cells were routinely grown in suspension in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin (10,000 U/mL penicillin and 10 mg/mL streptomycin) and 1% glutamine, grown at 37°C under a humidified 5% CO2 atmosphere. In all experiments 3 × 105 cells/mL were seeded, and after 72 h the cells were treated with fisetin for 24 h. Fisetin dissolved in dimethyl sulfoxide (DMSO) was added to the culture medium and adjusted to a final DMSO concentration of 0.1%.
Cell viability
Cell viability was assessed by the trypan blue dye exclusion and the MTT reduction assays as previously described [Citation17].
Western blotting
Cells (3 × 107) were lysed in 200 μL cell lysis buffer (50 mM Tris-HCl pH 7.4, 1% Tween 20, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM o-vanadate, 1 mM sodium fluoride, and protease inhibitors (1 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride (PMSF)) for 2 h on ice. Protein extracts were cleared by centrifugation, and the protein concentration was determined using the Lowry method [Citation18]. Twice the volume of sodium dodecyl sulfate (SDS) gel loading buffer (100 mM Tris-HCl, pH 6.8, 200 mM dithiothreitol, 4% SDS, 0.1% bromophenol blue, and 20% glycerol) was added to the samples and the mixture boiled for 10 min. Cell extracts, corresponding to 3 × 105 cells, were resolved by SDS-polyacrylamide gel (12%) electrophoresis (PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 1 h in 1% fat-free dried milk or bovine serum albumin (2%) in Tris-buffered saline (TBS) - Tween 20 (0.05%) and incubated overnight at 4°C with appropriate primary antibody at 1:1000 dilution. After washing in TBS-Tween 20 (0.05%), membranes were incubated with antirabbit or antimouse horseradish peroxidase-conjugated secondary antibodies, at 1:2000 dilutions (in all Western blotting assays), in blocking buffer for 1 h. Detection was performed by using enhanced chemiluminescence (ECL).
Antioxidant enzyme activities
Total superoxide dismutase (SOD) activity was determined from the rate of inhibition of ferrocytochrome c oxidation, at 550 nm, in a standard reaction medium [Citation19]. The manganese superoxide dismutase (MnSOD) activity was measured after inhibition of the Cu/Zn isoenzyme by addition of 1 mM KCN [Citation20]. Catalase activity was determined by measuring the decrease in absorption of H2O2 at 240 nm [Citation21]. Glutathione peroxidase (GPX) activity was determined by measuring the NADPH oxidation rate in the presence of GSH and GSH reductase [Citation22].
Marker enzyme activities for oxidative stress
Aconitase activity was measured at 25°C by following the change in the absorption at 340 nm, due to NADP+ reduction [Citation23]. Fumarase activity was measured at 25°C by following the increase in absorbance at 240 nm at 25°C in a standard reaction mixture [Citation24]. All the measurements were carried out in a UV-VIS spectrophotometer (Hitachi, model U-2001).
Statistical evaluation
The Western blots represent three independent experiments. Cell viability was expressed as the mean ± standard error of three independent experiments run in triplicate. Data for each assay were statistically evaluated by analysis of variance (ANOVA).
Results and discussion
Differential effect of fisetin on HL60 cells and normal human lymphocyte viabilities
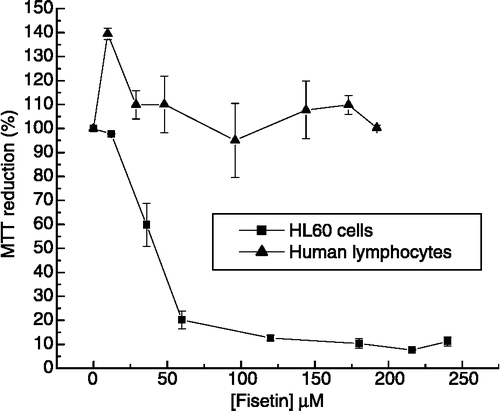
We have previously described cytotoxic effects and mechanism of action of different compounds on cancer cells Citation25-27. Other natural products have also been reported as important sources of potential chemotherapeutic agents Citation28-30. Flavonoids, widely distributed in vegetables, fruits, and wine, have been shown to exert anticarcinogenic effects [Citation10,Citation13,Citation15,Citation31]. However, the molecular mechanisms by which flavonoids can act against cancer cells need to be elucidated. To establish the specificity of fisetin action on HL60 cells we checked, in parallel, the effect of this compound on normal human lymphocytes viability using the MTT assay. It was observed that after 24 hours of fisetin-treated HL60 cells, the mitochondrial activity was decreased, displaying an IC50 value around 30 μM (). In agreement with other authors [Citation13], it was also observed that fisetin induced HL60 cells death by apoptosis. Interestingly, human lymphocyte viability remained unchanged, even in the presence of fisetin at concentrations up to 200 μM. These results suggest that fisetin can be an interesting candidate for cancer treatment with a cellular-specific mechanism of action.
Figure 2 Cytotoxicity of fisetin in leukemic cells and normal human lymphocytes. HL60 cells (▪) and normal human lymphocytes (▴) were treated with different concentrations of fisetin for 24 h. In the absence of fisetin, the MTT reduction was considered as 100%. The experiment was performed in a 24-well plate. Results represent the means ± standard error of three experiments run in triplicate (P < 0.05).
Effect of fisetin on MAPKs phosphorylation and NFκB expression in HL60 cells
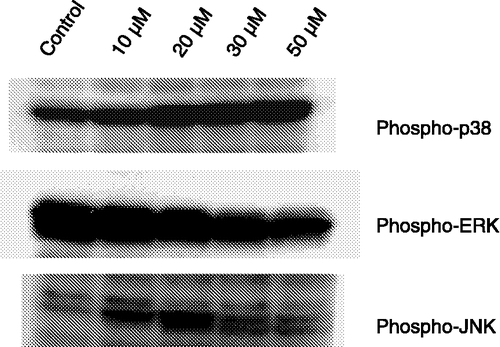
To obtain more insight into the molecular mechanisms mediated by fisetin on HL60 cells, we examined the phosphorylation state of total proteins and MAPKs, in response to fisetin at concentrations up to 50 μM. Fisetin caused activation of p38 and JNK MAPKs, while ERK was inhibited (). Williams et al. [Citation32] have demonstrated that flavonoids and their metabolites differentially acted on PI3-kinase, Akt/protein kinase B (Akt/PKB), tyrosine kinases, PKC, and MAPK signaling cascades. Inhibitory or stimulatory actions at these pathways are likely to profoundly affect cellular function by altering the phosphorylation state of target molecules and/or by modulating gene expression.
Figure 3 Effect of fisetin on MAPKs phosphorylation in HL60 cells. Cells were treated with different concentrations of fisetin (10, 20, 30 and 50 μM). Soluble lysates were matched for protein content and analyzed by Western blot. One representative immunoblot of three independent experiments is presented.
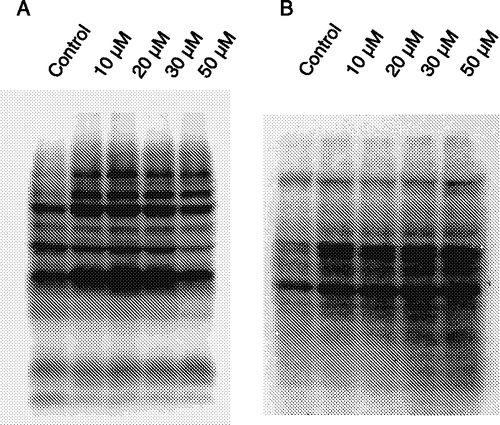
In addition, we also observed that cells treated with fisetin presented high expression of NFκB (). Decrease in MAPKs phosphorylation () and in the expression of NFκB p65 () in HL60 cells at fisetin concentrations higher than 20 μM could be ascribed to different steps of apoptosis. Our results indicate that depending on the fisetin concentration two steps of apoptosis can be reached: early and late apoptosis. Apparently, fisetin concentrations up to 20 μM caused early apoptosis, that was reinforced by the overexpression of NFκB. Recently, Kanno et al. [Citation33] demonstrated that the overexpression of NFκB is a pivotal event for apoptosis in HL60 cells induced by the flavonoid naringenin. It has been shown that the transcription factor NFκB participated in cell growth, differentiation, inflammatory responses induced by different signals related to the regulation of apoptosis and neoplastic transformation [Citation34,Citation35]. The pro- and antiapoptotic regulatory functions of NFκB have been shown to depend on the cell type, the differentiation state of the cell, and the nature of the apoptotic stimulus [Citation35]. Our data provided evidence that the overexpression of the subunit NFκB p65 in cell death was associated with ROS generation. Some authors observed that ROS per se were potent inducers of apoptosis [Citation36] and that the hydrogen peroxide-induced apoptosis required the release of mitochondria-derived ROS and the activation of NFκB [Citation37]. Our results demonstrating the ability of NFκB p65 overexpression to induce apoptosis, are in agreement with published data implicating NFκB to the induction of cell death in certain cells such as neurons, Schwann cells, prostate carcinoma, and embryonic kidney cells Citation38-42.
Effects of fisetin on protein phosphorylation in HL60 cells
In order to analyze the phosphorylation state in HL60 cells treated with fisetin, we examined the tyrosine and threonine phosphorylation on the cellular proteins. Phosphorylation of both residues increased in the cells treated with fisetin, except for a decrease in tyrosine phosphorylation at 50 μM fisetin (). Our results indicated that the fisetin action in HL60 cells was accompanied by an increase in tyrosine and threonine phosphorylations. We have observed that fisetin inhibited cytosolic phosphatase activities in HL60 cells (not shown).
Fisetin induces oxidative stress and decrease in antioxidant enzymes activities in HL60 cells
In order to analyze the cellular redox status after treatment of HL60 cells with fisetin we quantified the activities of aconitase, fumarase, catalase, glutathione peroxidase and two isoforms of superoxide dismutase (SOD), i.e. the MnSOD (mitochondrial isoform), and the CuZnSOD (cytosolic isoform). Treatment of HL60 cells with fisetin resulted in inactivation of mitochondrial aconitase, an enzyme sensitive to oxidative stress, but not fumarase, a mitochondrial enzyme sensitive to oxidative stress (). Fisetin caused also an expressive decrease in the antioxidant enzymes catalase, MnSOD, CuZnSOD and GPX. Our results suggest that fisetin can induce oxidative stress through ROS production. ROS can lead to cell death through inactivation of mitochondrial aconitase, an iron-sulfur (Fe-S) protein [Citation43]. Recent studies showed that ROS are emerging as obligatory mediators of cell death signaling in response to stimulation of TNF receptors and induction of JNK and p38 signaling Citation44-47. A MAPK phosphatase (MKP) was identified as a critical molecular target of ROS during TNFα-induced apoptosis, due to oxidation of an essential cysteine residue to sulfenic acid [Citation46]. MKP plays a critical role in the regulation of the activity of MAPKs [Citation48,Citation49]. Thus, ROS-dependent inhibition of MKPs caused persistent activation of JNK by TNFα, and, ultimately, programmed cell death via either a necrotic or an apoptotic pathway [Citation47]. These findings are in agreement with our results since, besides activating JNK and p38, fisetin also caused an increase of phosphoprotein levels which can be due to either inactivation of protein phosphatases or activation of protein kinases, activities which are highly sensitives to oxidant agents.
Table I. Effects of fisetin on antioxidant enzymes activities of HL60 cells. Cells were treated with fisetin (100 μM) and enzyme activities were determined as described in Materials and methods.
Conclusion
In summary, our results have reinforced the hypothesis that fisetin was likely to exert beneficial and/or toxic actions on cells not through its potential to act as antioxidant but rather through its modulation of protein kinase and phosphatase signaling cascades. Additionally, our results also indicated that the cellular effects of fisetin ultimately depended on the cell type, and on the extent to which it associated with the cells, either by interactions with the membrane or by uptake into the cytosol.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) - Proc. 04/15041-5, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), CAPES postgraduate scholarship to RRRS, and FAPESP postgraduate scholarships to KCSQ and ACSS.
References
- Middleton E, Jr, Kandaswami C. The impact of plant flavonoids on mammalian biology: Implications for immunity, inflammation and cancer. The Flavonoids: Advances in Research Since 1986, JH Harborne, AR Liss, New York 1993; 619–652
- Verbeek R, Plomp AC, van Tol EA, van Noort JM. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem Pharmacol 2004; 68: 621–629
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000; 52: 673–751
- Hendriks JJ, de Vries HE, van der Pol SM, van den Berg TK, van Tol EA, Dijkstra CD. Flavonoids inhibit myelin phagocytosis by macrophages; A structure-activity relationship study. Biochem Pharmacol 2003; 65: 877–885
- Ferriola PC, Cody V, Middleton E. Protein kinase C inhibition by plants flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem Pharmacol 1989; 38: 1617–1624
- Agullo G, Garnet-Payrustre L, Manenti S, Viala C, Rémésy C, Chap H, Payrustre B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-Kinase: A comparison with Tyrosine Kinase and Protein Kinase C inhibition. Biochem Pharmacol 1997; 53: 1649–1657
- Sagara Y, Vanhnasy J, Maher P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J Neurochem 2004; 90: 1144–1155
- Kimira M, Arai Y, Shimoi K, Watanabe S. Japanese intake of flavonoids and isoflavanoids from foods. J Epidemiol 1998; 8: 168–175
- Osheroff N, Zechiedrich EL, Gale KC. Catalytic function of DNA topoisomerase II. BioEssays 1991; 13: 269–275
- Olaharski AJ, Mondrala ST, Eastmond DA. Chromosomal malsegregation and micronucleus induction in vitro by the DNA topoisomerase II inhibitor fisetin. Mut Res 2005; 582: 79–86
- van Acker FAA, Schouten O, Haenen GRMM, van der Vijh WJF, Bast A. Flavonoids can replace alpha-tocopherol as an antioxidant. FEBS Lett 2000; 473: 145–148
- Pietta PG. Flavonoids as antioxidants. J Nat Prod 2000; 63: 1035–1042
- Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochem Pharmacol 2002; 63: 225–236
- Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wahala K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vivo angiogenesis. Cancer Res 1997; 57: 2916–2921
- Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis 2006; 9: 68–76
- Park JB. Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochem Biophys Res Commun 1999; 260: 568–574
- Bromberg N, Justo GZ, Haun M, Duran N, Ferreira CV. Violacein toxicity on human blood lymphocytes and effect on phosphatases. J Enz Inhib Med Chem 2005; 20: 449–454
- Hartree EF. Determination of proteins: A modification of the Lowry method that give a linear photometric response. Anal Biochem 1972; 48: 422–427
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969; 244: 6049–6055
- Beauchamp CO, Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochim Biophys Acta 1973; 317: 50–64
- Nelson DP, Kiesow LA. Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C. Anal Biochem 1972; 49: 474–478
- Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 1976; 71: 952–958
- Morton RL, Iklé D, White CW. Loss of lung mitochondrial aconitase activity due to hyperoxia in bronchopulmonary dysplasia in primates. Am J Physiol Lung Cell Mol Physiol 1998; 274: L127–L133
- Racker E. Spectrophotometric measurements of the enzymatic formation of fumaric acid and cis-aconitic acid. Biochim Biophys Acta 1950; 4: 211–214
- Freire AG, Melo PS, Haun M, Duran N, Aoyama H, Ferreira CV. Cytotoxic effect of the diterpene lactone dehydrocrotonin from Croton cajucara on human promyelocytic leukemia cells. Planta Med 2003; 69: 67–69
- Justo GZ, Ferreira CV. Coagulation and cancer therapy: The potential of natural compounds. Cur Genomics 2005; 6: 461–469
- De Fátima A, Modolo LV, Conegero LS, Pilli RA, Ferreira CV, Kohn K, de Carvalho JE. Styryl lactones and their derivatives: Biological activities, mechanisms of action and potential leads for drug design. Cur Med Chem, 2006 in the press
- O'Gorman DM, Cotter TG. Molecular signals in anti-apoptotic survival pathways. Leukemia 2001; 15: 21–34
- Makin G, Dive C. Apoptosis and cancer chemotherapy. Trends Cell Biol Sci 2001; 11: S22–S26
- Martelli AM, Tazzari PL, Tabellini G, Bortul R, Billi AM, Manzoli L, Ruggeri A, Conte R, Cocco L. A new selective AKT pharmacological inhibitor reduces resistance to chemotherapeutic drugs, TRAIL, all-trans-retinoic acid, and ionizing radiation of human leukemia cells. Leukemia 2003; 17: 1794–1805
- Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, Shih CM, Yang LL. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol 2002; 76: 351–359
- Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: Antioxidants or signaling molecules?. Free Radic Biol Med 2004; 36: 838–849
- Kanno S, Tomizawa A, Ohtake T, Koiwai K, Ujibe M, Ishikawa M. Naringenin-induced apoptosis via activation of NF-κB and necrosis involving the loss of ATP in human promyeloleukemia HL-60 cells. Toxicol Lett 2006; 166: 131–139
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999; 18: 6853–6866
- Ricca A, Biroccio A, Trisciuoglio D, Cippitelli M, Zupi G, del Bufalo D. relA overexpression reduces tumorigenicity and activates apoptosis in human cancer cells. Brit J Cancer 2001; 85: 1914–1921
- Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today 1997; 18: 45–51
- Dumont A, Hehner SP, Hofmann TG, Ueffing M, Dröge W, Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kB. Oncogene 1999; 18: 747–757
- Lin KI, Lee SH, Narayanan R, Baraban JM, Hardwick JM, Ratan RR. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-kB. J Cell Biol 1995; 131: 1149–1161
- Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm MR, Baeuerle PA, Barde YA. Selective activation of NF-kappaB by nerve growth factor through the neurotrophin receptor p75. Science 1996; 272: 542–545
- Grimm S, Bauer MK, Baeuerle PA, Schulze Osthoff K. Bcl-2 downregulates the activity of transcription factor NF-kappaB induced upon apoptosis. J Cell Biol 1996; 134: 13–23
- Lin B, Williams-Skipp C, Tao Y, Schleicher MS, Cano LL, Duke RC, Scheinman RI. NF-kB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Diff 1999; 6: 570–582
- Grilli M, Memo M. Nuclear factor kappa B/Rel proteins: A point of convergence of signaling pathways relevant in neuronal function and dysfunction. Biochem Pharmacol 1999; 57: 1–7
- Liang LP, Patel M. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson's disease. J Neurochem 2004; 90: 1076–1084
- Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NFkB inhibits TNFα-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 2003; 22: 3898–3909
- Pham CG, Bubici C, Zazzeroni F, Papa S, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Feritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 2004; 119: 529–542
- Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev 2004; 18: 2905–2915
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005; 120: 649–661
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–252
- Sumbayev VV, Yasinska IM. Regulation of MAP kinase-dependent apoptotic pathway: Implication of reactive oxygen and nitrogen species. Arch Biochem Biophys 2005; 436: 406–412