Abstract
Glutathione reductase (GR, type IV, Baker's yeast, E.C 1.6.4.2) is a flavoprotein that catalyzes the NADPH-dependent reduction of oxidized glutathione (GSSG) to reduced glutathione (GSH). In this study some metal ions have been tested on GR; lithium, manganese, molybdate, aluminium, barium, zinc, calcium, cadmium and nickel. Cadmium, nickel and calcium showed a good to moderate inhibitory effect on yeast GR. GR is inhibited non-competitively by Zn2 + (up to 2 mM) and activated above this concentration. Ca2 + inhibition was non-competitive with respect to GSSG and uncompetitive with respect to NADPH. Nickel inhibition was competitive with respect to GSSG and uncompetitive with respect to NADPH. The inhibition constants for these metals on GR were determined. The chelating agent EDTA recovered 90% of the GR activity inhibited by these metals.
Introduction
Glutathione reductase (E.C 1.6.4.2) is a pivotal enzyme of the antioxidant system in the cells [Citation1] which utilize molecular oxygen and generate highly reactive oxygen-derived free radicals. Endogenous cellular oxidants inactivate oxidant free radicals and protect aerobic cells from oxidant injury. Glutathione reductase (GR) and superoxide dismutase are key components of this antioxidant defence and inhibition of these antioxidant components would be expected to result in cell injury [Citation2]. GR has a central role in glutathione (GSH) metabolism and as such is a potential target for chemotherapy [Citation3]. Metal ions have diverse functions on organisms and a number of metals which act as a prosthetic group for enzymes and are activators and some are inhibitors. Zn2 + is a trace element known to be an essential nutrient for life and functions as a cofactor for numerous enzymes [Citation4]. However, excess in Zn2 + the body interacts with free thiol groups on macromolecules, so blocking the active sites of enzymes, co-enzymes and membrane receptors [Citation5]. GR is an important factor in cellular zinc susceptibility. Zn2 + toxicity has been linked to decreased reduced GSH and increased GSSG contents, which might be caused by GR inhibition by Zn2 + [Citation6]. 6-Phosphogluconate dehydrogenase, like many fungal dehydrogenases, was inhibited by Zn 2 + [Citation7]. Ca2 + is the most abundant mineral in the body and regulates many cellular process and has important structural roles in living organisms [Citation8]. However, overdoses of certain vitamins and minerals can produce toxic effects as they are inhibitors of some enzymes [Citation9]. Ni2 + and Cd2 + are carcinogenic to humans and/or animals, but the underlying mechanisms are poorly understood [Citation10]. Ni2 + is well-known inhibitor of Fe(II)/alpha-ketoglutarate (alphaKG)-dependent hydroxylases [Citation11], yeast hexokinase [Citation12], horseradish peroxidase [Citation13] and microsomal epoxide hydrolase [Citation14]. Inhibition of some enzymes by lithium, such as inositol monophosphatase and glycogen synthase kinase-3, probably results in its mood-stabilizing effects [Citation15]. Mn2 + is an important trace element and may be essential for some metalloenzymes [Citation16]. Anaemia, one consequence of Al3 + toxicity, may be due to inhibition of delta-ALA dehydratase which occurs in the heme biosynthetic pathway [Citation17]. The ability of Al3 + to inhibit (Na+/K+) ATPase activity has been observed by several investigators [Citation18].
The present study aims at investigating the influence of Li+, Mn2 + , Mo6 + , Al3 + , Ba2 + , Zn2 + , Ca2 + and Ni2 + on the activity of Saccharomyces cerevisiae GR as well as the kinetic behaviour and type of inhibition of GR inhibition observed.
Materials and methods
Materials
Nicotinamide adenine dinucleotide phosphate reduced form (NADPH), oxidized glutathione (GSSG), Baker's yeast glutathione reductase (specific activity 1.25 U/mg), barium acetate and calcium chloride were obtained from Sigma Chemical Co., MO, USA. Nickel sulphate was obtained from NEEDHAM project. Zinc sulphate was obtained as ANALAR (Hopkin & Williams Ltd). EDTA was obtained from SERVA Feinbiochemica GmbH & Co. Aluminium chloride, Lithium carbonate, were from Fischer; Manganese (MnSO4) from Merck and Molybdenum (Na2MoO4) from BDH Chemicals Ltd.
Assay of glutathione reductase activity
Glutathione reductase activity was determined according to the modified Stall method [Citation19]. The incubation mixture contained 100 mM sodium phosphate buffer, pH 7.4, 1 mM GSSG, 200 mM NADPH and Baker's yeast glutathione reductase. Decrease in the absorbance of NADPH at 340 nm was monitored spectrophotometrically, at 37°C. Assays were carried out in duplicate and the activities were followed for 40 s. The reaction was linear during this time period.
A unit of activity (U) was defined as the amount of enzyme that catalyses the oxidation of 1 μmole of NADPH in 1 min under these conditions. Specific activity is defined as units per mg of protein.
Inhibition studies
Activities were measured after adding different concentrations Li+, Mn2 + , Mo6 + , Al3 + , Ba2 + , Zn2 + , Ca2 + and Ni2 + to the assay mixture given above for glutathione reductase measurement. Assays of GR in the presence of heavy metal ions were performed without enzyme-inhibitor preincubation in that the reactions were initiated by adding enzyme to the substrate-inhibitor mixture.
Recovery of glutathione reductase activity
EDTA (0–12 mM) concentrations were added to the above assay mixtures containing 2.5 mM CaCl2, 2 mM ZnSO4, and 1 mM NiSO4, respectively, and the initial velocities were determined.
Statistical analysis of kinetic data
The data were analyzed and the kinetic constants were calculated using the following equations [Citation20] by means of a nonlinear curve-fitting program of Statistica.
where V = Reaction rate, [S] = Substrate concentration, Vm = Maximum rate, and Km = Michaelis-Menten constant (substrate concentration at half the maximal velocity (Vm)).
Results
In this study we have investigated the effects of several metal ions on Baker's yeast glutathione reductase. The values of the kinetic parameters of GR in a non-inhibited reaction were determined as KmGSSG 90 ± 12 μM, Km NADPH 30 ± 4 μM. In other kinetics studies, yeast GR KmGSSG and KmNADPH values were found to be 55 μM and 3.8 μM respectively [Citation21]. The E.coli GR values were KmGSSG 97 ± 12 μM and Km NADPH 22 ± 2 μM [Citation22], Cyanobacterium Anabaena sp. Strain 7119 GR values were KmGSSG 210 μM, Km NADPH 9.4 μM [Citation23] and rat liver values were KmGSSG 56.7 ± 0.4 μM, Km NADPH 7.9 ± 0.6 μM [Citation24].
The inhibition kinetics of Saccharomyces cerevisiae GR was studied without enzyme-inhibitor pre-incubation.
We found that some metals Li+, Mn2 + , Mo6 + , Al3 + , Ba2 + had no effect the GR activity but Cd2 + , Ni2 + and Ca2 + inhibited the enzyme in a concentration-dependent manner with IC50 values of 0.025, 0.8 and 5 mM respectively. Whereas zinc was both an inhibitor and activator of the enzyme. We established that yeast GR is inhibited by Zn2 + (0.1–2 mM) and activated above this concentration (2.5–5 mM). The IC50 value of Zn2 + could not be determined because at 2 mM Zn2 + concentration the enzyme lost its activity by 36% and above this concentration GR was activated in a concentration-dependent manner (i.e. 3.5 fold at 5 mM Zn2 + concentration). Kinetic characterization of the inhibition effects of Zn2 + on GR from Saccharomyces cerevisiae have been investigated and no inhibitory effect was found with Zn2 + (30–90 μM concentration) on this enzyme [Citation25]. The obtained IC50 values of calcium are 5 mM, and nickel 0.8 mM and Yeast GR is inhibited by much lower concentrations of Cd2 + ion than the other metals (IC50 of Cd2 + is 0.025 mM). Cd2 + is a very potent enzyme inhibitor; it inhibits many enzymes such as in our previous study we have reported that Cd2 + is also a potent inhibitor of glucose-6-phosphate dehydrogenase (G-6-PD) from lamb kidney cortex [Citation26].
The kinetic characterization of the inhibitory effects of these metals on GR was also investigated. Kinetic characterization of the inhibition of ZnSO4 on GR is shown in and ; the inhibition is non-competitive with respect to both GSSG and NADPH with KiGSSG 0.476 ± 0.085 mM and KiNADPH 0.96 ± 0.134 mM. Zn2 + , at low levels, has several basic housekeeping functions in metalloenzymes, transcription factors, immunoregulation, growth, and cytoprotection, displaying antioxidant, anti-apoptotic, and anti-inflammatory roles. At high levels, however, the metal can be highly toxic [Citation27]. Toxic doses of Zn2 + inhibit intestinal alkaline phosphatase [Citation28], mitochondrial cytochrome c oxidase [Citation29], glyceraldehyde-3-phosphate dehydrogenase [Citation30], beta amylase [Citation31] and G-6-PD from lamb kidney cortex [Citation26]. Zn2 + homeostasis in bacteria is achieved by export systems and uptake systems which are separately regulated by their own regulators. Three types of Zn2 + export systems that protect cells from high toxic concentrations of Zn2 + have been identified [Citation32].
Figure 1 Lineweaver-Burk double reciprocal plot of initial velocity against GSSG as varied substrate and ZnSO4 (0.05–1 mM) as inhibitor at a fixed NADPH (0.1 mM) concentration. *0.1 mM NADPH (constant); □ 0.05 mM ZnSO4 ;○ 0.1 mM ZnSO4; Δ 0.5 mM ZnSO4; ⋄1 mM ZnSO4.
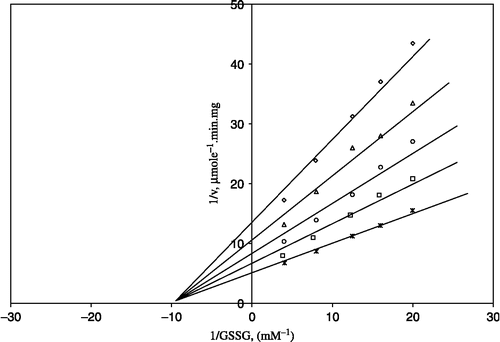
Figure 2 Lineweaver-Burk double reciprocal plot of initial velocity against NADPH as varied substrate and ZnSO4 (0.05–1 mM) as inhibitor at different fixed GSSG (0.7 mM) concentrations. ⋄ 0.7 mM GSSG (constant); □ 0.05 mM ZnSO4;Δ 0.1 mM ZnSO4;*0.5 mM ZnSO4;○ 1 mM ZnSO4.
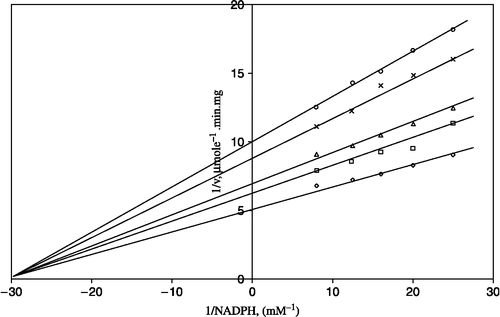
Kinetic characterization of the inhibition effects of CaCl2 on glutathione reductase is shown in and ; the inhibition is non-competitive with respect to GSSG and uncompetitive with respect to NADPH with KiGSSG 1.476 ± 0.195 mM and KiNADPH 2.993 ± 0.227 mM respectively. Ca2 + is essential to maintaining total body health although high levels of Ca2 + may be harmful. Hypercalcemia may also provoke acute renal failure or hypertension, or aggravate tubular necrosis [Citation33].
Figure 3 Lineweaver-Burk double reciprocal plot of initial velocity against GSSG as varied substrate and CaCl2 (0.8–1.6 mM) as inhibitor at a fixed NADPH (0.1 mM) concentrations. *0.1 mM NADPH (constant); □ 0.8 mM CaCl2; ○ 1 mm CaCl2; Δ 1.2 mM CaCl2; ⋄1.6 mM CaCl2.
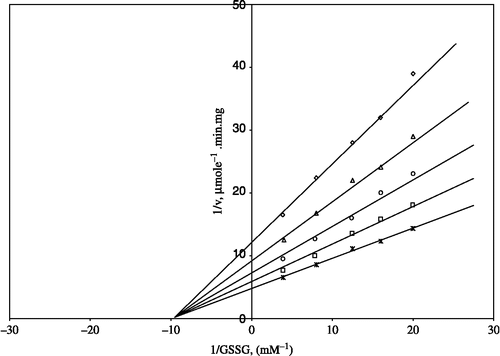
Figure 4 Lineweaver-Burk double reciprocal plot of initial velocity against NADPH as varied substrate and CaCl2 (0.8–1.6 mM) as inhibitor at different fixed GSSG (0.7 mM) concentrations. ⋄ 0.7 mM GSSG (constant); □ 0.8 mM CaCl2; Δ 1 mm CaCl2; *1.2 mM CaCl2; ○ 1.6 mM CaCl2.
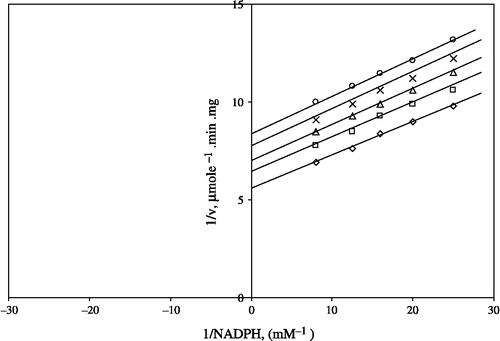
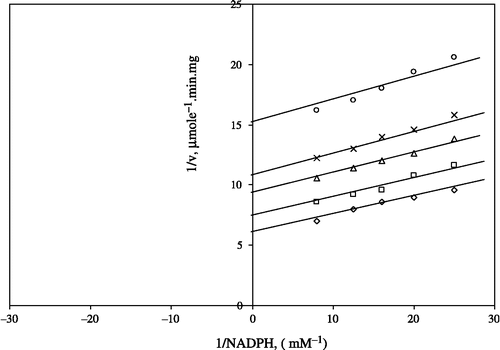
Nutrient minerals are essential yet are potentially toxic and homeostatic mechanisms are required to regulate their intracellular levels [Citation34]. Although Ni2 + is an essential cofactor for a number of enzymatic reactions in prokaryotes and eukaryotes [Citation35], this metal ion can inhibit GR in a concentration-dependent manner. Ni 2 + is a widely distributed metal that is industrially applied in many forms. Accumulated epidemiological evidence confirms that occupational exposures to nickel compounds are mostly associated with increased nasal and lung cancer incidence [Citation36]. DNA damage in the form of strand breaks and DNA-protein cross-links resulted in vivo following injection of nickel carbonate in rats [Citation37]. The interaction between Ni2 + and yeast hexokinase has been studied and kinetic studies showed that Ni2 + caused a non-competitive inhibition when glucose was the variable substrate and competitive inhibition when ATP was the variable substrate [Citation12]. Here, we found that Ni2 + was an inhibitor of GR giving to a competitive inhibition pattern when GSSG () was the varied substrate and uncompetitive pattern when NADPH () was the varied substrate.
Figure 5 Lineweaver-Burk double reciprocal plot of initial velocity against GSSG as varied substrate and NiSO4 (0.1–0.4 mM) as inhibitor at different fixed NADPH (0.1 mM) concentrations. *0.1 mM NADPH (constant); ○ 0.1 mM NiSO4; ▪ 0.2 mM NiSO4; Δ 0.3 mM NiSO4; ⋄ 0.4 mM NiSO4.
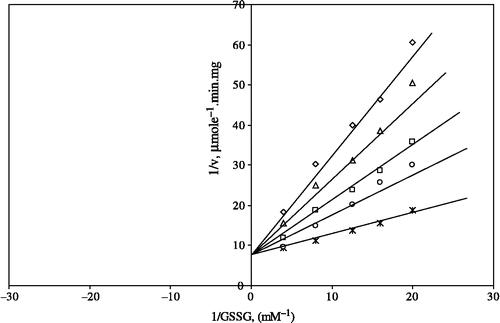
Figure 6 Lineweaver-Burk double reciprocal plot of initial velocity against NADPH as varied substrate and NiSO4 (0.1–0.4 mM) as inhibitor at different fixed GSSG (0.7 mM) concentrations. ⋄ 0.7 mM GSSG (constant); □ 0.1 mM NiSO4; Δ 0.2 mM NiSO4; *0.3 mM NiSO4; ○ 0.4 mM NiSO4.
EDTA is a chelating agent that binds divalent metal ions. When the divalent metals are chelated by the EDTA the toxic effects are lost. We suggest that EDTA has a recovering role in inhibition of yeast GR with metal ions and we found that when EDTA (0–12 mM) concentrations are added to the assay mixtures containing 2.5 mM CaCl2, 2 mM ZnSO4 and 1 mM NiSO4, respectively, GR activity was recovered by approximately 90%.
Conclusions
We have found that Zn2 + , Ca2 + and Ni2 + ions are potent inhibitor of baker's yeast GR. This is probably due to the interactions of the metal ions with aminoacids of the enzyme. GR was inhibited with NiSO4 competitively with respect to GSSG, so there may be a competition between substrate GSSG and Ni2 + ions for the active site of this enzyme. A non-competitive inhibitor may bind to a non-substrate binding site on a protein and distort it to the point of non-functionality [Citation20]. The inhibition of GR with ZnSO4 was found non-competitive.
Glutathione reductase is a important enzyme that catalyzes the reduction of GSSG using NADPH as a cofactor. The enzyme is a major component of cellular defense mechanisms against oxidative injury [Citation38] and is an attractive target for the development of antimalarial agents, agents to decrease malarial drug resistance and anticancer agents. In addition, inhibition of the enzyme has been employed as a tool in research for various purposes [Citation39]. Investigation of the inhibitors of this enzyme is important for antimalarial and anticancer researches. Because of GR is a crucial enzyme in the antioxidant system, this study may be useful for understanding the mechanisms for oxidative damage associated with heavy metal toxicity.
References
- Paulikova H, Petrickova I, Antalik M, Podhradsky D. Effect of heparin and dextran sulfate on the activity of glutathione reductase from yeast. Biochem Mol Biol Int 1996; 38(6)1117–1126
- Puglia CD, Powell SR. Inhibition of cellular antioxidants: A possible mechanism of toxic cell injury. Environ Health Perspect 1984; 57: 307–311
- McCallum MJ, Barrett J. The purification and properties of glutathione reductase from the cestode moniezia expansa. Int J Biochem Cell Biol 1995; 27(4)393–401
- McClung JP, Scrimgeour AG. Zinc: An essential trace element with potential benefits to soldiers. Mil Med Dec 2005; 170(12)1048–1052
- Naab F, Volcomirsky M, Burlon A, Caraballo ME, Debray M, Kesque JM, Kreiner AJ, Ozafran MJ, Schuff JA, Stoliar P, Vazquez ME, Davidson J, Davidson M, Fonovich de Schroeder TM. Metabolic alterations without metal accumulation in the ovary of adult bufo arenarum females, observed after long-term exposure to Zn(2+), followed by toxicity to embryos. Arch Environ Contam Toxicol 2001; 41(2)201–207
- Walther UI, Wilhelm B, Walther SC, Muckter H, Forth W. Zinc toxicity in various lung cell lines is mediated by glutathione and GSSG reductase activity. In Vitr Mol Toxicol 2000; 13(2)145–152
- Niehaus WG, Richardson SB, Wolz RL. Slow-binding inhibition of 6-phosphogluconate dehydrogenase by zinc ion. Arch Biochem Biophys 1996; 333(2)333–337
- Tandoğan B, Ulusu NN. Importance of calcium. Tr J Med Sci 2005; 35: 197–201
- Somer E. In: The essential guide to vitamins and minerals. NY Harper Perennial, New York 1995; 89–94
- Kasprzak KS, Bialkowski K. Inhibition of antimutagenic enzymes, 8-oxo-dGTPases, by carcinogenic metals. Recent developments. J Inorg Biochem 2000; 79(1–4)231–236
- Kalliri E, Grzyska PK, Hausinger RP. Kinetic and spectroscopic investigation of CoII, NiII, and N-oxalylglycine inhibition of the FeII/alpha-ketoglutarate dioxygenase, TauD. Biochem Biophys Res Commun. 2005; 338(1)191–197
- Romero CS, Olmo R, Teijon C, Blanco MD, Teijon JM, Romero A. Structural and functional implications of the hexokinase-nickel interaction. J Inorg Biochem. 2005; 99(12)2395–2402
- Mahmoudi A, Nazari K, Mohammadian N, Moosavi-Movahedi AA. Effect of Mn 2+, Co 2+. Ni 2+, and Cu 2+ on horseradish peroxidase: activation, inhibition, and denaturation studies. Appl Biochem Biotechnol 2003; 104(1)81–94
- Draper AJ, Hammock BD. Inhibition of soluble and microsomal epoxide hydrolase by zinc and other metals. Toxicol Sci 1999; 52(1)26–32
- Bachmann RF, Schloesser RJ, Gould TD, Manji HK. Mood stabilizers target cellular plasticity and resilience cascades: Implications for the development of novel therapeutics. Mol Neurobiol 2005; 32(2)173–202
- Frealle E, Noel C, Viscogliosi E, Camus D, Dei-Cas E, Delhaes L. Manganese superoxide dismutase in pathogenic fungi: An issue with pathophysiological and phylogenetic involvements. FEMS Immunol Med Microbiol 2005; 45(3)411–422
- Schroeder TM, Caspers ML. Kinetics of aluminum-induced inhibition of delta-aminolevulinic acid dehydratase in vitro. Biochem Pharmacol 1996; 52(6)927–931
- Silva VS, Duarte AI, Rego AC, Oliveira CR, Goncalves PP. Effect of chronic exposure to aluminium on isoform expression and activity of rat (Na+/K+)ATPase. Toxicol Sci 2005; 88(2)485–494
- Acan NL, Tezcan EF. Sheep brain glutathione reductase: Purification and general properties. FEBS Lett 1989; 250: 72–74
- Segel IH. Enzyme Kinetics (3rd ed.), Chapter 3. John Wiley and Sons, Toronto 1975; 100–159
- Massey V, Williams CH, Jr. On the reaction mechanism of yeast glutathione reductase. J Biol Chem 1965; 240(11)4470–4480
- Bashir A, Perham RN, Scrutton NS, Berry A. Altering kinetic mechanism and enzyme stability by mutagenesis of the dimer interface of glutathione reductase. Biochem J 1995; 312: 527–533
- Serrano A, Rivas J, Losada M. Purification and properties of glutathione reductase from the cyanobacterium Anabaena sp. strain 7119. J Bacteriol 1984; 158(1)317–324
- Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 1975; 250(14)5475–5480
- Serafini MT, Romeu A, Arola L. Zn(II), Cd(II) and Cu(II) interactions on glutathione reductase and glucose-6-phosphate dehydrogenase. Biochem Int 1989; 18(4)793–802
- Tandogan B, Ulusu NN. Effects of cadmium and zinc ions on purified lamb kidney cortex glucose-6-phosphate dehydrogenase activity. J Enz Inhib Med Chem 2006; 21(2)225–230
- Franco JL, Trivella DB, Trevisan R, Dinslaken DF, Marques MR, Bainy AC, Dafre AL. Antioxidant status and stress proteins in the gills of the brown mussel Perna perna exposed to zinc. Chem Biol Interact 2006; 160(3)232–240
- Yora T, Sakagishi Y. Comparative biochemical study of alkaline phosphatase isozymes in fish, amphibians, reptiles, birds and mammals. Comp Biochem Physiol B 1986; 85(3)649–658
- Kuznetsova SS, Azarkina NV, Vygodina TV, Siletsky SA, Konstantinov AA. Zinc ions as cytochrome C oxidase inhibitors: Two sites of action. Biochemistry (Mosc) 2005; 70(2)128–136
- Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: Contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci 2000; 20(9)3139–3146
- Dahot MU, Saboury AA, Moosavi-Movahedi AA. Inhibition of beta-amylase activity by calcium, magnesium and zinc ions determined by spectrophotometry and isothermal titration calorimetry. J Enz Inhib Med Chem 2004; 19(2)157–160
- Hantke K. Bacterial zinc transporters and regulators. Biometals 2001; 14(3–4)239–249
- Moyses-Neto M, Guimaraes FM, Ayoub FH, Vieira-Neto OM, Costa JA, Dantas M. Acute renal failure and hypercalcemia. Ren Fail 2006; 28(2)153–159
- Eide DJ, Clark S, Nair TM, Gehl M, Gribskov M, Guerinot ML, Harper JF. Characterization of the yeast ionome: A genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol 2005; 6(9)R77
- Eitinger T, Mandrand-Berthelot MA. Nickel transport systems in microorganisms. Arch Microbiol 2000; 173(1)1–9
- Lu H, Shi X, Costa M, Huang C. Carcinogenic effect of nickel compounds. Mol Cell Biochem 2005; 279(1–2)45–67
- Ciccarelli RB, Wetterhahn KE. Molecular basis for the activity of nickel. IARC Sci Publ 1984; 53: 201–213
- Willmore WG, Storey KB. Purification and properties of glutathione reductase from liver of the anoxia-tolerant turtle. Trachemys scripta elegans. Mol Cell Biochem 2006, (Epub ahead of print)
- Seefeldt T, Dwivedi C, Peitz G, Herman J, Carlson L, Zhang Z, Guan XJ. cetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino)-phenylcarbamoylsulfanyl] propionic acid and its derivatives as a novel class of glutathione reductase inhibitors. Med Chem 2005; 48(16)5224–5231
