Abstract
Hyperhomocysteinemia is associated with a lot of diseases including cardiovascular diseases and neural tube defect, but it has not been clarified exactly which mechanism is responsible for occurence disease. Here, homocysteine (Hcy) and cysteine (Cys), which are thiol containing amino acids, were examined for their effect on glutathione peroxidase (GPx) activity. İt was observed that the GPx-1 activity was inhibited under severe hyperhomocysteinemia (50–500 μM Hcy) conditions, especially at low glutathione concentrations but that cysteine increased GPx-1 activity at low glutathione concentrations and inhibition clearly appeared at 500 μM Cys concentration.
Introduction
Homocysteine (Hcy) is a sulphur containing, nonessential amino acid biosynthesized from dietary methionine [Citation1]. It is present in plasma in several different forms. Approximately 70% is bound to plasma proteins, mainly albumin, by a disulphide link. The remaining homocysteine combines with other thiols, including cysteine, resulting in homocysteine-cysteine mixed disulphide (the most abundant disulphide species), and homocysteine itself, to form the dimer homocystine. Only a small proportion (approximately 1%) normally circulates as the free thiol compound. The different forms of homocysteine appear to be in a state of flux exchanging readily between the different forms [Citation2].
Homocysteine may either be catabolized to cysteine or remethylated to methionine [Citation3]. Under conditions of low protein intake, homocysteine is metabolised primarily to methionine by a remethylation pathway. When the remethylation pathway is saturated, or when cysteine is required, homocysteine is converted to cystathionine (and then cysteine) by cystathionine β-synthase. Vitamine B6 (pyridoxine) is an essential cofactor. Cysteine may be metabolised further to sulphate and excreted in the urine [Citation4].
Available results suggest that almost all human tissues have some capacity to convert methionine to homocysteine but that the apportionment of homocysteine to the transsulfuration or remethylation pathways may vary markedly from tissue to tissue. Because fetal tissues and placenta lack γ-cystathionase activity, it has been suggested that cyst(e)ine may be an essential amino acid at this stage of life. By 30 weeks gestation, hepatic γ-cystathionase attains 23 percent of the adult specific activity; by full term (39 weeks), 46 percent. The specific activity continues to rise postnatally for about 1 year [Citation5].
In the adult population, the normal plasma total homocysteine is 5–15 μmol/L, with a mean concentration of about 10μmol/L. Hyperhomocysteinemia is usually defined as a plasma tHcy >15μmol/L, and is denoted moderate (15–30 μmol/L), intermediate (30–100 μmol/L) or severe hyperhomocysteinemia (> 100 μmol/L) [Citation6].
An elevated plasma level of homocysteine is a common, independent risk factor for cardiovascular disease [Citation7]. Hyperhomocysteinemia is also associated with thrombosis, stroke [Citation8,Citation9], cerebrovascular disease [Citation10], Alzheimer's disease [Citation11,Citation12], neural tube defect [Citation13], placental abruption/infarction and pre-eclampsia [Citation14,Citation15,Citation16]. But the mechanisms responsible for the occurence of the disease are still incompletely understood.
One commonly held view is that oxidative stress may be an important contributing factor [Citation17]. Auto-oxidation of homocysteine in vitro generates reactive oxygen species (ROS), including hydrogen peroxide and superoxide, and promotes oxidation of low density lipoprotein. Whether or not homocysteine auto-oxidation is a major mechanism for the generation of ROS in vivo is uncertain. Conversion of homocysteine to it's disulfide forms in plasma is mediated mainly by thiol-disulfide exchange reactions rather than by copper-dependent oxidation, which suggests that homocysteine is unlikely to be a major source of hydrogen peroxide in vivo. Therefore, it is perhaps more likely that indirect mechanisms are responsible for the oxidative stress of hyperhomocysteinemia. Indirect oxidative effects of hyperhomocysteinemia may include generation of superoxide from xanthine oxidase or uncoupled endothelial nitric oxide synthase, downregulation of antioxidant enzymes (e.g.GPx-1) and depletion of intracellular glutathione [Citation18].
In some studies it has been indicated that homocysteine inhibits the expression of cellular glutathione peroxidase (GPx-1) [Citation19,Citation20,Citation21], which can lead to an increase in ROS and which would alone induce oxidative stress. Dayal et al. demonstrated that deficiency of GPx-1 exacerbates endothelial dysfunction in mice with moderate hyperhomocysteinemia [Citation18]. These in vivo findings provide support for the hypothesis that indirect oxidative mechanisms are responsible for the disease formation in hyperhomocysteinemia.
However, the questions of if and how homocysteine induces oxidative stress are not yet clear. In this study we examine the of effects homocysteine on GPx-1 activity, which is an antioxidant enzyme. And also we examine the effects of cysteine on this enzyme to compare with homocysteine effects.
Materials and methods
Materials
Glutathione peroxidase (G-6137), glutathione reductase (G-3664), L-cysteine (C-7352), DL-homocysteine (H-4628), β-NADPH (N-7505), FAD (F-6625), reduced glutathione (G-4251) and oxidized glutathione (G-4626) were purchased from Sigma and tert-butyl hydroperoxide (tBH, 820244) was purchased from Merck.
Biochemical measurements
GPx activity measurement
GPx activity measurements were carried out according to the Beutler method [Citation22].
This method is a coupled, kinetic, spectrophotometric assay in which the incubation mixture containing Tris- HCl (1M, pH 8.0), GSH (0.1–0.25–0.5–0.75–1.0 mM), GR(1 U/mL), NADPH (0.2 mM), GPx-1 (5 mU/mL) and H2O2 were preincubated at 37oC for 10 min. Then the t-BH (0.07 mM) was added and the decrease in absorbance at 340 nm was determined.
Determination of enzyme concentration
To determine the appropriate enzyme concentration for this study the absorbance changes at 340 nm during 10 min were recorded in the presence of different GPx-1 activity (50–0.01 mU/mL). At the end of this study, considering the case of application, it was concluded that 5 mU/mL GPx-1 was the most appropriate concentration.
GR activity measurement
GR activity was determined by a modification of the procedure of Beutler [Citation22]. GR activity catalyzes the reduction of oxidized glutathione (GSSG) by NADPH or NADH to reduced glutathione (GSH). The activity of the enzyme is measured by following the oxidation of NADPH spectrophotometrically at 340 nm.
GR is a flavin enzyme and the complete activation of apoenzyme requires the preincubation of enzyme with FAD, necessarily done before GSSG and NADPH are added to the reaction system since these seem to interfere with activation of the enzyme by FAD. Reaction system containing Tris- HCl (1M, pH 8.0), GR (1U/mL), FAD (1μM) and H2O were incubated at 37oC for 10 min. Then GSSG (0.5 mM) was added and the mixture incubated at 37oC for 10 min. Finally NADPH (0.2 mM) was added and the decrease in absorbance at 340 nm was determined.
Inhibition experiments
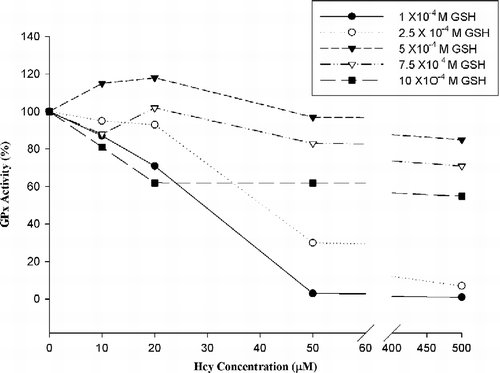
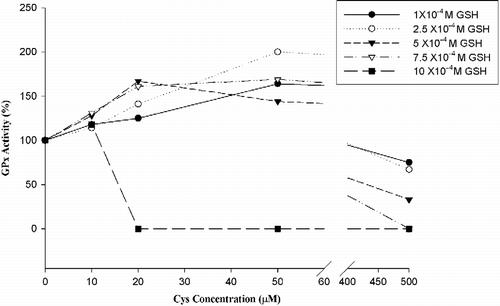
Hcy and Cys, which are thiol-containing amino acids, were used as inhibitors in our experiments. Inhibition experiments were carried out at different GSH concentrations (1x10-4 M , 10x10− 4 M GSH) in the presence of 10 μM, 20 μM, 50 μM, 500 μM inhibitor (Hcs or Cys) concentration. 5 mU/mL GPx-1% activity changes are shown in and .
To investigate the effects of Hcy and Cys on GR (10 U/mL) activity we examined the enzyme activity at 0μM, 50 μM, 500μM inhibitor concentrations in the presence of 5x10− 4M GSSG. Hcy effect on GR activity is shown in and Cys effect is shown in .
Table I. Hcy effect on GR Activity.
Table II. Cysteine effect on GR activity.
Experiment of NADPH consumption
Another important aspect of the work was to investigate whether or not NADPH was consumed by homocysteine's itself. For this purpose the absorbance change was recorded of NADPH (2 mM, 100 μL) in sodium phosphate (NaP) buffer (pH 7, 5 mM, 900 μL) at 340 nm during 10 min and compared with the absorbance changes of mixtures which contained NaP buffer, NADPH (2 mM, 100 μL) and Hcy at different concentrations (50 μM, 500 μM, 1 mM, 1.5 mM, 2 mM) at 340 nm during 10 min. The results are summarized in .
Table III. Investigation of the whether or not NADPH is consumed by homocysteine itself at 340 nm
In all measurements pure water was used as blank and measurements were repeated three times.
Results
According to Hcy inhibits GPx-1 activity in hyperhomocysteinemia conditions (50–500 μM Hcy).
The results in showed that Cys increased GPx-1 activity in low glutathione concentrations, and inhibition clearly appeared at 500 μM Cys concentration.
The results in showed that Hcy had no inhibitory effect on GR activity and actually increases enzyme activity.
The results in showed that Cys had no inhibitory effect on GR activity.
showed that the end of this experiment, NADPH was not consumed by homocysteine's itself.
Discussion
Glutathione peroxidases (Gpxs) are selenoproteins [Citation23,Citation24], containing Se in the form of the amino acid selenocysteine at their active site [Citation25,Citation26]. GPxs catalyze the reduction of hydrogen peroxide and various organic hydroperoxides into water and alcohols, respectively [Citation27]. There are at least six GPx isoenzymes found in mammals; 1) cellular glutathione peroxidase (GPx-1) [Citation28], 2) gastrointestinal glutathione peroxidase (GPx-2) [Citation29], 3) plasma glutathione peroxidase (GPx-3) [Citation30], 4) phospholipid hydroperoxide glutathione peroxidase (GPx-4) [Citation31], 5) epididymal glutathione peroxidase (GPx-5) [Citation32], and, 6) Glutathione peroxidase 6 (GPx-6) [Citation33].
GPx-1 is a cytosolic enzyme expressed in every cell type and is thought to be one of the major antioxidant proteins in mammals [Citation23]. The enzyme was first described in 1957 and is found mainly in cytoplasm [Citation28].
The mechanism for the GPx reactions is shown in . The first step is an oxidation of the selenol group of the enzyme by a hydroperoxide to form a selenic acid derivative (ESeOH). The second step leads to the formation of covalent bonding between the sulfur of the GSH and the selenium of the enzyme to form a selenenyl sulfide adduct (ESeSG). The last step is the regeneration of the reduced enzyme via a second GSH that breaks the selenadisulfide bridge in ESeSG [Citation34] (see ).
Figure 3 The catalytic mechanism of natural GPx [Citation34,Citation35].
![Figure 3 The catalytic mechanism of natural GPx [Citation34,Citation35].](/cms/asset/3eb2ed2a-2018-42c8-809d-bfa3217ad1e5/ienz_a_216420_f0003_b.gif)
In previous studies, it has been shown that homocysteine inhibits the expression of GPx-1 [Citation19,Citation20,Citation21] but no study exists concerning the direct effects of Hcy on GPx-1 activity. The results of the present study indicate that high Hcy concentrations (50–500 μM), especially at low glutathione concentrations, inhibit GPx-1 activity. These observation can be a step at explaning of the responsible mechanism of Hcy on antioxidant system such as atherosclerosis formation at the endothelial bed. The red cells of normal adults contain 68.5 ± 10.8 mg/dL GSH [Citation22]. In our inhibition experiments we used low GSH concentrations to examine the effects of the thiol containing amino acids, Hcy and Cys, at low antioxidant concentrations.
Besides Hcy we also examined the effects of cysteine to compare with Hcy. Hcy inhibited GPx-1 activity in hyperhomocysteinemia conditions (50–500 μM Hcy). On the other hand cysteine increased GPx-1 activity in low glutathione concentrations, and it's inhibition clearly appeared at 500 μM Cys concentration.
These results can be explained by two possible mechanism; 1) In high concentrations, thiol metabolites (Hcy, Cys) are bound to Se, which is the active site of the enzyme, directly and block ESeSG formation in the reaction cascade so that the activity of the enzyme is decreased 2) Thiol metabolites can react with GSH in the last step of the GPx reaction so that the enzyme cannot be regenerated and activity is decreased.
GPx activity measurement were carried out in two stages. In the first stage; GPx detoxifies the hydroperoxide with the aid of GSH; in this step GSH is converted to GSSG. In the second stage GR reduces the GSSG by using NADPH as a cofactor. Hydroperoxide reduction was followed by a decrease in NADPH absorbance at 340 nm.
To investigate the effect of Hcy on GR activity we examine the enzyme activity at 0μM, 50 μM, 500μM Hcy concentrations. In the absence of Hcy (0μM Hcy) GR activity was 1.28 U/mL − 0.68 U/mL (with FAD-without FAD), under the conditions of 50μM and 500μM Hcy concentrations, GR activity was found 1.74 U/mL − 1.49 U/mL (with FAD-without FAD), 1.58 U/mL − 1.30 U/mL (with FAD-without FAD) respectively. These results showed that Hcy has no inhibitor effect on GR activity and actually increases enzyme activity. In consequence of this study it is demonstrated that Hcy inhibits GPx activity directly.
Another important aspect of the work was to investigate whether or not NADPH was consumed by homocysteine itself. For this purpose we recorded the absorbance change of NADPH in NaP buffer at 340 nm during 10 minutes in comparison with the absorbance changes of mixtures which contained NaP buffer, NADPH and Hcy at different concentrations over the same period. At the end of the study it was observed that Hcy alone did not consume NADPH.
In conclusion, all these observations indicated that Hcy inhibits GPx-1 activity directly in severe hyperhomocysteinemia (50–500 μM Hcy) conditions especially at low antioxidant status.
| Abbreviations | ||
| FAD+: | = | oxidized flavine adenine dinucleotide phosphate |
| GPx: | = | glutathione peroxidase |
| GPx-1: | = | cellular glutathione peroxidase |
| GPx-2: | = | gastrointestinal glutathione peroxidase |
| GPx-3: | = | plasma glutathione peroxidase |
| GPx-4: | = | phospholipid hydroperoxide glutathione peroxidase |
| GPx-5: | = | epididymal glutathione peroxidase |
| GPx-6: | = | Glutathione peroxidase 6 |
| GR: | = | glutathione reductase |
| GSH: | = | reduced glutathione |
| GSSG: | = | oxidized glutathione |
| Hcy: | = | homocysteine |
| NaP: | = | sodium phosphate |
| ROS: | = | reactive oxygen species |
| TBH: | = | tert-butylhydroperoxide |
Acknowledgements
The authors wish to thank Dr. Atilla Dikmen and Dr. Şeref Erdoğan for their expert opinions. This project was supported by Çukurova University Research Foundation (2003YL7).
References
- Apostolova MD, Bontchev PR, Ivanova BB, et al. Copper-homocysteine complexes and potential physiological actions. J Inorg Biochem 2003; 95: 321–333
- Still RA, McDowell IF. Clinical implications of plasma homocysteine measurement in cardiovascular disease. J Clin Pathol 1998; 51: 183–188
- Pastore A, Massoud R, Motti C, et al. Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin Chem 1998; 44: 825–832
- Hankey GJ, Eikelboom JW. Homocysteine and vascular disease. Lancet 1999; 354: 407–413
- Mudd SH, Levy HL, Skovby F. Disorders of transsulfuration. The metabolic and molecular bases of inherited disease8th, CR Scriver, AL Beaudet, WS Sly, D Valle. McGraw-Hill Companies, New York 2001; 2007–2043
- Refsum H, Fiskerstrand T, Guttormsen AB, Ueland PM. Assessment of homocysteine status. J Inher Metab Dis 1997; 20: 286–294
- Nygard O, Vollset SE, Refsum H, Brattström L, Ueland PM. Total homocysteine and cardiovascular disease. J Intern Med 1999; 246: 425–454
- Ragone R. Homocystine solubility and vascular disease. FASEB J 2002; 16(401)4
- Monsen AL, Ueland PM. Homocysteine and methylmalonic acid in diagnosis and risk assesement from infancy to adolescence. Am J Clin Nutr 2003; 78: 7–21
- Romerio SC, Linder L, Nyfeler J, et al. Acute hyperhomocysteinemia decreases NO bioavailability in healthy adults. Atherosclerosis 2004; 176(337)44
- Miller JW. Homocysteine and alzheimer's Disease. Nutr Rev 1999; 57: 126–129
- Ho PI, Collins SC, Dhitavat S, et al. Homocysteine potentiates β-amyloid neurotoxicity: Role of oxidative stress. J Neurochem 2001; 78(1)6
- Wenstrom KD, Johanning GL, Owen J, Johnston KE, Acton S, Tamura T. Role of amniotic fluid homocysteine level and of fetal 5,10-methylenetetrahydrafolate reductase genotype in the etiology of neural tube defects. Am J Med Genet 2000; 90(12)6
- Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: A systematic review. Placenta 1999; 20: 519–529
- Sohda S, Arinami T, Hamada H, Yamada N, Hamaguchi H, Kubo T. Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J Med Genet 1997; 34: 525–526
- Wang J, Trudinger BJ, Duarte N, Wilcken DE, Wang XL. Elevated circulating homocyst(e)ine levels in placental vascular disease and associated pre-eclampsia. BJOG 2000; 107: 935–938
- Heydrick SJ, Weiss N, Thomas SR, et al. L-Homocysteine and L-homocystine stereospecifically induce endothelial nitric oxide synthase-dependent lipid peroxidation in endothelial cells. Free Radic Biol Med 2004; 36: 632–640
- Dayal S, Brown KL, Weydert CJ, et al. Deficiency of glutathione peroxidase-1 sensitizes hyperhomocysteinemic mice to endothelial dysfunction. Arterioscler Thromb Vasc Biol 2002; 22: 1996–2000
- Weiss N, Zhang Y, Heydrick S, Bierl C, Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. PNAS 2001; 98: 12503–12508
- Upchurch GR, Welch GN, Fabian AJ, et al. Homocyst(e)ine decreases bioavaliable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 1997; 272: 17012–17017
- Outinen PA, Sood SK, Pfeifer SI, et al. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood 1999; 94: 959–967
- Beutler E. Red cell metabolism3rd ed. Grune and Stratton, Orlando 1984
- Tapiero H, Townsend DM, Tew KD. The antioxidant role of selenium and selenocompounds. Biomed Pharmacother 2003; 57: 134–144
- Holben DH, Smith AM. The diverse role of selenium within selenoproteins: A review. J Am Diet Assoc 1999; 99: 836–843
- Takebe G, Yarimizu J, Saito Y, et al. A comperative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein. P J Biol Chem 2002; 277: 41254–41258
- Kelner MJ, Bagnell RD, Montoya MA, Lanham KA. Structural organization of the human gastrointestinal glutathione peroxidase (GPX2) promoter and 3′-nontranscribed region: Transcriptional response to exogenous redox agents. Gene 2000; 248: 109–116
- Haan JB, Bladier C, Griffiths P, et al. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stres-inducing agents paraquat and hydrogen peroxide. J Biol Chem 1998; 273: 22528–22536
- Tan M, Li S, Swaroop M, Guan K, Oberley LW, Sun Y. Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem 1999; 274: 12061–12066
- Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem 1993; 268: 2571–2576
- Maddipati KR, Marnett LJ. Characterization of the major hydroperoxide-reducing activity of human plasma. J Biol Chem 1987; 262: 17398–17403
- Puglisi R, Tramer F, Carlomagno G, et al. PHGPx in spermatogenesis: How many functions?. Contraception 2005; 72: 291–293
- Hall L, Williams K, Perry ACF, Frayne J, Jury JA. The majority of human glutathione peroxidase type 5 (GPX5) transcripts are incorrectly spliced: Implications for the role of GPX5 in the male reproductive tract. Biochem J 1998; 333: 5–9
- Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science 2003; 300: 1439–1443
- Yu H, Liu J, Liu X, Zang T, Luo G, Shen J. Kinetic studies on the glutathione peroxidase activity of selenium-containing glutathione transferase. Comp Biochem Physiol 2005; 141: 382–389
- Flohé L. The selenoprotein glutathione peroxidase. Coenzymes and cofactors, G Dolphin, R Poulson, O Avramovic. John Wiley and Sons, New York 1989; vol. 3.: 644–731