Abstract
The inhibition of two human carbonic anhydrase (HCA, EC 4.2.1.1) isozymes, the cytosolic HCA I and II, with heavy metal salts of Pb(II), Co(II) and Hg(II)has been investigated. Human erythrocyte CA-I isozyme was purified with a specific activity of 920 EUmg− 1 and a yield of 30% and CA-II isozyme was purified with a specific activity of 8000 EUmg− 1 and a yield of 40% using Sepharose-4B-L tyrosine-sulfanilamide affinity gel chromatography. The overall purification was approximately 104-fold for HCA-I and 900-fold for HCA-II. The inhibitory effects of different heavy metals (lead, cobalt and mercury) on CA activity were determined at low concentrations using the esterase method under in vitro conditions. Ki values for these metals were calculated from Lineweaver-Burk graphs as 1.0, 3.22 and 1.45 mM for HCA-I and 0.059, 1.382 and 0.32 mM for HCA-II respectively. Lead was a noncompetitive inhibitor for HCA-I and competitive for HCA-II, cobalt was competitive for HCA-I and noncompetitive for HCA-II and mercury was uncompetitive for both HCA-I and HCA-II. Lead was the best inhibitor for both HCA-I and HCA-II.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are widespread metalloenzymes in higher vertebrates including humans [Citation1]. These metalloproteins catalyze the equilibration between CO2 and HCO3– using zinc as co-factor.
Sixteen isozymes of the zinc binding enzyme have been described that differ in their subcellular localization, catalytic activity and susceptibility to different classes of inhibitors. Some of these isozymes are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), others are membrane bound (CA IV, CA IX, CA XII and CA XIV), two are mitochondrial (CA VA and CA VB), and one is secreted in saliva (CA VI). Hilvo et al. (2005) have reported that CA XV isoform is not expressed in humans or in other primates but it is abundant in rodents and other higher vertebrates [Citation2]. There are also three acatalytic forms called CA-related proteins (CARPs): CARP VIII, CARP X and CARP XI [Citation1].
It is known that carbonic anhydrase isozymes are present in various tissues of living organisms. In humans CAs exist in the gastrointestinal tract, the reproductive tract, the nervous system, kidneys, lungs, skin and eyes, among others [Citation1].
CA isozymes are involved in important physiological and pathological functions, such as pH and CO2 homeostasis, respiration and transport of CO2/HCO3− between metabolizing tissues and the lungs, electrolyte secretion in different tissues/organs and biosynthetic reactions (such as gluconeogenesis, lipogenesis and ureagenesis). The two major CA isozymes (CAI and CAII) are present at high concentrations in the cytosol in erythrocytes, and CAII has the highest turnover rate of all the CAs [Citation3].
As well as physiological functions, the carbonic anhydrase enzymes also catalyze some non-physiological reactions [Citation4], for instance, it was observed that the purified enzyme has esterase activity in in vitro conditions [Citation5].
![]()
The enzyme has been purified from many tissues including human erythrocytes and its kinetic properties have been examined in human erythrocytes [Citation6], fish gills [Citation7] and erythrocytes [Citation8], rat saliva and erythrocytes [Citation9], Plasmodium falciparum [Citation10], insect [Citation11], bovine bone [Citation12], bovine leucocytes [Citation13]. Morover, it has been reported that CA has been partially characterized from plants, yeast and bacteria [Citation10].
Exposure to heavy metals is an important problem of environmental toxicology. Most heavy metals are toxic to humans, animals and plants. Man is at great risk of suffering from health hazards associated with toxic metals because of bioaccumulation [Citation14].
Hura and Hura 2005 has evaluated the heavy metal content in the food of the Eastern Romania area. They have found that meat, vegetables, bakery products and diets included lead and cadmium. Radwan and Salama 2005 carried out a survey to assess the levels of lead, cadmium, copper and zinc in various fruits and vegetables sold in Egyptian markets. They detected the highest mean levels of Pb, Cd, Cu and Zn in strawberries, cucumber, dates and spinach, respectively [Citation15]. Sivaperumal et al. 2006 evaluated the concentration of heavy metals in commercially important species of fish, shellfish and fish products from fish markets in and around the Cochin area. The study showed that different metals (Cd, Pb, Hg, Cr, As, Zn, Cu, Co, Mn, Ni, and Se) were present in the samples at different levels [Citation16]. In another study the concentrations of nickel and cobalt in refined beet sugar samples were evaluated [Citation17].
It is clear that different food may contain heavy metals at different levels. Determination of these substances in food is important in environmental monitoring for the prevention, control and reduction of pollution as well as for health.
Many chemical susbstances and synthesized drugs affect metabolisms by changing enzyme activities. Chemicals are generally known to activate or inhibit several body enzymes in vivo [Citation18] and affect the metabolic pathways.
In the present study we have purified carbonic anhydrase I and II from human erythrocytes and examined the in vitro inhibition effects of some heavy metals on these important enzymes.
Materials and methods
Chemicals
CNBr-activated Sepharose 4B, protein assay reagents, 4-nitrophenylacetate and chemicals for electrophoresis were purchased from Sigma-Aldrich Co. (Sigma-Aldrich Chemie GmbH Export Department Eschenstrasse 5, 82024 Taufkirchen, Germany). Para-aminobenzenesulfonamide and L-tyrosine were from E. Merck (Merck KGaA Frankfurter strasse 250, D-64293 Darmstadt Germany). All other chemicals were analytical grade and obtained from either Sigma-Aldrich or Merck.
Purification of carbonic anhydrase isozymes from human erythrocytes by affinity chromatography
Erythrocytes were purified from fresh human blood obtained from the Blood Center of the Research Hospital at Atatürk University. The blood samples were centrifuged at 1500 rpm for 15 min and the plasma and buffy coat were removed. The red cells were isolated and washed twice with 0.9% NaCl, and hemolysed with 1.5 volumes of ice-cold water. The ghost and intact cells were removed by centrifugation at 20 000 rpm for 30 min at 4°C. The pH of the hemolysate was adjusted to 8.7 with solid Tris. The hemolysate was applied to the prepared Sepharose 4B-L-tyrosine-sulfanylamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The human carbonic anhydrase (HCA-I and CA-II) isozymes were eluted with 1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6), respectively. All procedures were performed at 4°C [Citation19].
Hydratase activity assay
Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson (1976). CO2-Hydratase activity as an enzyme unit (EU) was calculated by using the equation where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
Esterase activity assay
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBİOS UV-VIS) according to the method described by Verpoorte et al. [Citation5]. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1 mL 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution.
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the standard [Citation21].
SDS polyacrylamide gel electrophoresis
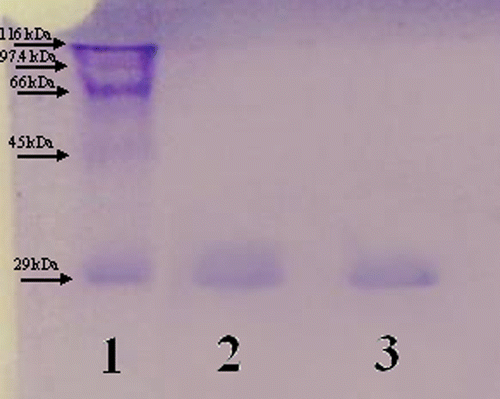
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli. A 20 μg sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coommassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without the dye. The electrophoretic pattern was photographed (see ).
In vitro inhibition studies with heavy metals
The inhibitory effects of lead, cobalt and mercury salts (Merck) were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used. HCA-I enzyme activities were measured for Pb(NO3)2 (2 × 10− 4–1 × 10− 3M), CoCl2 × 6H2O (1 × 10− 3–5 × 10− 3 M), HgCl2 (1 × 10− 3–5 × 10− 3M) at cuvette concentrations and HCA-II enzyme activities were measured for Pb(NO3)2 (2 × 10− 4–1 × 10− 3 M), CoCl2 × 6H2O (2 × 10− 5–3 × 10− 3 M), HgCl2 (1 × 10− 3–5 × 10− 3M) at cuvette concentrations. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor an Activity%-[Inhibitor] graph was drawn. To determine Ki values, three different inhibitor concentrations were tested; Pb(NO3)2: 2 × 10− 4–1 × 10− 3 M for HCA-I; 2 × 10− 4–5 × 10− 4 M for HCA-II, CoCl2 × 6H2O: 1 × 10− 3–5 × 10− 3M for HCA-I; 2 × 10− 5–2 × 10− 3 for HCA-II, HgCl2: 1 × 10− 3–4 × 10− 3M for HCA-I; 2 × 10− 3–5 × 10− 3 M for HCA-II. In these experiments, 4-nitrophenylacetate was used as substrate at five different concentrations (0.3–0.9 mM). The Lineweaver–Burk curves obtained were used for the determination of Ki and the inhibitor type.
Results
The purification of the enzymes was performed with a simple one step method by a Sepharose-4B-L tyrosine-sulfanilamide affinity column. HCA-I enzyme was purifed ∼104-fold with a specific activity of 920 EUmg− 1 and overall yield of 30% and the HCA-II enzyme was purifed ∼900-fold with a specific activity of 8000 EUmg− 1 and overall yield of 40% (). shows the SDS-PAGE obtained for determining the purity of the enzymes.
Table I. Summary of purification procedure for HCA-I and HCA-II.
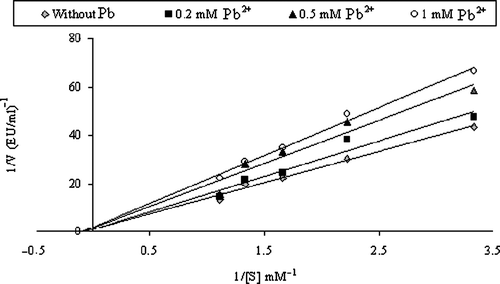
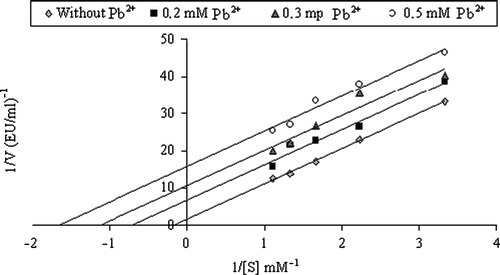
Inhibitory effects of heavy metals on enzyme activities were tested under in vitro conditions and Ki values were calculated from Lineweaver–Burk graphs and are given in and the representative graphs for lead are shown in and .
Table II. Ki values and inhibition types for 3 inhibitors of HCA-I and HCA-II.
Discussion
Carbonic anhydrase which is a widespread metalloenzyme has previously been purified and characterized from many living organisms including animals Citation10Citation24-26. The isozymes of CA play important roles in different tissues [Citation27,Citation28]. The similarities of CAs from various sources have been determined from their crystal structures [Citation29]. It is known that carbonic anhydrase has been purified many times from different organisms and the effects of various chemicals, pesticides and drugs on its activity have been investigated Citation30-32. Hundreds of pollutants in the form of metals, acids, bases and other toxic compounds are being added to rivers, seas and the atmosphere a situation which has resulted in the destruction of the natural balance [Citation30].
In this study, CA-I and II isoenzymes from human eyrtrocytes were purified by a simple one step procedure by using Sephrarose 4B-L-tirozin-sulfanilamide affinity column. The activity of the effluents were determined by the hydratase method [Citation20] and other further kinetic studies were performed using the esterase activity method [Citation5]. HCA-I was purified 104-fold with specific activity (920 EUmg− 1) and yield (30%). Similarly, HCA-II was purified 900-fold with specific activity (8000 EUmg− 1) and yield (40%). SDS-PAGE of the enzymes showed a single polypeptide band ().
Generally, the hydratase activity method is used in kinetic studies on CA and, we have not encountered any studies on the inhibitory effects of heavy metals on CA esterase activity. In fact, most heavy metals inhibit CA I/II in the micromolar range as determined by the CO2 hydratase method. However the esterase method usually gives much higher Ki values with all inhibitors. Vitale et al. investigated the in vitro effects of some heavy metals on estuarine crab CA using the CO2 hydration reaction and calculated the IC50 values [Citation31]. When these results are compared with those here obtained using the esterase method, it is clearly seen that the inhibition values obtained from the CO2 hydration reaction are lower. This shows the importance of the use of the physiological substrate in kinetic studies. For example, Gervais and Tufts used CO2 as a substrate of CA under physiological conditions to determine the Kcat value [Citation32]. However, using the esterase method for the same reason in another study found that the Kcat value from the hydratse method was higher than that of the esterase method (Unpublished Data). However, the advantage of the esterase activity determination is that it is a spectrophotometric and easy method.
Inhibition effects of many substances such as medical drugs, various metals, anions and pesticides have been reported in the literature Citation27Citation33-36. Many chemicals affect metabolism by changing normal enzyme activity, particularly inhibition of a specific enzyme [Citation37,Citation38], and the effects can be dramatic and systemic [Citation39]. Hence, heavy metals have various toxicological effects on living organisms. For example, it has been reported that heavy metals, such as mercury and cadmium, exert a toxic action in a synergistic fashion with salinity [Citation31,Citation37,Citation39]. Also, it has been expressed that the binding of heavy metals with membrane transport ligands can alter their catalytic function [Citation31,Citation42]. § Lead, cobalt and mercury salts were chosen for investigation of their inhibitory effects on CA in this study and it was important that heavy metals inhibited the enzyme activity at low concentrations. Ki parameters of these metals for HCA-I and HCA-II were determined and it was found that Pb2 + ,Co2 + and Hg2 + were potent inhibitors of CA. Lineweaver-Burk plots showed that Pb2 + inhibited HCA-I in a noncompetitive manner; HCA-II in an uncompetitive manner (, , ), Co2 + inhibits HCA-I in a competitive manner; HCA-II in a noncompetitive manner () and Hg2 + inhibits both HCA-I and HCA-II in an uncompetitive manner (). As can be clearly seen from the Ki values, the best inhibitor for both HCA-I and HCA-II was lead.
| Abbreviations | ||
| CA: | = | carbonic anhydrase |
| HCA-I: | = | human carbonic anhydrase I |
| HCA-II: | = | human carbonic anhydrase II |
References
- Supuran CT. Carbonic anhydrases: Catalytic and inhibition mechanisms, distribution and physiological roles carbonic anhydrase. Carbonic anhydrase. Its inhibitors and activators, CT Supuran, Conway J Scozzafara. CRC Press, London 2004; 1–23
- Hilvo M, Tolvanen M, Clark A, Shen BR, Shah GN, Waheed A, Halmi P, Hanninen M, Hamalainen JM, Vihinen M, Sly WS, Parkkila S. Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase. Biochem J 2005; 392: 83–92
- Ozensoy O, Arslan O, Oznur Sinan S. A new method for purification of carbonic anhydrase isozymes by affinity chromatography. Biochemistry - Moscow 2004; 69: 216–219
- Supuran CT, Scozzafava A. Applications of carbonic anhydrase. inhibitors and activators in therapy. Expert Opin Ther Pat 2002; 212: 217–242
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases. B and C J Biol Chem 1967; 242: 4221–4229
- Beydemir S, Çiftçi M, Kufrevioglu OI, Buyukokuroglu ME. Effects of gentamisin sulfate on enzyme activities of carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. Biol Pharm Bull 2002; 25: 966–969
- Bone Q, Marshall NB, Blaxter JHS. Sensory systems and communication, USA. Biology of Fishes, Q Bone, NB Marshall, JHS Blaxter. Chapman Hall, New York, NY 1995; 219–261
- ARENA (Applied Research Ethics National Association). Institutional animal care and use committee guidebook2nd Ed. Boston ARENA; 2002; 121–125
- Feldstein JB, Silverman DN. Purification and characterization of carbonic anhydrase from the saliva of the rat. J Biol Chem 1984; 259: 5447–5453
- Krungkrai SR, Suraveratum N, Rochanakij S, Krungkrai J. Characterization of carbonic anhydrase in plasmodium falciparium. Int J Parasıtol 2001; 31: 661–668
- Burt E, Darlington MV, Graf G, Meyer HJ. Isolation, purification and characterization of an insect carbonic anhydrase. Insect Bıochem Mol Biol 1992; 22: 285–291
- Sender S, Bottcher K, Cetin Y, Gros G. Carbonic anhydrase in the gills of seawater- and freshwater-acclimated flounders platichthys flesus: Purification, characterization, and immunohistochemical localization. J Hıstochem Cytochem 1999; 47: 43–50
- Gervais MR, Tufts BL. Characterization of carbonic anhydrase and anion exchange in the erythrocytes of bowfin (Amia calva), a primitive air-breathing fish. Comp Biochem Phys 1999; 23A: 343–350
- Hura C, Hura BA. Assessment of the heavy metals in the food from romania, 2005. Toxicology Letters 2006; 164: 270
- Radwan MA, Salama AK. Market basket survey for some heavy metals in egyptian fruits and vegetables. Food Chem Toxicol 2006; 44: 1273–1278
- Sivaperumal TV, Sankar PG. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chem 2006, in press
- Sancho D, Deban L, Campos I, Pardo R, Vega M. Determination of nickel and cobalt in refined beet sugar by adsorptive cathodic stripping voltammetry without sample pretreatment. Food Chem 2000; 71: 139–145
- Çiftçi M, Beydemir Ş, Yılmaz H, Bakan E. Effects of some drugs on rat erythrocyte 6-phosphogluconate dehydrogenase: An in vitro and in vivo study. Pol J Pharm 2002; 54: 275–280
- Arslan O, Nalbantoglu B, Demir N, Ozdemir H, Küfrevioğlu Öİ. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Trop J Med Sci 1996; 26: 163–166
- Wilbur KM, Anderson NG. Electrometric and colorometric determination of carbonic anhydrase. J Biol Chem 1976; 176: 147–151
- Bradford MM. A rapid and sensitive method for the quantition of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–251
- LaemmLi DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970; 227: 680–683
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934; 57: 685
- Bottcher K, Waheed A, Sly WS. Membrane-associated carbonic anhydrase from the crab gill: Purification, characterization, and comparison with mammalian CAs. Arch Biochem Biophys 1994; 312: 429–435
- Beydemir S, Gülçin I. Effects of melatonin on carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. J Enz Inhib Med Chem 2004; 19: 193–197
- Zhenyan Y, Liping X, Seunghwan L, Rongqing ZA. Novel carbonic anhydrase from the mantle of the pearl oyster (Pinctada fucata). Comp Biochem Physiol B 2006; 143: 190–194
- Bülbül M, Hisar O, Beydemir S, Çiftçi M, Küfrevioğlu ÖI. The in vitro and in vivo inhibitory effects of some sulfonamide derivatives on rainbow trout (Oncorhynchus Mykiss) erythrocyte carbonic anhydrase activity. J Enz Inhib Med Chem 2003; 18: 371–375
- Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents?. Bioorg Med Chem 2001; 9: 703–714
- Huang S, Xue Y, Sauer-Eriksson E, Chirica L, Lindskog S, Jonsson BH. Crystal structure of carbonic anhydrase from Neisseria gonorrhoeae and its complex with the inhibitor acetazolamide. J Mol Biol 1998; 283: 301–310
- Çelik I, Çamaş H, Arslan O, Küfrevioğlu ÖI. The effects of some pesticides on human and bovine erythrocyte carbonic anhydrase enzyme activities in vitro. J Envıron Scı Heal A 1996; 31: 2651–2657
- Vitale AM, Monserrat JM, Castilho P, Rodriguez EM. Inhibitory effects of cadmium on carbonic anhydrase activity and ionic regulation of the estuarine crab Chasmagnathus granulata (Decapoda, Grapsidae). Comp Biochem Phys C 1999; 122: 121–129
- Gervais MR, Tufts BL. Characterization of carbonic anhydrase and anion exchange in the erythrocytes of bowfin (Amia calva), a primitive air-breathing fish. Comp Biochem Phys; 1999; 23A: 343–350
- Innocenti A, Antel J, Wurl M, Vullo D, Firnges MA, Scozzafavaa A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of isozymes I, II, IV, V and IX with complex fluorides, chlorides and cyanides. Bioorg Med Chem Lett 2005; 15: 1909–1913
- Beydemir S, Gülçin I. Effects of melatonin on carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. J Enzy Inhib Med Chem 2004; 19: 193–197
- Casini A, Mincione F, Vullo D, Menabuoni L, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors with strong topical antiglaucoma properties incorporating a 4-(2-aminopyrimidin-4-yl-amino)-benzenesulfonamide scaffold. J Enz Inhib Med Chem 2002; 17: 9–18
- Casini A, Scozzafava A, Mincione F, Menabuoni L, Supuran CT. Carbonic anhydrase inhibitors: Synthesis of water soluble sulfonamides incorporating a 4-sulfamoylphenylmethylthiourea scaffold, with potent intraocular pressure lowering properties. J Enz Inhib Med Chem 2002; 17: 333–343
- Hochster RM, Kates M, Quastel JH. Metabolic inhibitors. 1973; Vols. 3 and 4: 66–82, New York: Academic Press; 71–89
- Özdemir H, Uğuz MT. In vitro effects of some anesthetic drugs on lactoperoxidase enzyme activity. J Enz Inhib Med Chem 2005; 20: 491–495
- Christensen GM, Olson D, Riedel B. Chemical effects on the activity of eight enzymes: A review and a discussion relevant to environmental monitoring. Environ Res 1982; 29: 247–255
- O'Hara J. Cadmium uptake by fiddler crabs exposed to temperature and salinity stress. J Fish Res Board Can 1973; 30: 846–848
- Bianchini A, Gilles R. Toxicity and accumulation of mercury in three species of crabs with different osmoregulatory capacities. Bull Environ Contam 1996; 57: 91–98
- Rainbow PS, Dallinger R. Metal uptake, regulation and excretion in freshwater invertebrates. Ecotoxicology of metals in invertebrates, R Dallinger, PS Rainbow. Lewis Publishers, Boca Raton, FL 1993; 119–131