Abstract
We have previously reported on the synthesis of novel indole derivatives where some compounds showed significant antioxidant activity. Here, we report the synthesis of novel N–H and N-substituted indole-2- and 3-carboxamide derivatives and investigated their antioxidant role in order to identify structural characteristics responsible for activity. Although all compounds showed a strong inhibitory (95–100%) effect on superoxide anion (SOD) only compounds 4, 5 and 6 showed simliar potency for the inhibition of lipid peroxidation (81–94%) which revealed that compounds 4, 5 and 6 possessed highly potent antioxidant properties. Substitution in the 1-position of the indole ring caused the significant differences between the activity results regarding lipid peroxidation inhibition.
Introduction
In recent years much evidence has proved the link existing between the development of human diseases and oxidative stress, oxidative stress being caused by increased free radical generation. Free radicals are capable of altering all major classes of biomolecules, such as lipids, nucleic acids and proteins. There has been an intensive interest in the role of oxygen-free radicals, more generally known as reactive oxygen species (ROS) along with reactive nitrogen species (RNS) [Citation1]. ROS and RNS are formed regularly as a result of normal organ functions, or as a result of excess oxidative stress [Citation2]. Prime targets of free radicals are the polyunsaturated fatty acids in cell membranes and their interaction results in lipid peroxidation [Citation3]. Oxidative stress has been implicated in the development of neurodegenerative diseases like Parkinson's [Citation4] and Alzheimer's disease [Citation5], aging [Citation6] and some types of cancer [Citation7].
Many publications have covered the antioxidant role of several indole derivatives. Among them, indole-3-carbinol has been reported as a free radical scavenger and it has been associated with a variety of benefits including chemoprevention of cancers of the endometrium, lung, mammary tissue, tongue, colon and liver in various animal models [Citation8]. Amine triazole-containing indole derivatives showed protective activity against the oxidative injury of ischemic myocardium and they scavenged superoxide anion and hydroxyl radical [Citation9]. The combination of indole-3-acetic acid (IAA) and horseradish peroxidase (HRP) has recently been proposed as a novel cancer therapy. It has been reported that IAA activated by HRP produces free radicals such as indolyl, skatolyl and peroxyl radicals. In addition, it has been proposed that IAA stimulates the production of ROS which initiates cellular damage and apoptosis. Thus, the proposed IAA/HRP combination may enhance cellular oxidative stress, and lead to cell death [Citation10]. Some di-indolylmethane (DIM) derivatives possessed potent radical scavenging activities and some of them showed inhibitory activities in a primary anticancer assay in vitro [Citation11]. Modified analogues of trolox (indolinic nitroxides) were also found as efficient antioxidants, protecting both lipids and proteins from peroxidation [Citation12]. Another study showed that 5-methoxy-2-(N-acetylaminoethyl)indole maintained a significant antioxidant and cytoprotective effect [Citation13]. More recently, several benzimidazole-containing indoles and tetrahydro-naphthalene-indole derivatives were reported as strong inhibitors of lipid peroxidation and superoxide anion formation [Citation14,Citation15].
Recent data from our laboratory showed the important antioxidant effects of N-substituted amide [Citation16,Citation17] and ester [Citation18] derivatives of indole. It was found that indole amides can scavenge oxygen free radicals, and some of them also inhibited the 1O2 which were produced by cyclooxygenases [Citation17]. Another study demonstrated that a series of 3-(substituted-benzylidene)-1, 3-dihydro indolin-2 one and thione derivatives were effective as radical scavengers and may be considered as an important source for combating oxidative damage [Citation19]. More recently, we found that some N-substituted indole 2- and 3-carboxamides, N-H and N-substituted indole 3-propanamide derivatives had strong antioxidant activity (unpublished results). In the initially prepared compounds, several halo substituents were present at different positions on the aromatic side chain. Here, for a better and wider definition of the structure-activity relationships (SAR), we have synthesized similiar derivatives, paying particular attention to the role of the different lipophilic group and its position on the aromatic ring and we also explored the activity pattern of congeners at the 2- or 3-positions of the indole ring.
Materials and methods
Chemistry
Indole-3-carboxylic acid, 4-chloroaniline, 3-chloro-4-fluoroaniline, 1,1'-carbonyldiimidazole (CDI) were from Aldrich; p-fluoro benzyl bromide, dichloromethane, anhydrous sodium sulphate, anhydrous tetrahydro furane, anhydrous dimethylformamide and hexane were from Merck; indole-2-carboxylic acid, deuterated dimethylsulfoxide and, deuterated chloroform were from Acros; methanol, ethyl acetate and toluene from Riedel–de Häen; sodium hydride was from Fluka; ethanol was from Ildam; nitrogen gas was from Kargaz, Cytochrome c, α-tocopherol (Vit E) and thiobarbituric acid (TBA) were purchased from Sigma Chemicals Co. Male Wistar rats (200–220 g) were used for the assay of lipid peroxidation and received a standart diet. Procedures involving the animals and their care conformed to Institutional guidelines, in compliance with National and International laws and guidelines for the use of animals in biomedical research. Melting points were measured with a capillary melting point apparatus (Electrothermal 9100). 1H-NMR spectra were recorded on a Varian Mercury 400 NMR spectrometer (400 MHz) with Me4Si as internal standard. Chemicals shifts (δ) were reported in parts per million (ppm) and signals were reported as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). Mass spectra were recorded on a Waters ZQ Micromass LC-MS Spectrometer Electrosprey Ionization (ESI). Infrared (IR) spectra were measured on a Jasco FT/IR-420. Analytical TLC was carried out on Merck 0.2 mm precoated silica gel (60 F-254) aluminium sheets, with visualization by irradiation with a UV lamp. Flash column chromatography was accomplished on silica gel 60 (230–400 mesh, Merck).
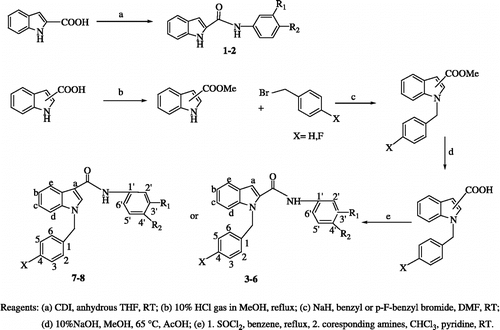
Synthesis of compounds (1–2)
1, 1'-carbonyldiimidazole (0.005 mol) was added under stirring in a nitrogen atmosphere, to a solution of indole-2-carboxylic acid (0.005 mol) in anhydrous THF. The mixture was stirred at room temperature for 1 h. After carbon dioxide evolution had ceased, a solution of the appropriate amine (0.005 mol) in THF was added and the reaction mixture was stirred at room temperature overnight. The mixture was diluted with water and extracted with ethyl acetate (3 × 50 mL). The combined organic phase was dried over anhydrous Na2SO4 and evaporated to dryness to give crude compounds, which were crystallized from MeOH.
General procedure for synthesis of N-substituted indole-2-and 3-carboxamide derivatives (3–8)
1-Benzyl- and p-fluorobenzyl indole-2-carboxylic acids and 1-benzyl indole-3-carboxylic acids (0.002 mol) were refluxed in 5 mL benzene (CARE-CARCINOGEN) with 2.5 mL SOCl2 for 2 h at 80°C. The solvent and SOCl2 were removed by co-evaporation with toluene (3 × 10 mL). The residue was dissolved in 10 mL chloroform and an equivalent amount of pyridine and the corresponding amine derivatives were added. The mixture was stirred at room temperature overnight. The solvent was evaporated to dryness to give crude compounds, which were purified by silicagel column chromatography (hexane: EtOAc = 8:2) and recrystallized with EtOH.
N-(4-chlorophenyl)-1H-indole-2-carboxamide (1)
Recrystallization from methanol gave pure 1.453 g of compound 1: Yield 82.1%; m.p. 274.6°C; IR (ν, cm− 1) 1639 (CO), 3334 (amide NH), 3419 (indole NH); H1-NMR (400 MHz, DMSO-d6) δ 11.78 (s, 1H, NH), 10.34 (s, 1H, CONH), 7.84 (d, 2H, J = 8.8, H-3', 5'), 7.67 (d, 1H, J = 8.8, H-e), 7.46 (d, 1H, J = 8.0, H-d), 7.44-7.41 (m, 3H, H-2', 6', a), 7.22 (t, 1H, J = 16.0, H-b or c), 7.06 (t, 1H, J = 14.8, H-c or b); C15H11ClN2O: 271.08 (M++1).
N-(3-chloro-4-fluorophenyl)-1H-indole-2-carboxamide (2)
Recrystallization from methanol gave pure 1.453 g of compound 2: Yield 84.4%; m.p. 228.9°C; IR (ν, cm− 1) 1651 (CO), 3322 (amide NH), 3405 (indole NH); H1-NMR (400 MHz, DMSO-d6) δ 11.79 (s, 1H, NH), 10.40 (s, 1H, CONH), 8.09 (dd, 1H, J = 9.2, 2.4, 2.8, H-2'), 7.76 (m, 1H, H-5'), 7.68 (d, 1H, J = 8.0, H-e), 7.47 (d, 1H, J = 6.8, H-d), 7.44-7.41 (m, 2H, H-6', a), 7.23 (t, 1H, J = 16.4, H-b or c), 7.07 (t, 1H, J = 14.8, H-c or b); C15H10ClFN2O: 289.05 (M++1).
1-Benzyl-N-(4-chlorophenyl)-1H-indole-2-carboxamide (3)
Recrystallization from ethanol gave pure 0.232 g of compound 3: Yield 42.7%; m.p. 163.4°C; IR (ν, cm− 1) 1651 (CO), 3282 (amide NH); H1-NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H, CONH), 7.79 (d, 2H, J = 8.8, H-3', 5'), 7.73 (d, 1H, J = 7.6, H-e), 7.55 (d, 1H, J = 7.6, H-d), 7.41-7.07 (m, 10H, aromatic protons), 5.81 (s, 2H, CH2Bz); C22H17ClN2O: 361.13 (M++1).
1-Benzyl-N-(3-chloro-4-fluorophenyl)-1H-indole-2-carboxamide (4)
Recrystallization from ethanol gave pure 0.225 g of compound 4: Yield 39.5%; m.p. 162.5°C; IR (ν, cm− 1) 1646 (CO), 3280 (amide NH); H1-NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H, CONH), 8.03 (dd, 1H, J = 9.6, 2.4, 1.4, H-2'), 7.71 (d, 1H, J = 8.0, H-e), 7.67 (m, 1H, H-5'), 7.54 (d, 1H, J = 8.0, H-d), 7.42-7.04 (m, 9H, H-aromatic protons), 5.84 (s, 2H, CH2Bz); C22H16ClFN2O: 379.13 (M++1).
N-(4-chlorophenyl)-1-(4-fluorobenzyl)-1H-indole-2-carboxamide (5)
Recrystallization from ethanol gave pure 0.252 g of compound 5: Yield 30.1%; m.p. 171.9°C; IR (ν, cm− 1) 1647 (CO), 3301 (amide NH); H1-NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H, CONH), 7.77 (d, 2H, J = 9.2, H-3', 5'), 7.71 (d, 1H, J = 8.4, H-e), 7.55 (d, 1H, J = 8.4, H-d), 7.40-7.04 (m, 9H, H-aromatic protons), 5.81 (s, 2H, CH2Bz); C22H16ClFN2O: 379.13 (M++1).
N-(3-chloro-4-fluorophenyl)-1-(4-fluorobenzyl)-1H-indole-2-carboxamide (6)
Recrystallization from ethanol gave pure 0.312 g of compound 6: Yield 43.0%; m.p. 144.6°C; IR (ν, cm− 1) 1650 (CO), 3254 (amide NH); H1-NMR (400 MHz, CDCl3) δ 8.05 (dd, 1H, J = 8.4, 1.6, 2.0, H-2'), 7.82 (dd, 1H, J = 9.2, 2.8, 2.4, H-5'), 7.77 (s, 1H, H-a), 7.63 (s, 1H, CONH), 7.48–7.44 (m, 2H, H-2, 6), 7.37–7.01 (m, 7H, H-aromatic protons), 5.73 (s, 2H, CH2Bz); C22H15ClF2N2O: 397.10 (M++1).
1-Benzyl-N-(4-chlorophenyl)-1H-indole-3-carboxamide (7)
Recrystallization from ethanol gave pure 0.231 g of compound 7: Yield 36.2%; m.p. 217.2°C; IR (ν, cm− 1) 1636 (CO), 3312 (amide NH); H1-NMR (400 MHz, CDCl3) δ 8.01 (d, 2H, J = 8.4, H-3', 5'), 7.72 (s, 1H, H-a), 7.59 (s, 1H, CONH), 7.55 (d, 2H, J = 8.4, H-2', 6'), 7.32-7.08 (m, 9H, H-aromatic protons), 5.73 (s, 2H, CH2Bz); C22H17ClN2O: 359.17 (M++1).
1-Benzyl-N-(3-chloro-4-fluorophenyl)-1H-indole-3-carboxamide (8)
Recrystallization from ethanol gave pure 0.380 g of compound 8: Yield 34.7%; m.p. 165.7°C; IR (ν, cm− 1) 1644 (CO), 3297 (amide NH); H1-NMR (400 MHz, CDCl3) δ 7.81 (m, 2H, H-2', 5'), 7.70 (d, 1H, J = 8.0, H-e), 7.54 (d, 1H, J = 8.0, H-d), 7.33–7.14 (m, 10H, H-aromatic protons), 5.73 (s, 2H, CH2Bz); C22H16ClFN2O: 378.11 (M++1).
Antioxidant properties of novel indole derivatives
Assay of lipid peroxidation
The effect of synthesized compounds on rat liver homogenate which are induced by FeCl2-ascorbic acid and lipid peroxidation (LP) was determined by the modified method of Mihara et al. [Citation20]. Male albino Wistar rats (200–225 g) were fed with standard laboratory rat chow and allowed to drink tap water ad libitum. The animals were starved for 24 h prior to execution by decapitation under anesthesia. The livers were immediately removed and washed with ice-cold distilled water, and immediately homogenized with an ice chilled Teflon homogenizer. LP was measured spectrophotometrically by estimation of thiobarbituric acid reactant substances (TBARS). The amounts of TBARS were expressed in terms of nmol malondialdehyde (MDA)/g tissue. This optimized assay mixture contained 0.5 mL of liver homogenate, 0.1 mL of Tris-HCl buffer (pH 7.2), 0.05 mL of 0.1 mM ascorbic acid, 0.05 mL of 4 mM FeCl2 and 0.05 mL of various concentrations of synthesized compounds in DMSO/MeOH (5:95), or α-tocopherol. The mixture was incubated for 1 h at 37°C. After incubation, 3.0 mL of H3PO4 and 1.0 mL of 0.6% TBA were added and the mixture shaken vigorously and then boiled for 30 min. After cooling, n-butanol was added and whole mixture was again shaken vigorously. The n-butanol phase was separated by centrifugation at 3000 rpm for 10 min. The absorbance of the supernatant was measured at 532 nm against a blank, which contained all reagents except the liver homogenate.
Superoxide radical scavenging activity
The capacity of the compounds to scavenge superoxide anion formation was determined spectrophotometrically on the basis of inhibition of cytochrome c reduction as per the modified method of McCord et al. [Citation21]. Superoxide anion was generated in the xanthine/xanthine oxidase system. The reaction mixture contained in a final volume of 1.0 mL, 0.05 M phosphate buffer pH 7.8, 0.32 units/mL xanthine oxidase, 50 μM xanthine, 60 mM cytochrome c and different concentration of synthesized compounds in 100 μL. Xanthine oxidase was finally added to this mixture to start the reaction. The absorbance was measured spectrophotometrically at 550 nm for cytochrome c reduction. Each experiment was performed in triplicate, and the results were expressed as a percentage of the control.
Results and discussion
N-H indole-2-carboxamide derivatives 1–2 were prepared from the acid and appropriate amines, in anhydrous THF, under a nitrogen atmosphere, using 1, 1'-carbonyldiimidazole as the condensing agent () [Citation22]. This experimental procedure was not successful for the synthesis of the N-H indole-3-carboxamide derivatives. Well-established methodology was used to synthesize the amide derivatives of indole-2- and 3-carboxylic acids as indicated in [Citation23]. Initially, methyl indole-2-carboxylate was synthesized to protect the carboxylic acid. Substitution of the indole N-H with benzyl or p-fluorobenzyl was accomplished by reaction with NaH in DMF. N-substituted indole carboxylic acid derivatives were obtained by hydrolysis of the N-substituted methyl indole carboxylate and the final N-substituted indole-2 and 3-carboxamide derivatives 3–8 synthesized by converting the acid derivatives to acyl followed by the reaction with the appropriate amines in the presence of pyridine, essentially in accordance with our previous procedure [Citation23]. All products were purified by recrystallization from the appropriate solvent, after preliminary purification, when necessary, on a silica gel column. Their structure was confirmed by 1H-NMR, IR and MS spectral data. In the IR spectra, all compounds showed amide CO stretching in the range 1636–1651 cm− 1, while amide NH stretching of was found as 3280–3334 cm− 1 and indole NH stretching at 3405–3419 cm− 1. Numbering for NMR interpretation is shown in . N-substituted benzyl CH2 protons showed singlets at δ 5.73–5.84 ppm. All aromatic protons have shown chemical shifts at δ 6.87–8.19 ppm.
The in vitro antioxidant effects of the novel N-H and N-substituted indole-2 and 3-carboxamides 1–8 on rat liver microsomal NADPH-dependent lipid peroxidation levels and their free radicals scavenging properties were investigated and compared with Vit E (). Although all compounds showed a strong inhibitory effect on superoxide anion (O2·–) in the range of 95–100% at 10− 3M concentration, they had little or no inhibitory effect at the 10− 4M concentration. Only compounds 4, 5 and 6 inhibited LP in the range 81–94% at the 10− 3M concentration. Moreover all compounds had a higher inhibitory effect on LP than SOD at the 10− 4M concentration. Vit E caused 87% inhibition of superoxide anion production and 68% inhibition of lipid peroxidase at 10− 3 M concentration. Comparison of the activity results revealed that the compounds are equally active or slightly more active than Vit E.
Table I. Inhibition of SOD and LP by compounds 1–8.
The antioxidant profiles of congeners at the 2- or 3-positions of the indole ring were found slightly smiliar. The only difference was between identical congeners 4 and 8. While compound 4 showed 81% inhibiton for LP, compound 8 had 36% inhibition. On the other hand, compound 2, which is the congener of compound 4 without 1-benzyl substitution, showed 48% inhibition for LP. These results indicated that N-substituted indole-2-carboxamide derivatives were stronger inhibitors for LP than N-substituted indole-3-carboxamide derivatives. The special effect of the substitution feature at the 2-position might be due to the lipophilic groups being involved in interaction with the aromatic ring located at the 1-position. This special effect might play a positive role on the inhibitory effect for LP. However, more data is needed to prove this approach with several substitution patterns at both the 2- and 3-positions of indole.
The N-benzyl indole-3-carboxamide derivatives 7 and 8 showed lower inhibition for LP. For indole-2 carboxamide derivatives, both p-fluoro benzyl- and benzyl substitution at the 1-position of indole enhanced the inhibitory effect of LP except for compound 3. Compound 3 showed a different activity pattern to that of compound 4. The replacement of chloro with fluoro and, moreover, the presence of a second halo substituent in a different position of the aniline ring (compound 4) resulted in better inhibition of LP. Significant differences in activity resulted when the 1-position of the indole ring was substituted. Although compounds 4, 5 and 6 increased LP inhibition, other N-substituted compounds 3, 7 and 8 showed weak LP inhibition. The introduction of benzyl and p-fluorobenzyl in the 1-position of indole increased activity for both LP and SOD inhibition by indole-2 carboxamides. Parallel results were not obtained for indole-3-carboxamide derivatives. The reason for this difference may be due to the positions of carboxamide moiety, halo substitutions on the aromatic side chain and the diversity of oxidative stress production mechanism in the SOD and LP assays. Since compounds 4, 5 and 6 showed potent inhibitory activity for both SOD and LP, it can be concluded that these compounds possess highly potent antioxidant properties.
Acknowledgements
This work was partially supported by a grant from the Turkish Scientific and Technical Research Institute (106S127 SBAG-HD-141).
References
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biol Interac 2006; 160: 1–40
- Iemma F, Trombino S, Puoci F, Cirillo G, Spizzirri UG, Muzzalupo R, Picci N. Synthesis and antioxidant efficiency of a new copolymer containing phosphorylated myo-inositol. Macromol Biosci 2005; 5: 1049–1056
- Gonenc A, Erten D, Aslan S, Akıncı M, Simsek B, Torun M. Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Internat 2006; 30: 376–380
- Reiter RJ, Acuna CD, Tan DX. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann N Y Acad Sci 2001; 939: 200–215
- Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla M, Chain DG. Development of indole-3-propionic acid (Oxigon) for alzheimer's diseses. J Mol Nurosci 2002; 19: 213–217
- Ames NB, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative disease of aging. Proc Natl Acad Sci USA 1993; 90: 7915–7922
- Collins AR. Antioxidant intervention as a route to cancer prevention. Eur J Cancer 2005; 41: 1923–1930
- Organesian A, Hendricks JD, Williams DE. Long term dietary indole-3-carbinol inhibits diethylnitrosamine-initiated hepatocarcinogenesis in the infant mouse model. Cancer Lett 1997; 118: 87–94
- Andreadou I, Tsantili-Kakoulidou A, Spyropoulou E, Siatra T. Reactions of indole derivatives with cardioprotective activity with reactive oxygen species. Comparison with melatonin. Chem Pharm Bull 2003; 51: 1128–1131
- Kim DS, Jeon SE, Park KC. Oxidation of indole-3-acetic acid by horsedish peroxidase induces apoptosis in G361 human melanoma cells. Cell Signal 2004; 16: 81–88
- Benanadhi SH, Zheng JB, Dong XC, Yuan SG. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacol Sin 2004; 25: 666–671
- Antosiewich E, Damiani E, Jassem W, Wozniak M, Orena M, Greci L. Influence of structure on the antioxidant activity of indolinic nitroxide radicals. Free Rad Biol Med 1997; 22: 249–255
- Spadoni G, Diamantini G, Bendini A, Tarzia G, Vacondio F, Silva C, Rivara M, Mor M, Plazzi PV, Zusso M, Franceschini D, Giusti P. Synthesis, antioxidant activity and structure-activity relationships for a new series of 2-(N-acylaminoethyl)indoles with melatonin-like cytoprotective activity. J Pineal Res 2006; 40: 259–269
- Ates-Alagoz Z, Kus C, Coban T. Synthesis and antioxidant properties of novel benzimidazoles containing substituted indole or 1, 1, 4, 4-tetramethyl-1, 2, 3, 4-tetrahydro-naphthalene fragments. J Enz Inhib Med Chem 2005; 20: 325–331
- Ates-Alagoz Z, Coban T, Buyukbingol E. Synthesis and antioxidant activity of new tetrahydro-napthalene-indole derivatives as retinoid and melatonin analogues. Arch Pharm Life Sci 2006; 339: 193–200
- Olgen S, Coban T. Antioxidant activity of N-substituted indole-2- and 3-carboxamides. J Fac Pharmacy Ankara 2004; 33: 109–116
- Aboul-Enein HY, Kruk I, Lichszteld K, Michalska T, Kladna A, Marczynski S, Olgen S. Scavenging of reactive species by N-substituted indole-2- and 3-carboxamides. Luminescence 2004; 19: 1–7
- Olgen S, Coban T. Antioxidant evaluation of novel N-H and N-substituted indole esters. Biol Pharm Bull 2003; 26: 736–738
- Aboul-Enein HY, Kruk I, Lichszteld K, Michalska T, Kladna A, Marczynski S, Olgen S. Scavenging of reactive oxgen species by novel 3 (substituted-benzylidine)-1, 3-dihydro-indoline-2-one and 3-(substituted-benzylidine)-1, 3-dihydro-indoline-2-thione derivatives. Biopolymers 2005; 78: 171–178
- Mihara M, Uchiyama MS, Fukuzawa K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem Med 1980; 23: 303–311
- McCord JM, Fridovich JM. Preparation and assay of superoxide dismutases. Meth Enzymol 1978; 53: 382–393
- Battaglia S, Boldrini E, Da Settimo F, Dondio G, La Motta C, Marini AM, Primofiore G. Indole amide derivatives: Synthesis, structure-activity relationships and molecular modelling studies of a new series of histamine H1-receptor antagonists. Eur J Med Chem 1999; 34: 93–105
- Olgen S, Guner E, Fabregat MA, Crespo MI, Nebioglu D. Syntheses and biological evaluation of indole-2 and 3-carboxamides: New selective cyclooxygenase-2 inhibitors. Pharmazie 2002; 57(4)238–242