Abstract
Five novel coordinated complexes of iron(II) with ciprofloxacin and neutral bidentate ligands have been prepared and characterized using elemental analyses, magnetic measurements, IR spectra, UV-VIS spectral, thermogravimetric analyses, 1H-NMR and 13C-NMR. The antimicrobial activity of the individual ligands, metal salt and metal complexes with respect to Bacillus subtilis, Escherichia coli, Bacillus cereus, Staphylococcus aureus, Salmonella typhi, Serratia marcescens, Aspergillus niger, Aspergillus flavus and Lasiodiplodia theobromae were evaluated by the agar-plate technique in comparison to reference standard drugs (ofloxacin, levofloxacin and fluconozole). Binding of the complexes to DNA was studied and is discussed.
Introduction
Metal ions such as iron(II) and (III) are necessary for a number of vital functions in life sciences [Citation1]. Iron complexes are known to be models for two stages of ferritin iron storage and biomineralization [Citation2]. Iron is the central atom of the heme complex, which is made of photoporphyrin IX and iron(II) [Citation3]. Apart from the porphyrins, a number of iron complexing ligands are found in aerobic microbial cells Citation4-5. Discovery of quinolones was reported in 1962 and subsequently due to its mode of action, chemical design and potential antimicrobial activity more than 10000 compounds related to this fluoroquinolone group have been launched as clinical drugs. Quinolones antibiotics are complexing agents for a variety of metal ions including alkaline earth metal ions [Citation6]. Many organic compounds used in medicine do not have a purely organic mode of action; some are activated or biotransformed by metal ions, others have a direct or indirect effect on metal ion metabolism [Citation7]. Even though the quinolone family has been widely studied there is no evidence for a precise mechanism of its action. Some reports suggest that the drugs interacts directly with DNA, blocks the activity of the DNA-gyrase repair enzyme [Citation8] or intercalate with the purine/pyrimidine bases of nucleic acids [Citation9]. d-Block metals are also well known to cleave and to bind DNA as only metal or as their complexes with different ligands Citation10-12. Several iron chelates have been reported for application in the treatment of thalassaemia, other transfusion-dependent diseases [Citation13], and also used as MRI contrast agents [Citation14]. Several iron complexes are well known for their antibacterial, antifungal and biomimetic activity Citation15-18 and here, five novel iron(II) complexes are examined for their antibacterial DNA-interactive activity.
Materials and methods
All the chemicals used were of analytical grade. 2,2′-Bipyridylamine (bipym), 1,8-diaminonaphthalene, and thiophene-2-carboxaldehyde were purchased from Lancaster (Morecambe, England). Ciprofloxacin hydrochloride was from Bayer AG (Wyppertal, Germany). 2-Aminopyridine, benzaldehyde, ethylenediamine, 3-chloro aniline, cyclohexanone, o-phenylenediamine, p-anisaldehyde and cupric nitrate were from E. Merck (India) Ltd. Mumbai, Luria broth and agar-agar were from SRL, India and Sperm herring DNA was from Sigma Chemical Co., India. The organic solvents were purified by standard methods [Citation19].
Infrared spectra were recorded on a FT-IR Shimadzu spectrophotometer as KBr pellets in the range 4000–400 cm–1. C, H and N elemental analyses were performed with a model 240 Perkin Elmer elemental analyzer. The reflectance spectra of the complexes were recorded in the range 1700–350 nm (as MgO discs) on a Beckman DK-2A spectrophotometer. The metal contents of the complexes were analyzed by EDTA titration [Citation25] after decomposing the organic matter with a mixture of HClO4, H2SO4, and HNO3 (1:1.5:2.5). Thermogravimetric analyses studies were obtained with a model 5000/2960 SDTA, TA instrument (USA). The 1H-NMR and 13C-NMR was recorded on a Bruker Avance (400 MHz). The electronic spectra were recorded on a Shimadzu UV-VIS spectrophotometer. The magnetic moments were measured by Gouy's method using mercury tetrathiocyanatocobaltate(II) as the calibrant (χg = 16.44 × 10− 6 cgs units at 20°C), Citizen Balance. The diamagnetic correction was made using Pascal's constant [Citation26].
Chemistry
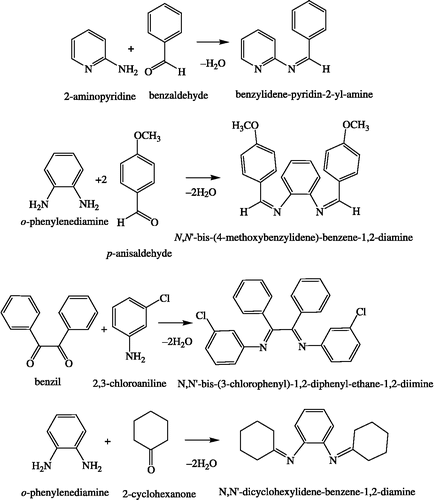
Preparation of Schiff bases
Benzylidene-2-aminopyridine (A2=bap)
2-Aminopyridine (0.94 g, 10 mmol) in ethanol was added slowly to benzaldehyde (1.06 g, 10 mmol) in ethanol (∼100 mL) and the mixture refluxed on a bath for 6 h, the solution concentrated to half volume and kept overnight under vacuum over P2O5. The separated ligand was washed with n-hexane and air-dried. Yield: 58%, m.p.: 142°C, Found %: C, 78.93; H, 5.47; N, 15.15. C12H10N2 (182.22) requires %: C, 79.10; H, 5.53; N, 15.37.
Thiophene-2-carboxaldeneaniline (A3=tca)
Thiophene-o-carboxaldeneaniline was synthesized by the reported procedure [Citation20].
Bis(benzylidene)-1,8-diaminonaphthalene (A4=bendan)
Bis(benzylidene)-1,8-diaminonaphthalene was synthesized by the reported procedure [Citation21].
Bis(benzylidene)ethylenediamine (A5=benen)
Bis(benzylidene)-ethylenediamine(benen) was synthesized according to the published procedure [Citation22].
N,N′-Bis-(4-methoxybenzylidene)-benzene-1,2-diamine (A6=bmbbd)
p-Anisaldehyde (2.72 g, 20 mmol) in ethanol was added drop wise to an ethanolic solution of o-phenylenediamine (1.08 g, 10 mmol) and refluxed on a water bath for 8 h. Fine yellow crystalline particles were obtained on filtration, which were further crystallized in ethanol, washed with n-hexane and air-dried. Yield: 45%, m.p.: 210°C, Found %: C, 76.72; H, 5.81; N, 8.09. C22H20N2O2 (344.41) requires %: C, 76.72; H, 5.85; N, 8.13%.
N,N′-Bis-(3-chlorophenyl)-1,2-diphenylethane-1,2-diimine(A7=bcpded)
An ethanolic solution of benzil (2.10 g, 10 mmol) and 3-chloroaniline (2.54 g, 20 mmol) was refluxed on a water bath for 24 h, concentrated to one third volume and kept overnight over sulfuric acid in a desiccator. The fine crystalline particles obtained on filtration were washed with ether : hexane (1:1) and air-dried. Yield: 56%, m.p.: 230°C, Found %: C, 72.93; H, 4.47; N, 16.15. C26H18Cl2N2 (429.34) requires %: C, 72.73; H, 4.23; N, 16.51%.
N,N′-Dicyclohexylidenebenzene-1,2-diamine (A8=dcbd)
An ethanolic solution of cyclohexanone (1.96 g, 20 mmol) was added to o-phenylenediamine (1.08 g, 10 mmol) in ethanol. The mixture was stirred continuously for 4 h when fine yellow crystalline particles were obtained which were washed with n-hexane and air-dried. Yield: 68%, m.p.:135°C, Found %: C, 80.47; H, 8.93; N, 10.40. C18H24N2 (268.40) requires %: C, 80.55; H, 9.01; N, 10.44%. The reaction schemes for the preparation of the ligands are shown in Scheme .
Synthesis of metal complexes
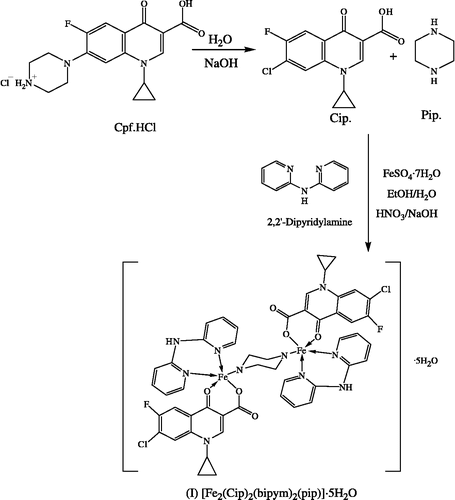
[Fe2(Cip)2(bipym)2(pip)]·5H2O (I)
A methanolic solution of FeSO4·7H2O (2.78 g, 10 mmol) was added to bipym (1.71 g, 10 mmol) in methanol, followed by addition of a previously prepared solution of Cpf·HCl (3.67 g, 10 mmol) in water and the pH was adjusted to 4.5 ∼ 6.0 pH with dilute HNO3 or NaOH solution. During reaction the piperazine ring of ciprofloxacin was substituted by chloride ion in the presence of NaOH solution [Citation23]. The resulting red solution was refluxed for 5 h, heated on a steam bath for 3–4 h, and then was kept overnight at room temperature. A fine colored crystalline product was obtained which was washed with ether and dried in a vacuum desiccator. Yield: 75%, m.p.: 352°C, Found %: C, 50.29; H, 4.31; N, 11.83; Fe, 9.40. C50H52Cl2F2Fe2N10O11 (1189.60) requires %: C, 50.48; H, 4.41; N, 11.77; Fe, 9.39%.
The proposed reaction scheme for the preparation of the complex is shown in Scheme .
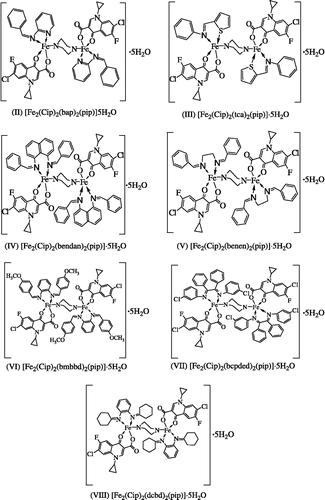
[Fe2(cip)2(bap)2(pip)]5h2o (Ii)
Prepared from bap (1.82 g, 10 mmol). Yield: 65%, m.p.: 210°C, Found %: C, 53.48; H, 4.51; N, 9.19; Fe, 9.20. C54H54Cl2F2Fe2N8O11 (1211.65) requires %: C, 53.53; H, 4.49; N, 9.25; Fe, 9.22.
[Fe2(cip)2(tca)2(pip)]·5h2o (Iii)
Prepared from tca (1.87 g, 10 mmol). Yield: 60%, m.p.: >360°C, Found %: C, 51.07; H, 4.18; N, 6.91; Fe, 9.15. C52H52Cl2F2Fe2N6O11S2 (1221.73) requires %: C, 51.12; H, 4.29; N, 6.88; Fe, 9.14.
[Fe2(cip)2(bendan)2(pip)]·5h2o (Iv)
Prepared from bendan (3.34 g, 10 mmol). Yield: 57%, m.p.: 305°C, Found %: C, 61.70; H, 4.62; N, 7.44; Fe, 7.35. C78H70Cl2F2Fe2N8O11 (1516.03) requires %: C, 61.80; H, 4.65; N, 7.39; Fe, 7.37.
[Fe2(Cip)2(benen)2(pip)]·5H2O (V)
Prepared from benen (2.36 g, 10 mmol). Yield: 56%, m.p.: 303°C, Found %: C, 56.46; H, 4.98; N, 8.45; Fe, 8.49. C62H66Cl2F2Fe2N8O11 (1319.83) requires %: C, 56.42; H, 5.04; N, 8.49; Fe, 8.46.
[Fe2(cip)2(bmbbd)2(pip)]·5h2o (Vi)
Prepared from bmbbd (3.44 g, 10 mmol). Yield: 59%, m.p.: 230°C, Found %: C, 57.77; H, 4.90; N, 7.34; Fe, 7.21. C74H74Cl2F2Fe2N8O15 (1536.02) requires %: C, 57.86; H, 4.86; N, 7.30; Fe, 7.27.
[Fe2(cip)2(bcpded)2(pip)]·5h2o (Vii)
Prepared from bcpded (4.29 g, 10 mmol). Yield: 50%, m.p.: 300°C, Found %: C, 57.69; H, 4.05; N, 6.51; Fe, 6.48. C82H70Cl6F2Fe2N8O11 (1705.88) requires %: C, 57.73; H, 4.14; N, 6.57; Fe, 6.55.
[Fe2(cip)2(dcbd)2(pip)]·5h2o (Viii)
Prepared from dcbd (2.68 g, 10 mmol). Yield: 58%, m.p.: 180°C, Found %: C, 57.14; H, 6.02; N, 8.07; Fe, 7.97. C66H82Cl2F2Fe2N8O11 (1384.00) requires %: C, 57.28; H, 5.97; N, 8.10; Fe, 8.07.
In-vitro antimicrobial study
Preparation of stock solution
A stock solution of 2.5 ppm was made in 5% DMSO solution.
Determination of MIC value
The antimicrobial screening concentration to be used was estimated from the minimal inhibitory concentration (MIC). MIC was determined using the method of progressive double dilution in liquid media containing 1ppm to 50ppm of the compound being tested. All the compounds were more effective with MIC value at 2.5ppm ≈ 2.5μg/mL. Consequently the biological screening on solid media of all the compounds was carried out at this MIC (2.5μg/mL) and the results are expressed as zone of inhibition in mm. The antimicrobial activity using the Agar-Plate technique [Citation24] of ofloxacin, levofloxacin, fluconozole, ligands, FeSO4·7H2O, and the complexes were analyzed against various gram-negative and gram-positive microbial cultures of Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Salmonella typhi, Escherichia coli and Serratia marcescens and three fungi cultures namely Aspergillus niger, Aspergillus flavus, Lasiodiplodia theobromae.
Preparation of agar plates
The media was made up by dissolving bacteriological agar (20 gm) and Luria broth (20 gm) in 1 L distilled water. The mixture was autoclave for 15 minutes at 120 oC and then dispensed into sterilized petri dishes, allowed to solidify, and then inoculated.
Inoculation procedure
The target microorganism cultures were prepared separately in 15 mL of liquid LB medium. Inoculation was done with the help of a micropipette with sterilized tip; 100 μL of culture was placed onto the surface of an agar plate, and spread evenly over the surface by means of a sterile, bent glass rod. Then two wells (d = 10 mm) were based in each plate with a sterilized borer.
Application of discs
Sterilized stock solutions (2.5μg/mL) were applied on discs in the wells of the inoculated agar plates which were then incubated at 37 oC for 24 h. The zone of inhibition was then measured (in mm) around the disc and the results are shown in . Control experiments were performed with only equivalent volume of solvents without added test compounds. All experiments were performed in triplicate and ofloxacin, levofloxacin, and fluconozole were used as a standard drug. The growth was compared with the control and is expressed as zone of inhibition.
Absorption titration
The DNA binding affinity study was performed on a UV-VIS spectrophotometer. Absorption titration of compounds in DMSO, and the whole system in buffer (phosphate, pH 7.2) was done by keeping fixed the amount of the iron complexes (where compound: I = 11.89, II = 12.11, III = 12.21, IV = 15.16, V = 13.19, VI = 15.36, VII = 17.05, VIII = 13.84 μgm) and varying the amount of DNA i.e. 3 − 7 μgm. Compound-DNA solutions were employed to record absorption spectra.
Results and discussion
Chemistry
The Schiff bases A2–A8 were prepared by condensation of the amine and aldehyde/ketone in ethanol. The structural characterizations of all Schiff bases have been done using IR spectra, 1H- and 13C-NMR spectra, and elemental analyses (). The complexes under investigation have been characterized using IR spectra, magnetic measurements and electronic spectra and their data are presented in Tables and . All the complexes were insoluble in ether, hexane, chloroform, while partially soluble in water, methanol and dimethyl formamide, but were completely soluble in dimethyl sulphoxide.
1H- and 13C-NMR spectra of Schiff bases
The 13C-NMR spectra and 1H-NMR spectra of the ligands were recorded in DMSO-d6. The 13C-NMR and 1H-NMR spectral data are reported along with the possible assignment in . In the 13C-NMR, the spectra peaks observed at 114.5–136.4, 113.5–144.5, and 128.5–143.0 ppm were assigned to aromatic, pyridine, and thiophene carbons respectively. Peaks observed at 157.9–180.5, 155.3–161.2, 123.5–155.3 ppm were assigned to C = N, CH = N, and C–N carbons respectively. In the 1H-NMR spectra of the ligands, peaks observed at 7.0–8.0 ppm were assigned to the aromatic protons and the singlet peak appearing at 7.8–9.1 ppm was assigned to the azomethine proton (–CH = N–).
Table I. 1H NMR and 13C NMR spectral data of Schiff basesa.
IR spectra
The IR spectra of the ligands and complexes are shown in . The peak observed at 3520 cm− 1 in ciprofloxacin [Citation27] is due to hydrogen bonding which contributed to the ionic resonance structure. This peak was absent in the spectra of the metal complexes signifying deprotonation of the carboxylic proton. This data is supported by ν(M–O) [Citation28] band appearing at about 505 ∼ 512 cm− 1. The ν(C = O) band appears at 1708 cm− 1 in the spectra of ciprofloxacin; the complexes show this band at 1626–1633 cm− 1, a shift towards lower energy suggesting that coordination occurs through the carbonyl oxygen atom [Citation29]. The frequency separation (Δν = ν COOasy–ν COOsym) in investigated complexes is greater than 200 cm− 1, suggesting a unidentate bonding nature for the carboxylate group Citation30-32. In 2,2′ bipyridylamine the ν (C = N) band appears at 1585 cm− 1. This band shifts to a higher frequency at 1618 cm− 1 [Citation33] in the complexes indicating the bidentate N–N coordination of the ligand. Similarly for benzylidene-2-aminopyridine the two strong bands at 1615 and 1593 cm− 1 are assigned to ν (C = N) of the azomethine and pyridine ring, respectively. On complexation these bands are shifted to 1565 cm− 1 and 1620 cm− 1, respectively, suggesting the bidentate N–N involvement in coordination Citation34-35. The ν (C = N) peak for the synthesized Schiff bases A3–A8 is observed at 1602–1629 cm− 1 and is shifted to 1570–1593 cm− 1, indicating the N–S or N–N bidentate coordination of ligand Citation36-39. These data are further supported by ν (M–N) [Citation40] which appears at about 530 ∼ 540 cm− 1. In [Fe2(Cip)2(tca)2(pip)]·5H2O, the ν (C–S) band of the ligand (tca) observed at 765 cm− 1 is shifted lower to 752 cm− 1 in the spectra of the complex indicating the participation of the sulfur atom of thiophene ring. This data is further supported by a new band observed at 420 cm− 1 which may be assigned to the ν (M–S) Citation41-43 mode.
Table II. Infrared spectral data of complexesa.
Electronic spectra and magnetic properties
Electronic spectral data and magnetic moments are presented in . Fe(II) complexes are an intense greenish brown in color, but the origin of this color is doubtful. The spectrophotometrically characterized five coordinated Fe(II) complexes are rarely reported [Citation44]. The diffuse reflectance spectra of the diiron(II) complexes [Fe2(L)2(An)2(pip)] ·5H2O exhibited three band at about ∼265 nm, ∼550 nm, and ∼700 nm[Citation45,Citation46]. These bands are assigned to different transitions of ∼265 nm for π → π*, ∼550 nm for d–d, and ∼700 nm for MLCT in the d6-system of Fe(II) atom. The magnetic moments of all compounds was in the range 4.73–5.17 BM which is in good agreement for a five coordinated dinuclear Fe(II) mixed ligand system and consistent with the presence of four unpaired electrons [Citation47,Citation48] suggesting a paramagnetic nature. The magnetic moment and electronic spectra suggest that Fe(II) is in a distorted square pyramidal coordination environment.
Table III. Electronic spectral data of the ligands and complexesa.
TGA
The thermal stability of the complexes was investigated using thermogravimetric analyses. The TGA curves obtained at a heating rate of 10oC/min in a N2 atmosphere over the temperature range of 50–800oC showed that all the complexes had a loss in weight corresponding to five water molecules in the range of 50–100oC indicating that these water molecules are water of crystallization. In the temperature range 100–800oC the ligand molecules are lost. In all cases the final products were metal oxides. These results are in good agreement with the composition of the complexes. The suggested structures of the complexes are shown in .
Antimicrobial activity
The increase in antimicrobial activity of the complexes over the ligands A1–A8 may be due to the effect of the metal coordination and as a consequence of their structures and additional > C = N– bond [Citation49]. A possible explanation for the increase in antimicrobial activity may be considered in the light of Overtone's concept [Citation50] and the Tweedy's chelation theory [Citation51]. According to Overtone's concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid-soluble materials so that liposolubility is an important factor controlling the antimicrobial activity [Citation52]. On chelation, the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups [Citation53,Citation54]. Further, the mode of action of the compounds may involve the formation of a hydrogen bond through the azomethine group with the active centre of cell constituents, resulting in interference with the normal cell process [Citation55]. Comparative analysis showed a higher antimicrobial activity for the complexes than the free ligands, metal salt and the control (DMSO). The complexes exhibited higher activities as compared to the standard drugs ofloxacin, levofloxacin, and flucanozole for B. subtilis and E. coli, while for S. aureus, B. cereus, S. typhi and S. marcescens all the complexes possessed good activity (). With A. niger, A. flavus and L. theobromae, there was no significant antifungal activity.
Table IV. Antimicrobial activity of compounds.
DNA binding
Absorption spectroscopy is extensively used to confirm the binding of complexes with the DNA helix. Complexes bound to DNA through interaction result in a bathochromic (red) shift and hypochromic (blue) shift due to interaction between chromophores and the base pair of the DNA helix. The level of hypochromism is commonly consistent with the strength of the intercalative interaction Citation56-57. The DNA binding data for the complexes are shown in . The maxima at about 272 nm is observed in the spectrum of the complex in the absence of DNA, but this decreases as the amount of DNA increases and is observed at about 247 nm in the presence of 7μgm DNA. The absorption spectra of the complex [Fe2(Cip)2(bipym)2(pip)].5H2O is shown in .
Table V. DNA interaction data for the complexes.
Similarly for the variable ligands (A1–A8), FeSO4.7H2O, and ciprofloxacin the maxima was observed at c. 260 nm in the absence of DNA but in the presence of 7μgm DNA maxima was observed at c. 245 nm. All data leads to the suggestion that in the presence of 7 μgm of DNA (maxima at about 245 nm) the whole complex dissociates, and free Fe(II), constant ligand(Cip), and variable ligands(A1–A8) bind with DNA.
Bathochromic wavelength shifts and hypochromic absorption are characteristic of the electronic spectra of many DNA bound groove binders and most if not all DNA–bound intercalators [Citation58].
Acknowledgements
We thank Prof. J. S. Parmar, Head, Department of Chemistry, and Prof. D. Madammvar, Head, Department of Biosciences, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India for providing the laboratory facilities.
References
- Brown DA. Metal Ions Biol Syst 1982; 14: 125
- Iron biomineralization, EC Theil, RB Frenkel. Plenum Press. 1999
- Seitz JF. The biochemistry of cells of blood and bone marrow. Thomas CC, Springfield, III 1969; 10
- Neilands JB. Struc Bonding 1966; 1: 100
- Sigal H. Metal ions in biological systems, 1982; 14, Brown DA, Chidambaram MV, Iron containing drugs. 5,125.
- Turel I. Coord Chem Rev 2002; 27: 232
- Guo Z, Sadler PJ. Adv Inorg Chem 1999; 49: 183
- Quinolone antimicrobial agents, LL Shen, DC Hopper, JS Wolfson. American Society for Microbiology, Washington DC 1993; 77, 2nd Esit.,
- Chaohan ZH, Supuran CT, Scozzafava A. J Enz Inhib Med Chem 2004; 20: 303
- Pedreño E, López-Contreras AJ, Cremades A, Peñafiel R. J Inorg Biochem 2005; 99: 2074–2080
- Inoue S, Yamamoto K, Kawnishi S. Chem Res Toxicol 1990; 3: 144
- Sundquist WT, Lippard SJ. Coord Chem Rev 1990; 100: 293
- Barman Balfour JA, Foster RH. Drugs 1999; 58: 553–578
- Schewert DD, Nicholas R, Ji G, Radüchel B, Ebert W, Heffner PE, Keck R, Davies JA. J Med Chem 2005; 48: 7482–7485
- Esmelindro MC, Oestreicher EG, Márqer-Alvarer H, Dariva C, Egues SMS, Fernandes C, Bortoluzzi AJ, Drago V, Antunes OAC. J Inorg Biochem 2005; 99: 2054–2061
- Patel MN, Patel KM, Patel KN, Patel NH. Synth React Inorg Met-Org Chem 2001; 31(2)239–246
- Chohan ZH, Pervez H, Khan KM, Supuran CT. J Enz Inhib Med Chem 2005; 20: 81–88
- Nam W, Kim J, Suh J, Seo MS, Kim KM, Kim YS, Jang HG, Tosha T, Kitagawa T. J Inorg Biochem 2006; 627
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Vogel's textbook of practical organic chemistry. 5th ed. 2004.
- Panchal PK, Pansuriya PB, Patel MN. J Enz Inhib Med Chem 2006; 21(4)453–458
- Parekh HM, Pansuriya PB, Patel MN. Pol J Chem 2005; 79: 1843–1851
- Parekh HM, Mehta SR, Patel MN. Russ J Inorg Chem 2006; 51(1)67–72
- Wu G, Wang G, Fu X, Zhu L. Molecules 2003; 8: 287–296
- Stokes EJ, Ridgway GL. In Clinical Bacteriology: 5th ed. Edward Arnold Publisher: Baltimore, Maryland; USA. 1980; 205, and M.J. Pelczar, E.C.S. Chan, N.R. Krieg, Microbiology, 5th ed. 23 (1993) 488.
- Vogel AI. Textbook of quantitative inorganic analysis4th ed. ELBS and Longman, London 1978
- Pascal P. Compt Rend 1944; 57: 218
- Silverstein RM, Webster FX. Spectrometric identification of organic compounds6th ed. John Wiley & Sons, Inc. 2004
- Chohan ZH, Supuran CT. J Enz Inhib Med Chem 2005; 20(5)463–468
- Turel I, Leban I, Bukovec N. J Inorg Biochem 1999; 241–245
- Anacona JR, Rodriguez I. J Coord Chem 2004; 57(15)1263–1269
- Nakamoto K, Infrared and Raman Spectra of inorganic and coordination compounds. (1986) 4th ed. A Wiley Interscience Publication.
- Patel NH, Panchal PK, Pansuriya PB, Patel MN. J Macro Science Part-A Pure and App Chem 2006; 43: 1083–1090
- Panchal PK, Patel MN. Synth React Inorg Met-Org Chem 2004; 34(7)1277–1289
- Gudasi KB, Goudar TR. Synt React Inorg Met-Org Chem 2000; 30(10)1859–1869
- Mandal SS, Ghorai PC, Ray S, Saha HK. J Indian Chem Soc 1995; 72: 807
- Raman N, Kulandaisamy A, Jayasubramananian K. Pol J Chem 2002; 76: 1085–1094
- Parekh HM, Panchal PK, Pansuriya PB, Patel MN. Pol J Chem 2006; 80: 989–992
- Panchal PK, Pansuriya PB, Patel MN. Toxicol Env Chem 2005; 88(1)57–64
- Parekh HM, Panchal PK, Patel MN. J Therm Anal Cal 2006; 86(3)803–807
- Chohan ZH, Humayun P, Rauf A, Khan KM, Supuran CT. J Enz Inhib Med Chem 2004; 19(5)417–423
- Sargent AL, Titus EP, Riordan CG. Inorg Chem 1996; 35(21)7095
- Chattopadhayay SK, Benerjee T, Roychoudhury P. Trans Met Chem 1997; 22: 216
- Zenglu L. Synth React Inorg Met-Org Chem 2004; 34: 469
- Morassi R, Bertini I, Sacconi L. Coord Chem Rev 1973; 343–402
- Britovsek GJP, Bruce M, Gibson VC, Kimberley BS, Maddox PJ, Mastroianni S, McTavish SJ, Redshaw C, Solan GA, White AJP, Williams DJ, Strömberg S. J Am Chem Soc 1999; 121: 8728–8740
- Curnow OJ, Holze R, Lang H, Böhme U, Klaib S, Fern GM. J Electroanal Chem 2005; 167–171
- Bouwkamp MW, Bart SC, Hawrelak EJ, Trovitch RJ, Lobkovsky E, Chirik P. Chem Commun 2005; 3409
- Zhang J, Gandelman M, Herrman D, Leitus G, Shimon LJW, Ben-David Y, Milstein D. Inorg Chim Acta 2006; 1955–1960
- Arif M, Shafiq Z, Chaohan ZH, Yaqub M, Supuran CT. J Enz Inhib Med Chem 2006; 21: 95–103
- Pansuriya PB, Panchal PK, Parekh HM, Patel MN. J Enz Inhib Med Chem 2006; 21: 203–209
- Tweedy BG. Phytopathology 1964; 55: 910
- Chaohan ZH, Hassan M, Khan KM, Supuran CT. J Enz Inhib Med Chem 2005; 20: 183–188
- Iqbal HS, Iqbal MS, Chohan ZH, Scozzafava A, Suparan CT. J Enz Inhib Med Chem 2002; 17: 87
- Rehman SU, Chaohan ZH, Gulnaz F, Supuran CT. J Enz Inhib Med Chem 2005; 20: 333
- Dharmaraj N, Viswanathamurthi P, Natarajan K. Trans Met Chem 2001; 26: 105
- Barton JK, Danishefsky AT, Goldberg JM. J Am Chem Soc 1984; 106: 2172
- Kelly TM, Tossi AB, McConnell DJ, Strekas TC. Nucl Acid Res 1985; 13: 6017
- Santra BK, Reddy PAN, Neelakanta G, Mahadevan S, Nethaji M, Chakravarty AR. J Inorg Biochem 2002; 89: 191

![Figure 2 Absorption spectra of complex [Fe2(L)2(A1)2(pip)]·5H2O in absence and presence of DNA.](/cms/asset/3bb7f17d-e6ea-498f-811c-71ab6cb36b3e/ienz_a_222817_f0004_b.gif)