Abstract
A series of N1-nicotinoyl-3- (4-hydroxy-3-methyl phenyl)-5-(substituted phenyl)-2-pyrazolines were synthesized by the reaction between isoniazid (INH) and chalcones and were tested for their in vitro anti-viral activity. Among the compounds, the electron withdrawing group substituted analogues 5-(4-chlorophenyl)-3-(4-hydroxy-3-methylphenyl)-4, 5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4b), 5-(2-chlorophenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4i), 5-(4-fluorophenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4h) and 5-(2,6-dichlorophenyl)-3-(4-hydroxy-3-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridyl methanone (4j) were the most promising and the halogeno function appeared to be essential for antiviral activity.
Keywords::
Introduction
The incidence of viral infections have been constantly emerging and re-emerging on a global scale. Research on viral diseases of medical and public health importance could greatly contribute to the quality of human lives.
Effective antiviral drugs have been developed much more slowly than other types of anti-infective chemotherapy. One of the major limitations has been the absence of specific viral ‘targets’, because host cell pathways are used predominantly for viral replication. However, improved understanding of viral replication has led to rapid advances in antiviral therapy, and important new drugs are being developed annually, more than half of the currently available antiviral drugs having been introduced into therapy since 1990.The most common mechanism of action of the antiviral drugs involves inhibition of viral nucleic acid synthesis. Agents that have been developed may be active against both DNA and RNA viruses, depending on the viral enzyme (s) affected by the drug. Recently we reported that pyrazoline derivatives possess antituberclosis activityCitation1-2. Also, 1,3, 5 trisubstituted pyrazoline derivatives have been evaluated for their antiproliferative activity against human lung cancer A549[Citation3]. Pyrazoline derivatives are incorporated into a wide variety of therapeutically important compounds possessing a broad spectrum of biological activitiesCitation4-10. In view of these findings and in continuation of our previous work on the synthesis of pyrazoline derivatives we report here the synthesis and evaluation of antiviral activity of novel isoniazid (INH) substituted pyrazoline derivatives.
Materials
All the chemicals were supplied by E.Merck (Germany) and S.D Fine Chemicals (India). Melting points were determined by the open tube capillary method and are uncorrected. Purity of the compounds was checked on thin layer chromatography (TLC) plates (silica gel G) in the solvent system toluene-ethyl formate- formic acid (5:4:1) and benzene (CARE-CARCINOGENIC)-methanol (8:2), the spots being located under iodine vapors or UV light. IR spectra were obtained on a Perkin-Elmer 1720 FT-IR spectrometer (KBr Pellets). 1H NMR spectra were recorded or a Bruker AC 300 MHz, spectrometer using TMS as internal standard in DMSO-d6.
Methods
Chemistry
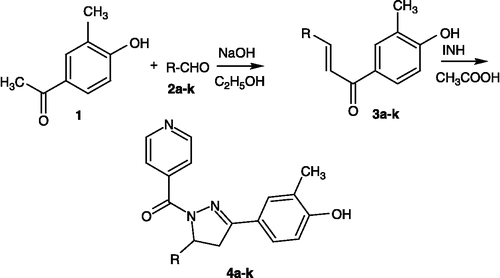
General method for the preparation of compounds 1-(4-hydroxy-3-methylphenyl)-3-[(sub) phenyl]-2-propen-1-ones (3a–k)
1-(4-hydroxy-3-methylphenyl)-3-[(substituted) phenyl]-2-propen-1-one derivatives were synthesized by condensing 4-hydroxy-3methylacetophenone with the appropriate aromatic aldehydes according to the Claisen-Schmidt condensation.
1-(4′-hydroxy-3′-methyl phenyl-3-(4″-methoxy phenyl)-2-propen-1-one (3a)
IR: (KBr) cm − 1: 3200 (OH), 3042 (CH), 1686 (C = O). 1H NMR (DMSO-d6 ppm): 2.2(3H,s,CH3), 3.9 (3H,s, OCH3), 6.8–6.9(1H × 2,d J = 7.5 Hz, 8.5 Hz, CH = CH), 7.2–7.9 (7H,s, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3-(4″-chlorophenyl)-2-propen-1-one (3b)
IR: (KBr) cm − 1: 3210 (OH), 3030 (CH), 1676 (C = O). 1H NMR (DMSO-d6 ppm): 2.2(3H,s, CH3), 6.7–6.8(1H × 2,d J = 8.34 Hz, 6.79 Hz, CH = CH), 7.7–8.0(7H,m, aromatic), 9.2(1H,s,OH).
1-(4′-hydroxy-3-methylphenyl)-3-(4″-dimethylamino phenyl)-2-propen-1-one (3c)
IR: (KBr) cm − 1: 3200 (OH), 3040 (CH), 1680 (C = O). 1H NMR (DMSO-d6 ppm): 2.2(3H,s,CH3), 2.83(6H,s, N (CH3 × 2), 6.8–6.9(1H × 2,d J = 7.61 Hz, 7.63 Hz, CH = CH), 7.6–8.1(7H,m, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3-phenyl-2-propen-1-one (3d)
IR: (KBr) cm − 1: 3210 (OH), 1680 (C = O), 3042 (CH). 1H NMR (DMSO-d6 ppm): 2.2(3H,s,CH3), 6.8–7.4(1H × 2,d J = 8.28 Hz, 6.70 Hz, CH = CH), 7.7–8.2(8H,m, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3-(3″,4″-dimethoxyphenyl)-2-propen-1-one (3e)
IR: (KBr) cm − 1: 3232(OH), 3046 (CH), 1680 (C = O). 1H NMR (DMSO-d6) ppm: 2.2(3H,s, CH3), 3.9(6H,s, OCH3 × 2), 6.9–7.3(1H × 2,d J = 7.45 Hz, 7.29 Hz, CH = CH), 7.6–8.1(6H,m, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3- (3″,4″,5′-tri-methoxyphenyl)-2-propen-1-one (3f)
IR: (KBr) cm − 1:3220(OH), 3036 (CH), 1686 (C = O). 1H NMR (DMSO-d6 ppm): 2.2(3H,s,CH3), 3.9 (9H,s,OCH3 × 3), 6.9–7.5(1H × 2,d J = 7.55 Hz, 7.27 Hz, CH = CH), 7.7–8.1 (5H,m, aromatic), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3-furfuryl-2-propen-1-one (3g)
IR: (KBr) cm − 1: 3200(OH), 3040(CH), 1680(C = O). 1H NMR (DMSO-d6 ppm): 2.2 (3H,s, CH3), 7.7–8.2 (6H,m, aromatic), 6.4–7.4 (3H,m,furan), 6.8–6.9 (1H × 2,d J = 3.0 Hz, 8.36 Hz, CH = CH), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3-(4″-fluorophenyl)-2-propen-1-one (3h)
IR: (KBr) cm − 1:3200 (OH), 3040 (CH), 1680 (C = O). 1H NMR (DMSO-d6 ppm): 2.2 (3H,s,CH3), 7.7–8.2(7H,m, aromatic), 6.9–7.5 (1H × 2,d J = 7.24 Hz, 7.29 Hz, –CH = CH), 9.2 (1H,s, OH).
1-(4″-hydroxy-3″-methylphenyl)-3-(2″-chlorophenyl)-2-propen-1-one (3i)
IR: (KBr) cm − 1:3200 (OH), 3042(CH), 1684 (C = O). 1H NMR (DMSO-d6 ppm): 2.2 (3H,s, CH3), 7.6–8.0(7H,m, aromatic), 6.9–7.5 (1H × 2,d J = 8.35 Hz, 3.63 Hz, –CH = CH), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3-(2″,6″-dichlorophenyl)-2-propen-1-one (3j)
IR: (KBr) cm − 1:3210 (OH), 3040 (CH), 1670 (C = O). 1H NMR (DMSO-d6 ppm): 2.2 (3H,s, CH3), 7.7–8.0 (6H,m, aromatic), 6.9 − 7.5(1H × 2,d J = 5.41 Hz, 15.68 Hz, CH = CH), 9.2 (1H,s, OH).
1-(4′-hydroxy-3′-methylphenyl)-3-(3′-nitrophenyl)-2-propen-1-one (3k)
IR: (KBr) cm − 1:3200 (OH), 3040 (CH), 1680(C = O). 1H NMR (DMSO-d6 ppm): 2.2 (3H,s, CH3), 2 7.7–8.2 (7H,m, aromatic), 6.9–7.5 (1H × 2,d J = 5.46 Hz, 16.3 Hz, CH = CH), 9.2 (1H,s, OH).
General method for the preparation of compounds (4a–k)
Synthesis of 5-(4′-hydroxy-3′-methylphenyl)-5-(substituted phenyl)-4, 5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone derivatives (4a–k)
To a solution of 0.002 mol of the appropriate (4a–k) derivatives in 15mL of glacial acetic acid, 0.002 mol isoniazid was added and the reaction mixture was refluxed for 12 h and cooled. Excess of solvent was removed under reduced pressure and the reaction mixture was cooled, and poured onto crushed ice (20 gm). The product was filtered, washed with water and recrystallized from methanol.
5-(4′-hydroxy-3′-methylphenyl)-5-(4″-methoxyphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4a)
IR: (KBr) cm − 1:3210(OH), 3034(CH), 1682(C = O); 1H NMR(DMSO-d6)ppm: 1.4 (3H,s, CH3), 2.3(2H,s, CH2), 3.7(3H,s, OCH3), 5.78(1H,s, CH), 6.6–7.3(7H,m, aromatic), 7.98–8.1(4H,m, pyridine), 9.2 (1H,s, OH); EISMS: m/z: 388(M+1); Cal/Ana[C (71.30) 71.31,H (5.46) 5.41,N (10.85) 10.82]
5-(4′-chlorophenyl)-3-(4″-hydroxy-3″-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridyl methanone (4b)
IR: (KBr) cm − 1: 3200(OH), 3040(CH), 1680(C = O), 780(C–Cl); 1H NMR(DMSO-d6)ppm: 2.2 (3H,s, CH3), 2.3(2H,s, CH2), 4.1(1H,s,CH), 6.5–7.3(7H,m, aromatic), 7.9–8.9(4H,m,pyridine), 11.49 (1H,s, OH) EISMS: m/z: 392(M+1); Cal/Ana[C (67.43) 67.51,H (4.63) 4.67,N (10.72) 10.72]
5-(4′-dimethylaminophenyl)-3-(4′-hydroxy-3′-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4c)
IR: (KBr) cm − 1: 3230(OH), 3020(CH), 1676(C = O); 1H NMR(DMSO-d6)ppm: 2.1(3H,s,CH3), 2.6(2H,s,CH2), 2.9(6H,s,N-(CH3)2), 4.1(1H,s,CH), 6.5–7.5(7H,m, aromatic), 7.6–8.6(4H,m, pyridine), 11.2 (1H,s, OH); EISMS: m/z: 400(M+); Cal/Ana[C (71.98) 71.96,H (6.04) 6.08,N (13.99) 13.93].
3-(4′-hydroxy-3′-methylphenyl)-5-phenyl-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4d)
IR: (KBr)cm − 1: 3200(OH), 3040(CH), 1680(C = O); 1H NMR(DMSO-d6)ppm: 2.1(3H,s, CH3), 2.5(2H,s, CH2), 4.1(1H,s, CH), 6.5–7.8(8H,m, aromatic), 7.9–8.9(4H,m, pyridine), 11.49 (1H,s, OH); EISMS: m/z: 357(M+); Cal/Ana[C (73.53) 73.50,H (5.36) 5.41,N (11.76) 11.72].
5-(3′,4′-dimethoxyphenyl)-3-(4″-hydroxy-3″-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4e)
IR: (KBr)cm − 1: 3220(OH), 3044(CH), 1686(C = O); 1H NMR(DMSO-d6)ppm:2.2(3H,s, CH3), 2.4 (2H, s,CH2),3.8(6H,s,2 × OCH3),4.1(1H,s,CH),6.7–7.3(6H,m,aromatic),7.6–7.9 (4H,m, pyridine), 11.90 (1H,s, OH); EISMS: m/z: 418(M+1); Cal/Ana[C (69.05) 69.08,H (5.55) 5.41,N (10.07) 10.02].
3-(4′-hydroxy-3′-methylphenyl)-5-(3″,4″,5′-trimethoxyphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4f)
IR:(KBr)cm − 1:3200(OH),3040(CH),1680(C = O);1HNMR(DMSO-d6)ppm: 2.2(3H,s,CH3), 2.4(2H,s, CH2),3.8(9H,s,3 × OCH3),4.1(1H,s,CH),6.7–7.5(5H,m,aromatic),7.6–7.9(4H,m,pyridine),11.84 (1H, s,OH); EISMS: m/z: 448(M+1); Cal/Ana[C (67.10) 67.51,H (5.63) 5.61,N (9.39) 9.36].
5-(2′-furyl)-3-(4″-hydroxy-3″-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4g)
IR:(KBr)cm − 1:3200(OH),3040(CH),1680(C = O);1H NMR(DMSO-d6)ppm:2.0(3H,s,CH3), 2.7(2H, s,CH2),5.8(1H,s,CH),6.3–6.9(3H,m,aromatic),7.4–7.7(3H,m,furan),8.7–9.0(4H,m, pyridine), 10.38 (1H,s,OH);EISMS: m/z: 348(M+1); Cal/Ana[C (69.15) 69.21,H (4.93) 4.86,N (12.10) 12.12]
5-(4″-fluorophenyl)-3-(4″-hydroxy-3″-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridylmethanone (4h)
IR: (KBr) cm − 1:3200(OH), 3040(CH), 1680(C = O); 1H NMR(DMSO-d6)ppm: 2.2(3H,s, CH3), 2.3(2H,s, CH2), 4.1(1H,s, CH), 6.5–7.3(7H,m, aromatic), 7.9–8.9(4H,m, pyridine), 11.49 (1H,s, OH); EISMS: m/z: 376(M+1); Cal/Ana[C (70.39) 70.41,H (4.83) 4.81,N (11.19) 11.72]
5-(2′-chlorophenyl)-3-(4″-hydroxy-3″-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridyl methanone (4i)
IR: (KBr) cm − 1:3200(OH), 3040(CH), 1680 (C = O); 1H NMR(DMSO-d6)ppm: 2.2(3H,s, CH3), 2.9(2H,s,CH2),4.0(1H,s,CH),6.9–7.3(7H,m, aromatic),7.5–8.2(4H,m,pyridine),8.8(1H,s,OH); EISMS: m/z: 392(M+1); Cal/Ana[C (67.43) 67.51,H (4.63) 4.61,N (10.72) 10.73]
5-(2″,6′-dichlorophenyl)-3-(4″-hydroxy-3″-methylphenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridyl methanone (4j)
IR: (KBr) cm − 1:3200(OH), 3040(CH), 1680(C = O); 1H NMR(DMSO-d6)ppm: 2.2 (3H,s,CH3), 2.5(2H,s, CH2), 4.4(1H,s, CH), 6.5–7.3(6H,m, aromatic), 7.9–8.4(4H,m, pyridine), 11.49 (1H,s, OH); EISMS: m/z: 427(M+1); Cal/Ana[C (61.98) 61.96,H (4.02) 4.08,N (9.86) 9.84]
3-(4′-hydroxy-3′-methylphenyl)-5-(3″-nitrophenyl)-4,5-dihydro-1H-1-pyrazolyl-4-pyridyl methanone (4k)
IR: (KBr) cm − 1:32740(OH), 3042(CH), 1678(C = O); 1H NMR(DMSO-d6)ppm: 2.2(3H,s,CH3), 2.5 (2H,s,CH2), 4.4(1H,s, CH), 6.5–7.3(6H,m, aromatic), 7.9–8.4(4H,m, pyridine), 10.49 (1H,s, OH); EISMS: m/z: 402(M+); Cal/Ana[C (65.67) 65.69,H (4.51) 4.53,N (13.92) 13.82]
Antiviral activity assay
MDBK cells were seeded at a density of 5 × 03 per well in 96-well cell culture plates in MEM-FCS. Following 24 h incubation at 37°C under 5% CO2, the medium was removed and 5-fold serial dilutions of the test compounds were added in a total volume of 100 μL, after which the virus inoculum [100 CCID50 (50% cell culture infective dose) in 100 μL] was added to each well. This inoculum resulted in a greater than 90% destruction of the cell monolayer after 4–5 days of incubation at 37°C. Uninfected cells and cells receiving virus without compound were included in each assay plate. After 5 days, the medium was removed, and 90 μL of MEM-FCS supplemented with 10 μL of MTS/PMS solution (Promega, Leiden, and The Netherlands) was added to each well. Following a 2 h incubation period at 37°C, the absorbance of each well was read at 498 nm in a microplate reader. The 50% effective concentration (EC50) was defined as the concentration at compound of which 50% cell viability was protected from the virus-induced cytopathogenic effect (CPE).
Cytostatic assay
MDBK cells were seeded at a density of 5 × 03 cells per well of a 96-well plate in MEM-FCS. 24 h later, serial dilutions of the test compounds were added. Cells were allowed to proliferate for 3 days at 37°C, after which the cell number was determined by means of the MTS/PMS method (Promega). The 50% cytotoxic concentration (CC50) was defined as the concentration that inhibited the proliferation of exponentially growing cells by 50%.
Results and discusion
Chemistry
The physical constants and anti-vival testing results for the N1-nicotinoyl-3-(4-hydroxy-3-methylphenyl)-5-(substituted phenyl)-2-pyrazolines 4a–k described in this study are shown in Tables I –IV, and a reaction sequence for the preparation is outlined in Scheme . The required chalcones were prepared by reacting 4-hydroxy-3-methylacetophenone with the appropriate aldehyde in presence of base by a conventional Claisen-Schmidt condensation. Reaction between the chalcones and isonicotinyl hydrazide in ethanolic solution in the presence glacial acetic acid, reaction time varied from 8–14 hours, afforded the titled pyrazolines 4a–k in 65–94% yield after recrystallization from methanol. The purity of the compounds was confirmed by TLC and elemental analyses. Both analytical and spectral data (1H NMR, IR) of all the synthesized compounds were in full agreement with the proposed structures. In general, the IR Spectra revealed OH, C = O, C = N, and C–N peaks at 3307, 1640, 1590 and 1320 cm− 1 respectively. In the 1H NMR spectra the signals of the respective protons of the compounds were verified on the basis of their chemical shifts, multiplicities and coupling constants. The spectra showed a singlet at δ 2.2 ppm corresponding to the methyl group, a singlet at δ 2.57 ppm corresponding to the C4-methylene group, a singlet at δ 5.78 ppm corresponding to the C5 proton[Citation17] and a multiplet at δ 8.22–8.5 ppm for the pyridyl protons. The elemental analysis results were within ± 0.4% of the theoretical values.
Table I. Physical constants of the synthesized compounds. .
.
Table II. Cytotoxicity and antiviral activity of synthesized compounds in HeL cell cultures. .
.
Table III. Cytotoxicity and antiviral activity of synthesized compounds in HeLa cell cultures. .
.
Table IV. Cytotoxicity and antiviral activity of synthesized compounds in Vero cell cultures. .
.
Antiviral activity
Antiviral activity was tested against Herpes simplex virus-1 (KOS), Herpes simplex virus-2 (G), Vaccinia virus, Vesicular stomatitis virus, Herpes simplex virus-1 TK- KOS ACVr (in HeL cell culture), Vesicular stomatitis virus, Coxsackie virusB4, Respiratory syncytial virus (in HeLa cell culture), Para-influenza-3 virus, Reovirus-1, Sindbis virus, Coxsackie virusB4, Punta Toro virus (in Vero cell culture). For each compound, the 50% Minimum Cytotoxic Concentration (MCC) and the Minimum Inhibitory Concentration (MIC) were obtained. Ribavirin, Brivudin, Acyclovir, Ganciclovir and (S)-DHPA were used as the standards in the study. Both antiviral activity and cytotoxicity were determined by means of the MTS method. The results of the antiviral assay revealed that of the compounds tested specific antiviral activity was exhibited only by the derivatives bearing an INH substituted pyrazoline moiety. The results of the antiviral assays are shown in. Cytotoxicity towards uninfected host cells was determined under the same conditions as antiviral activity i.e. microscopic evaluation of the cell morphology of the confluent cell monolayer which either had or had not been inoculated with virus. The criterion of specific antiviral activity was taken as the inhibition of virus induced cytopathogenicity at a concentration that was at least 5- fold lower than theconcentration required to alter the morphology of uninfected host cells Citation11-16.
According to this criterion, derivatives 4b, 4i, 4h and 4j were active against Herpes simplex virus-1, Herpes simplex virus-2 (G), Vaccinia virus,Vesicular stomatitis virus and TK KOS ACVr (in HeL cells), 4c against Vesicular stomatitis virus, Coxsackie virus B4 and Respiratory syncytial virus (in HeLa cells) and 4c against sindbis virus, 4h against Reovirus-1, sindbis virus, Coxsackie virus B4 and Punta Toro virus (in Vero cells) could be considered as an indication of antiviral specificity. This data clearly shows that electron withdrawing group in the substituted analogues 4b, 4i, 4h and 4j appears to be essential for antiviral activity. However the nitro-, methoxy phenyl and furfuryl substituted analogues were less active (Vero, HeLa, HeL cells) at a concentration below their toxicity threshold.
Among the newer derivatives compounds 4b, 4i, 4h and 4j showed a promising in vitro antiviral activity and it is conceived that these derivatives can be further modified to improve their potential as anti-viral chemotherapeutic agents. Further studies to acquire more QSAR information are in progress in our laboratory.
Acknowledgements
The author (M.Shahar Yar) wishes to express has thanks to the University Grant Commission-New Delhi, India for a research award, Rega Institute for Medical Research Katholieke Universiteit Leuven, B-3000 Leuven Belgium for anti-viral screening and Dr. Kiran Smith, National Cancer Institute-USA for valuable suggestions.
References
- Shaharyar M, Siddiqui AA, Ali MA, Sriram D, Yogeeswari P. Synthesis and in vitro antimycobacterial activity of N1-nicotinoyl-3- (4′-hydroxy-3′-methylphenyl)-5-[(sub) phenyl]-2-pyrazolines. Bioorg Med Chem Lett 2006; 16: 3947–3949
- Shaharyar M, Siddiqui AA, Ali MA. Synthesis and evaluation of phenoxy acetic acid derivatives as a anti-mycobacterial agents. Bioorg Med Chem Lett 2006; 16: 4571–4574
- Shaharyar M, Siddiqui AA, Ali MA, Murugan V, Raghu C. Synthesis and cytotoxic activity of novel pyrazoline derivatives against human lung tumor cell line. J Chin Chem Soc 2007, (In press)
- Kucukguzel SG, Rollas S. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Farmaco 2002; 57(7)583–587
- Bekhit AA, Ashour HM, Guemei AA. Novel pyrazole derivatives as potential promising anti-inflammatory antimicrobial agents. Arch: Pharm (Weinheim) 2005; 338(4)167–174
- Ruhoglu O, Ozdemir Z, Calis U, Gumusel B, Bilgin AA. Synthesis of and pharmacological studies on the antidepressant and anticonvulsant activities of some 1,3,5-trisubstituted pyrazolines. Arzneimittelforschung 2005; 55(8)431–436
- Ucar G, Gokhan N, Yesilada A, Bilgin AA. 1N-Substituted thiocarbamoyl-3-phenyl-5-thienyl-2-pyrazolines: a novel cholinesterase and selective monoamine oxidase B inhibitors for the treatment of Parkinson's and Alzheimer's diseases. Neurosci Lett 2005; 15: 327–331
- Rajendra Prasad Y, Lakshmana Rao A, Prasoona L, Murali K, Ravi Kumar P. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2″-hydroxy naphthalen-1″-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett 2005; 15: 5030–5034
- Turan Zitouni G, Ozdemir A, Guven K. Synthesis of some 1-[(N, N-disubstituted thiocarbamoyl thioacetyl]-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives and investigation of their antibacterial and antifungal activities. Arch Pharm (Weinheim) 2005; 338(2-3)96–104
- Amr Ael G, Abdel-Latif N-A, Abdalla MM. Synthesis and anti androgenic activity of some new 3-substituted androstano [17,16-c]-5′-aryl-pyrazoline and their derivatives. Bioorg Med Chem 2006; 15: 373–384
- De Clercq E, Descamps J, Verhelst G, Walker RT, Jones AS, Torrence P-F, Shugar D. Comparative efficacy of different antiherpes drugs against different strains of herpes simplex virus. J Infect Dis 1980; 141: 563–574
- De Clercq E, Holy A, Rosenberg I, Sakuma T, Balzarini J, Maudgal PC. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986; 323: 464–467
- De Clercq E. Antiviral and antimetabolic activities of neplanocins. Antimicrob Agents Chemother 1985; 28: 84–89
- Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Meth 1988; 20: 309–321
- Balzarini J, Naesens L, Slachmuylders J, Niphuis H, Rosenberg I, Holy A, Schellekens H, De Clercq E. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS 1991; 5: 21–28
- Balzarini J, Pauwels R, Baba M, Herdewijn P, De Clercq E, Broder S, Johns DG. The in vitro and in vivo anti-retrovirus activity and intracellular metabolism of 3′-azido-2′,3′ dideoxythymidine and 2′,3′-dideoxy cytidine are highly dependent on the cell species. Pharmacol 1988; 37: 897–903
- Shahayar M, Siddiqui AA, Ali MA, Srinam D, Yogeeswari P. Bioorg Med Chem Lett 2006; 16: 3947–3949, Yar MS, Siddiqui AA, Ali MA, Bioorg Med Chem Lett 2006;16: 4571–74; Yar MS, Ali MA, Acta Pol Pharm 2006;6:491–496