Abstract
The crude methanolic extract of Andrachne cordifolia Muell. (Euphorbiaceae) and its various fractions in different solvent systems (chloroform, ethyl acetate and n- butanol) were screened for antibacterial and antifungal activities. Crude extract and subsequent fractions demonstrated moderate to excellent antibacterial activities against the tested pathogens. Highest antibacterial activity was displayed by both chloroform and ethyl acetate fractions (100%) followed by the crude extract (68%) against Salmonella typhi. Similarly, crude extract and its subsequent fractions showed mild to excellent activities in antifungal bioassay with maximum (76%) antifungal activity against Microsporum canis by the chloroform fraction followed by the crude extract (65%).
Introduction
Plants, as extracts and in various other forms, have been used for centuries in different traditional systems of medicine for the treatment of human ailments, particularly those caused by pathogenic bacteria and fungi. The antiseptic qualities of aromatic and medicinal plants and their extracts have been recognized since antiquity, while attempts to characterize these properties in the laboratory date back to the early 1900s Citation1-3. The growing antimicrobial resistance of certain microorganisms is now a worldwide concern. Acquisition and further spread of antibiotic resistance determinants among virulent bacterial populations is the most relevant problem for the treatment of infectious diseases [Citation4,Citation5]. It is because of these reasons that the search for plant products having antimicrobial properties has intensified in recent years [Citation6,Citation7].
In herbal medicine, the genus, Andrachne is used for the treatment of eye sores and to improve eyesight [Citation8] and also has a stimulating action on respiration and the blood pressure of the dog and cat, spasmolytic activity on the tracheal muscle of the cat and anti histaminic activity a guineapig illium [Citation9]. Interest in the phytochemical exploration of Andrachne cardifolia Muell began from 1983 when M.Ikram et al., isolated two bisbenzylisoquinoline alkaloids, cocsulin and penduline, from the roots of Andrachne cardifolia Muell [Citation10]. This achievement was followed by the isolation of a new triterpene alcohol glut-5 (10)-en-1β -ol [Citation11] and a pentacyclic triterpenic ketone, Glut-5 (10)-en-one [Citation12], from the whole plant of Andrachne cardifolia Muell. Since then, this plant has not been extensively subjected to isolation of its active constituents and characterization, though it deserves to be thoroughly investigated for its beneficial uses in the light of present scientific advancement in the field of natural products. The methanolic extract and subsequent fractions of Andrachne cardifolia Muell have been screened for their enzyme inhibitory activites in our laboratory and showed outstanding activity against lipoxygenase [Citation13]. Here, these extracts are examined for their antibacterial and antifungal activities.
Methods and materials
Plant material
Andrachne cordifolia Muell, locally known as Krachay (Pushto) belong to the Euphorbiaceae family. The whole plant was collected from Dir district, N.W.F.P (Pakistan) in 2004, and identified by Professor Dr. Jahander Shah, Plant Taxonomist, Vice Chancellor University of Malakand, Chakdara Dir. A voucher specimen (CA-012) has been deposited in the herbarium of the University of Malakand.
Extraction
The shade dried plant material was chopped into small pieces and pulverized into a fine powder. The plant material (15 kg) was soaked in methanol with occasional shaking, at room temperature. After 15 days, the methanol soluble materials were filtered off. The filtrate was concentrated under vacuum at low temperature (40°C) using a rotary evaporator. A blackish crude extract (295 g) was obtained.
Fractionation
The crude methanolic extract (295 g) was suspended in distilled water (500 mL) and sequentially partitioned with n-hexane (3 × 500 mL), chloroform (3 × 500 mL), ethyl acetate (3 × 500 mL) and n-butanol (3 × 500 mL) to yield the n-hexane (33 g), chloroform (69 g), ethyl acetate (36 g), n-butanol (41 g) and aqueous (75 g) fractions, respectively.
Antibacterial activity
The crude extract along with its fractions was screened against various human pathogens including Escherichia coli, Bacillus subtilis, Klebsiella pneumonae, Shigella flexenari, Staphylococcus aurous, and Salmonella typhi by the agar well diffusion method [Citation14]. In this method, 10 mL aliquots of nutrient broth (Sigma-Aldrich, Germany) was inoculated with the test organism and incubated at 37°C for 24 h. Using a sterile pipette, 0.6 mL of the broth culture of the test organism was added to 60 mL of molten agar, which had been cooled to 45°C, mixed well and poured into a sterile Petri dish (for the 9 cm Petri dish, 0.2 mL of the culture was added to 20 mL of agar). Duplicate plates of each organism were prepared. The agar was allowed to set and harden and the required number of wells were dug in the medium with the help of a sterile metallic cork borer ensuring proper distribution of the wells in the periphery and one in the center. Agar plugs were removed. Stock solutions of the test samples at a concentration of 1 mg/mL were prepared in the sterile dimethyl sulfoxide (DMSO) and 100 μL and 200 μL of each dilution was added to the respective wells. The control well received only 100 μL and 200 μL of DMSO. Imipinem was used as a standard drug. The plates were left at room temperature for 2 h to allow diffusion of the samples and then incubated face upwards at 37°C for 24 h. The diameter of the zones of inhibition was measured to the nearest mm (the well size also being noted).
Antifungal activity
The antifungal activity of the extracts was evaluated by the agar tube dilution method [Citation14]. The samples, 24 mg/ mL, were dissolved in sterile (autoclaved) dimethyl sulfoxide (DMSO, Merck), which served as a stock solution. Sabouraud dextrose agar (SDA, Sigma-Aldrich, Germany) was prepared by mixing 32.5 g sabouraud, 4% glucose agar and 4.0 g of agar-agar in 500 mL distilled water thoroughly with a magnetic stirrer. Then a 4 mL aliquot was dispensed into screw cap tubes, which were autoclaved at 120°C for 15 min and then cooled to 15°C. The non-solidified SDA media was mixed with stock solution (66.6 μL) giving a final concentration of 400 μg of the extract per mL of SDA. The tubes were then allowed to solidify in the slanted position at room temperature and then inoculated with a piece (4 mm diameter) of an inoculum removed from a seven days old culture of fungi to determine non-mycelial growth; an agar surface streak was employed. Other media supplemented with dimethyl sulfoxide (DMSO) and reference antifungal drugs served as negative and positive control respectively. Inhibition of fungal growth was observed visually after 7 days of incubation at 28 ± 1°C. Humidity (40–50%) was controlled by placing an open pan of water in the incubator.
Results and discusion
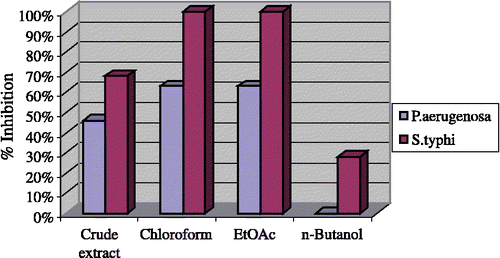
The crude methanolic extract and fractions of Andrachne cordifolia Muell were screened against various human pathogens and the results of their antibacterial activities are shown in . The results show that the crude extract displayed low activity against E. coli (43%), P. aeruginosa (46%), B. subtilis (46%) and S. typhi (68%). No activity against S. aurous and S. flexenari was seen.
Table I. Antibacterial Activities Of Crude Extracts and Various fractions of Andrachne Cardifolia Muell.
The chloroform fraction presented outstanding activity against S. typhi (100%) and P. aeruginosa (63%) (). However it exhibited no activity against S. aurous, B. subtilis, S. flexenari and E. coli. The ethyl acetate fraction had similar activity to that of the chloroform fraction i.e. against S. typhi (100%) and P. aeruginosa (63%). The n-butanol fraction showed moderate activity against E. coli (53%) and S. typhi (28%), while against S. aurous, B. subtilis, S. flexenari and P. aeruginosa it exhibited no activity.
Figure 1 Antibacterial activity towards P. aerugenosa and S. typhi of crude extract and subsequent fractions of Andrachne Cardifolia Muell. The extracts were used at a concentration of 3 mg/mL of DMSO.
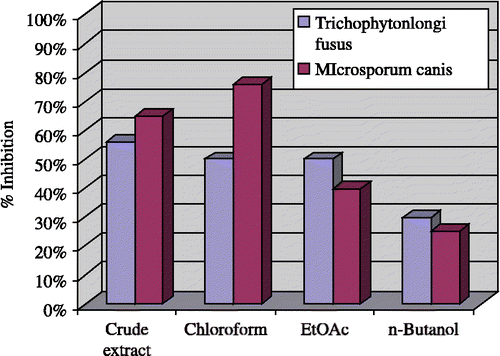
Antifungal activities of the crude extract and various fractions of Andrachne cardifoliaI Muell were evaluated against Trichophyton longi fusus, Candida albicans, Aspergilus flavus, Icrosporum canis, Fusarium solani, and Candida glaberata, in comparison with miconazole and amphotericin-B and the results are shown .
Table II. Antifungal activities of crude extracts and various fractions of Andrachne cardifolia Muell.
The crude extract displayed good antifungal activity against T. longi fusus (56%), M. canis (65%) and F. solani (50%) () while inactive C. albicans, A. flavus and C. glaberata. The CHCl3 fraction showed good to excellent result against T. longi fusus (50%) and M. canis (76%) but showed no activity against C. albicans, F. solani, A. flavus and C. glaberata. The activities of the EtOAc Fraction were similar to those of the chloroform fraction except against the M. canis (40%). The n-butanol fraction also showed antifungal activity against T. longi fusus (30%), C. albicans (20%) and M. canis (25%) but no activity against A. flavus, C. glaberat and F. solani was observed.
Figure 2 Antifungal activity towards T. longifusus and M. cansis of crude extract and subseqent fractions of Andrachne cardifolia Muell. Final concentration of relavant extract was 24 mg/mL of DMSO.
The results presented in show that the chloroform and ethyl acetate fractions of Andrachne cordifolia Muell had an outstanding activity (100%) against S. typhi which is an intracellular pathogen that causes diseases ranging from self-limiting enteritis to typhoid fever, the latter being a global health problem although its real impact is difficult to estimate because the clinical picture is confused with those of many other febrile infections. Worldwide, enteric infections rank third among all causes of the disease burden, being responsible for some 1.7–2.5 million deaths per year, mostly in young children and infants in developing countries Citation15-18.
On the other hand, multidrug-resistant (MDR) Salmonella Typhi (resistant to chloramphenicol, ampicillin, and trimethoprim–sulphamethoxazole) and isolates with reduced susceptibility to fluoroquinolones (indicated by resistance to nalidixic acid) can be treated with alternative synthetic drugs which are reasonably effective but quite expensive and have many side effects [Citation19,Citation20]. Development of new effective and safe products for the treatment of typhoid fever is urgently required. Therefore, this plant species could be an excellent natural source for the treatment of typhoid fever and a potential target for the activity-guided isolation of its active constituents in order to explore the mechanism of this activity and potentially relevant uses.
References
- Martindale WH. Essential oils in relation to their antiseptic powers as determined by their carbolic coefficients. Perf Ess Oil Res 1910; 1: 266–296
- Hoffman C, Evans AC. The uses of spices as preservatives. Journal of Ind Eng Chem 1911; 3: 835–838
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J App Microbiol 2000; 88: 308–316
- Paul W Ament, Jamshed Namirah, horne John P. linezolid: Its role in the treatment of gram-positive, drug-resistant bacterial infections. Clin Pharmacol 2002; 65: 663–670
- Alonso Ana, Sanchez Patricia, Martinez Jose L. Environmental selection of antibiotic resistance genes. Env Microbiol 2001; 3: 1–9
- Ray AB, Sarma BK, Singh UP. Medicinal properties of plants: Antifungal, antibacterial and antiviral activities. International Book 2004; 600, v,
- Bassam AS, Ghaleb G, Gahood AS, Naser J, Kamel A. Antibacterial activities of some plant extracts utilized in popular medicine in palestine. Turk J Biol 2004; 28: 99–102
- Gopalan G. Medicinal plants of india Vol. 1 page 64, (Indian Council of Medical Reaserch, New Dehli (1976).
- Derasari HR, Khasa JH. Evaluation of Indigenous plants for the treatment of acute shigellosis. Indian J Pharm 1966; 28: 237
- Khan MI, Ikram M, Hussain SF. Bis(benzyl-isoquinolin) alkaloids from andrachne cordifolia. Plaqnta Medica 1983; 47: 191–193
- Mukharjee KS, Bhattachcharjee P, Mukharjee RK, Ghosh PK. Phytochem 1986; 11: 2669–2670
- Mukharjee KS, Bhattachcharjee P. Phytochemi 1987; 26: 1539–1540
- Basher Ahmad, Hassan Shah SM, Bashir Shumaila, Khan Haroon, Shah Jehandar. J Enz Inhib Med Chem 2006, In Press
- Atta-ur-Rehman Choudhary MI, Thomsen. Manual of bioassay techniques for natural product research. Harward Academic Press, Amsterdam 1991; 82–84
- Marc P Girard, Steele Duncan, Chaignat Claire-Lise, Kieny Marie Paule. A review of vaccine research and development: Human enteric infections. 2006; 24: 2732–2750
- Patel Jayesh C, Rossanese Olivia W, Galan Jorge E. The functional interface between Salmonella and its host cell: Opportunities for therapeutic intervention. Trend Pharmacol Sci 2005; 26: 564–570
- Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Rao M, Naficy A, Clemens J, Bhan MK. Typhoid fever in children aged less than 5 years. Lancet 1999; 354: 734–737
- Saha SK, Baqui AH, Hanif M, Darmstadt GL, Ruhulamin M, Nagatake T, Santosham M, Black R. Typhoid fever in Bangladesh: Implications for vaccination policy. Ped Infect Dis J 2001; 20: 521–524
- Christopher M Parry. The treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever in viet nam. Transactions of the Roy Soc Trop Med Hyg 2004; 98: 413–422
- Fiona J Cooke, Wain John. The emergence of antibiotic resistance in typhoid fever. Travel Med Infect Dis 2004; 2: 67–74
