Abstract
The crude methanolic extract and chloroform, ethyl acetate and n-butanol fractions of Teucrium royleanum were examined as inhibitors of actylcholinesterase, butyrylcholinesterase, lipoxygenase and urease. A significant enzyme inhibition activity (52–83%) was shown by the crude methanolic extract and its fractions against acetylcholinesterase, while low to outstanding enzyme inhibitory activity was shown (19–93%) against butyrylcholinesterase. The crude methanolic extract and its various fractions demonstrated low activity against lipoxygenase and inactive against urease.
Introduction
Cholinesterase inhibitors exert their effects by inhibiting acetylcholinesterase and thereby increasing the concentration of endogenous acetylcholine in the vicinity of cholinoreceptors [Citation1] According to the cholinergic hypothesis, memory impairment in patients with senile dementia of Alzheimer's type results from a deficiency in cholinergic function in the brain [Citation2] Hence the most promising therapeutic strategy for activating central cholinergic functions has been the use of cholinomimetic agents. The enzyme acetylcholinesterase (AChE) has long been an attractive target for rational drug design and discovery of mechanism-based inhibitors. Because of its role in the hydrolysis of the neurotransmitter acetylcholine [Citation3] the inhibition of the enzyme is considered as a promising approach for the treatment of Alzheimer's disease (AD) and for other therapeutic applications in the treatment of Parkinson's disease, ageing and myasthenia gravis and glaucoma [Citation4,Citation5,Citation6].
Butyrylcholinesterase (BChE) is produced in the liver and enriched in the circulation. The exact physiological role of this enzyme is still elusive, but it is generally viewed as a back up for the homologous AChE [Citation7]. Although the role of butyrylcholinesterase (BChE) in the normal ageing and diseased brain is still unknown, recently it has been found that BChE inhibition may also be an effective tool for the treatment of AD and related dementias [Citation8].
Plant background
Species of the Teucrium genus are bitter, astringent, antirheumatic herbs that reduce inflammation, stimulate the digestion and have been used as herbal medicines for coughs and asthma since ancient times. Several studies on the bacteriostatic, spasmolytic, antioxidant, antinflammatory and antifungal activity of Teucrium species have been reported in the literature Citation9-12. As a folk medicine, Teucrium royleanum is used as an antispasmodic, astringent, antipyretic, and for skin rashes [Citation13]. The plant exhibits aromatic or bitter-aromatic, stimulant, astringent, tonic and diaphoretic properties
The constituents of Teucrium montanum are montanins A, B, C and D; teucrins A and E are from Teucrium chamaedrys; 6-ketoteuscordin and teuscordinin are from Teucrium scordium [Citation14]; and from Teucrium fruticans are the neo-clerodanes, namely 7β-hydroxyfruticolone, 11-hydroxyfruticolone, deacetylfruticolone and 6- acetyl-10- hydroxyteucjaponin B [Citation15].
Interest in the phytochemical exploration of Teucrium royleanum began in 1992 when Mashooda Hassan isolated triterpenoids, aliphatic, steroidal and acidic compounds, a mixture of triaocontane and hentriacontane, lupeol and β-amyrin, hexacosianic acid and stigmastatrien-3-ol [Citation16]. Since then keeping in view its uses as a folk medicine, this plant, although deserved, has not been extensively subjected to phytochemical and pharmacological investigation. Here, we examine its inhibitory properties towards a number of enzymes i.e, Ache, Bche, lipocygenase and urease.
Methods and materials
Plant material
Teucrium royleanum (Labiatea), as a whole plant, was collected from the Dir district, N.W.F.P (Pakistan) in June-August 2004 and identified by Professor Dr. Jahander Shah, Plant Taxonomist, Vice Chancellor, University of Malakand, Chakdara Dir. A voucher specimen (CA-013) has been deposited in the herbarium of the University of Malakand.
Extraction
The shade dried plant material was chopped into small pieces and pulverized into a fine powder. The plant material (12 kg) was soaked in methanol with occasional shaking, at room temperature. After 15 days, the methanol soluble material was filtered off. The filtrate was concentrated under vacuum at low temperature (40°C) using a rotary evaporator. A blackish crude extract (350 g) was obtained.
Fractionation
The crude methanolic extract (350 g) was suspended in distilled water (500 mL) and sequentially partitioned with n-hexane (3 × 500 mL), chloroform (3 × 500 mL), ethyl acetate (3 × 500 mL) and n-butanol (3 × 500 mL) to yield n- hexane (41 g), chloroform (83 g), ethyl acetate (88 g), n-butanol (54 g) and aqueous (67 g) fractions, respectively.
In vitro cholinesterase inhibition assay
Acetylcholinesterase and butyrylcholinesterase inhibiting activities were measured by slightly modifying the spectrophotometric method previously developed by Ellman et al. [Citation17]. Electric-eel AChE (type VI-S, Sigma) and horse-serum BChE (Sigma) were used as source of both the cholinesterases and acetylthiocholine iodide and butyrylthiocholine chloride (Sigma), respectively, were used as substrates of their reactions. 5, 5 - ithiobis (2-nitrobenzoic acid) (DTNB, Sigma) was used for the measurement of cholinesterase activity. Briefly, 140 μL of 100 mM sodium phosphate buffer (pH 8.0), 10 μL of DTNB, 20 μL of the test samples solutions and 20 μL of acetylcholinesterase/ butyrylcholinesterase solution were mixed and incubated for 15 min at 25°C. The reaction was then initiated by the addition of l0 μL acetylthiocholine / butyrylthiocholine, respectively. The hydrolysis of acetylthiocholine and butyrylthiocholine was monitored at a wavelength of 412 nm by the formation of the yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine and butyrylthiocholine respectively. The samples and the control were dissolved in 50% ethanol. All the reactions were performed in triplicate.
In vitro lipoxygenase inhibition assay
Lipoxygenase inhibiting activity was conveniently measured by slightly modifying the spectrometric method previously developed [Citation18]. Lipoxygenase (1.13.11.12) type I-B and linoleic acid were purchased from Sigma (St. Loius, MO) and were used without further purification. All other chemicals were of analytical grade. Briefly 160 μL of 0.1 mM sodium phosphate buffer (pH 7.0), 10 μL of the sample solution and 20 μL of lipoxygenase solution were mixed and incubated for 5 min at 25°C. The reaction was initiated by the addition of 10 μL linoleic acid solution (substrate), with the formation of (9Z, 11E)-13S)-13-hydroperoxyoctadeca-9, 11-dienoate, and the change in reaction was followed for 10 min. The test sample and the control were dissolved in 50% ethanol. All the reactions were performed in triplicate. The IC5o values (concentrations of sample causing 50% reduction in activity relative to the control) were then calculated using the EZ-Fit Enzymes kinetics programme.
Results and discussion
Plants, over the years, provided nutritional as well as medicinal uses to the living creatures of this universe. Here the in vitro enzyme inhibitory activities of the crude methanolic extract and subsequent fractions of Teucrium royleanum were examined against acetylcholinesterase, butyrylcholinesterase, lipoxygenase and urease and the results are presented in and
Figure 1 Acetylcholinesterase Inhibition by the crude extract and subsequent fractions of Teucrium royleanum 40 μg/200 μL.
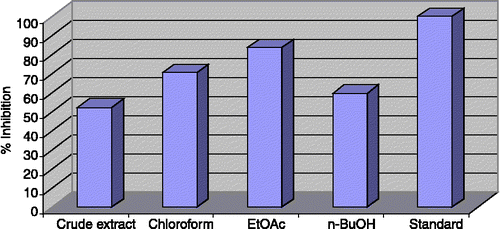
Figure 2 Butyrylcholinesterase Inhibition by the crude extract and subsequent fractions of Teucrium royleanum 40 μg/200 μL.
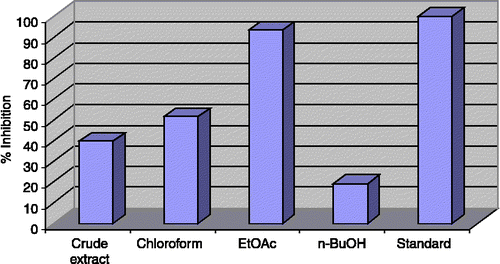
The results obtained with the crude extract of Teucrium royleanum exhibits moderate enzyme inhibitory activity against acetylcholinesterase (52.4%), butyrylcholinesterase (39.6%) and lipoxygenase (55.13%). The chloroform fraction exhibited significant enzyme inhibition activity (70.5%) against acetylcholinesterase and moderate activity against butyrylcholinesterase (51.41%) and lipoxygenase (39.22%).
The ethylacetate fraction showed outstanding inhibitory activity against acetylcholinesterase (83.62%) and butyrylcholinesterase (93.5%), but low activity against lipoxygenase (23.14%). The n-butanol fraction also showed moderate activity against acetylcholinesterase (59.55%) and low activity against butyrylcholinesterase (19.11%). But was inactive against lipoxygenase. The crude extract and all the fractions were inactive against urease.
As shown in, it can be seen that the crude extract and subsequent fractions of Teucrium royleanum are strong potential inhibitor of acetylcholinesterase and butyrylcholinesterase and provide an opportunity for the isolation of active medicinal agents for the treatment of various diseases.
References
- Bertram GKatzung. Basic and clinical pharmacology9th Edition. 2004; 102
- Robbins and Kummar. Basic pathology4th edition. 1987; 747
- Taylor P. Development of acetylcholinesterase inhibitors in the therapy of alzheimer's disease. Neurol 1998; 51: 30–35
- Ballard CG. Advances in the treatment of alzheimer's disease: Benefits of dual cholinesterase inhibitioneur. Neurol 2002; 47: 64–70
- Ruof J, Mittendorf T, Pirk O, von der Schulenburg JM. Diffusion of. innovations: Treatment of alzheimer's disease in germany health policy. 2002; 60: 59–66, (New York),
- Millard CB, Broomfield CA. Anticholinesterases: Medical applications of neurochemical principles. J Neurochem 1995; 64: 1909–1918
- Schwaez M, Glick D, Loewensten Y, Soreq H. Pharmacol 1995; 67: 283
- Yu QS, Holloway HW, Utsuki T, Brossi A, Greig NH. Synthesis of novel phenserinebased selective inhibitors of butyrylcholinesterase for alzheimer's disease. J Med Chem 1999; 42: 1855–1861
- Yang HO, Suh D, Han B, H. Isolation and characterization of platlet-activating factor receptor binding antagonist from Biota orientalis. Planta Medica 1995; 61: 37–40
- Harborne JB, Valdes B. Phytochemistry 1971; 10: 2850
- Youssef D, Frahm A, Constituents of the Egyptian Centaurea scoparia. III: Phenolic constituents of the aerial parts Constituents of the Egyptian Centaurea scoparia. III: Phenolic constituents of the aerial parts Planta Medica., 61 1995. p 570–573
- Baluchnejadmojarada Tourandokht, Roghanib Mehrdad, Roghani- Dehkordic Farshad. Journal of Ethnopharmacology 2005; 97: 207–210
- Kirtikar and Basu. Indian Medicinal Plant 2000; 8: 2696
- Eszter Gacs, Marton Kajtar. Hiterocycles 1982; 19: 3
- Coll Josep, Tandron Yudelsy. Isolation and structure elucidation of three neo-clerodane diterpenes from teucrium fruticans l. (LABIATAE). Phytochem 2005; 66: 2298–2303
- Mashooda Hasan M, Ramzan Maria C, Alvarez Garcia, Uddin Ahmad Viqar. Constituents of teucrium royleanum. Fitoterapia 1993; 64: 555
- Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem pharmacol 1961; 7: 88–95
- Tappel AL. Meth enzymol. Academic Press. 1962; Vol 5: 539–542
