Abstract
In this study, new 3-[(1(2H)-phthalazinone-2-yl(methyl/ethyl]-4-aryl-1,2,4-triazole-5-thione and 2-[[1(2H)-phthalazinone-2-yl]methyl/ethyl]-5-arylamino-1,3,4-thiadiazole derivatives were synthesized. Antimicrobial properties of the title compounds were investigated against two Gram (+) bacteria (S. aureus, B. subtilis), two Gram ( − ) bacteria (P. aeruginosa, E. coli) and two yeast-like fungi (C. albicans and C. parapsilosis) using the broth microdilution method. Generally the compounds were found to be active against B. subtilis and the fungi. Derivatives carrying a 1,3,4-thiadiazole ring generally showed higher antimicrobial activity against B. subtilis and the fungi when compared to other synthesized compounds.
Introduction
Since resistance of pathogenic bacteria towards available antibiotics is rapidly becoming a major world-wide problem, the design of new compounds to deal with resistant bacteria has become one of the most important areas of antibacterial research today. In addition, it is known that antifungal drugs do not have selective activity because of the biochemical similarity between human cell and fungi forms. Therefore, there are many studies focused on antibacterial and antifungal compounds Citation1-3.
Some compounds bearing a 1,2,4-triazole and 1,3,4-thiadiazole structure have been reported to have antimicrobial activity Citation4-8. In addition, many compounds carrying a phthalazinone ring have been reported to have diverse biological activities such as antimicrobial [Citation9,Citation10], antihypertensive [Citation11], analgesic and anti-inflammatory activities [Citation12].
In the design of new compounds, development of hybrid molecules through the combination of different pharmacophores in one structure may lead to compounds with increased antimicrobial activity. Therefore, these observations prompted us to synthesize new 1,2,4-triazole and 1,3,4-thiadiazole derivatives which were attached to position-2 of the phthalazinone ring through a methylene or ethylene bridge. Then, the synthesized compounds were tested against two Gram (+) bacteria (Staphylococcus aureus, Bacillus subtilis), two Gram ( − ) bacteria (Pseudomonas aeruginosa, Escherichia coli) and two yeast-like fungi Candida albicans and Candida parapsilosis using the broth microdilution method.
Experimental
Chemistry
All the chemicals used for the synthesis of the compounds were purchased from either Aldrich Chemicals or Merck AG. Melting points were determined with an Electrothermal-9300 digital melting point apparatus and are uncorrected. IR spectra were recorded on a Perkin–Elmer 1330 IR spectrophotometer and 1H-NMR spectra on a Mercury 400 FT NMR spectrometer using tetramethylsilane as the internal standard and DMSO-d6 as solvent. All chemical shifts were recorded as δ (ppm). Elemental analyses were performed with CHNS-932 (Leco) at Ankara University, Faculty of Pharmacy.
General procedure for [1(2H)-phthalazinone-2-yl]acetyl/propanoylthiosemicarbazides (6a–l)
To a solution of the [1(2H)-phthalazinone-2-yl]acetylhydrazine (0.0025 mol) in 50 mL methanol or the 3-[1(2H)-phthalazinone-2-yl]propanoylhydrazine (0.0025 mol) in 50 mL ethanol were added the appropriate isothiocyanate derivatives (0.0026 mol) and then each reaction mixture was refluxed until the starting material had been consumed. At the end of this period the precipitating product was filtered and crystallized from an appropriate solvent.
6a
IR (KBr) νmax (cm− 1) 1675, 1647; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.41 (1H, s, CONH), 9.78 (1H, s, NHNHCSNH), 9.51 (1H, s, NHNHCSNH), 8.47 (1H, s, H4), 8.22 (1H, d, H8), 7.97-7.88 (3H, m, H5,H6,H7), 7.50 (2H, d, phenyl-H2,6), 7.34 (2H, t, phenyl-H3,5), 7.16 (1H, t, phenyl-H4), 4.90 (2H, s, CH2).
6b
IR (KBr) νmax (cm− 1) 1679, 1656; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.23(1H, s, CONH), 9.52 (1H, s, NHNHCSNH), 8.45 (1H, s, NHNHCSNH), 8.42 (1H, s, H4), 8.15 (1H,d, H8), 7.95-7.84 (3H, m, H5, H6, H7), 7.27-7.26 (5H, m, phenyl-H), 4.85 (2H, s, CH2), 4.74 (2H, d, CH2C6H5).
6c
IR (KBr) νmax (cm− 1) 1685, 1650; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.22(1H, s, CONH), 9.46 (1H, s, NHNHCSNH), 8.48 (1H, s, H4), 8.26 (1H,d, H8), 8.06 (1H, s, NHNHCSNH), 8.00-7.88 (3H, m, H5, H6, H7), 7.32-7.18 (5H, m, phenyl-H), 4.87 (2H, s, CH2), 3.66-3.64 (2H, m, CH2CH2C6H5), 2.84 (2H, t, CH2CH2C6H5).
6d
IR (KBr) νmax (cm− 1) 1674, 1645; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.47 (1H, s, CONH), 9.93 (1H, s, NHNHCSNH), 9.59 (1H, s, NHNHCSNH), 8.50 (1H, s, H4), 8.26 (1H,d, H8), 8.00-7.89 (3H, m, H5, H6, H7), 7.58 (2H, d, phenyl-H2,6), 7.43 (2H, t, phenyl-H3,5), 4.93 (2H, s, CH2).
6e
IR (KBr) νmax (cm− 1) 1685,1648; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.38 (1H, s, CONH), 9.68 (1H, s, NHNHCSNH), 9.46 (1H, s, NHNHCSNH), 8.48 (1H, s, H4), 8.24 (1H,d, H8), 7.99-7.88 (3H, m, H5, H6, H7), 7.36 (2H, d, phenyl-H2,6), 6.93 (2H, t, phenyl-H3,5), 4.92 (2H, s, CH2), 3.76 (3H, s, OCH3).
6f
IR (KBr) νmax (cm− 1) 1675,1647; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.42 (1H, s, CONH), 9.75 (1H, s, NHNHCSNH), 9.48 (1H, s, NHNHCSNH), 8.49 (1H, s, H4), 8.24 (1H,d, H8), 8.01-7.89 (3H, m, H5, H6, H7), 7.38 (2H, d, phenyl-H2,6), 7.16 (2H, t, phenyl-H3,5), 4.92 (2H, s, CH2), 2.30 (3H, s, CH3).
6g
IR (KBr) νmax (cm− 1) 1701, 1632; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.03(1H, s, CONH), 9.56 (2H, s, NHNHCSNH), 8.41 (1H, s, H4), 8.16 (1H,d, H8), 7.93-7.81 (3H, m, H5, H6, H7), 7.42 (2H, d, phenyl-H2,6), 7.32 (2H, t, phenyl-H3,5), 7.16 (1H, t, phenyl-H4), 4.39 (2H, t, NCH2), 2.68 (2H, t, CH2CO).
6h
IR (KBr) νmax (cm− 1) 1706, 1637; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 9.93 (1H, s, CONH), 9.34 (1H, s, NHNHCSNH), 8.43 (1H, s, NHNHCSNH), 8.41 (1H, s, H4), 8.15 (1H,d, H8), 7.93-7.81 (3H, m, H5, H6, H7), 7.30-7.21 (5H, m, phenyl-H), 4.77 (2H, d, CH2C6H5), 4.39 (2H, t, NCH2), 2.65 (2H, t, CH2CO).
6i
IR (KBr) νmax (cm− 1) 1699, 1641; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 9.83 (1H, s, CONH), 9.21 (1H, s, NHNHCSNH), 8.43 (1H, s, H4), 8.24 (1H,d, H8), 7.97 (1H, s, NHNHCSNH), 7.94-7.83 (3H, m, H5, H6, H7), 7.29-7.15 (5H, m, phenyl-H), 4.37 (2H, t, NCH2), 3.64-3.60 (2H, m, CH2CH2C6H5), 2.79 (2H, t, CH2CH2C6H5), 2.63 (2H, t, CH2CO).
6j
IR (KBr) νmax (cm− 1) 1672, 1649; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.07 (1H, s, CONH), 9.69 (1H, s, NHNHCSNH), 9.61 (1H, s, NHNHCSNH), 8.45 (1H, s, H4), 8.20 (1H,d, H8),7.97-7.84 (3H, m, H5, H6, H7), 7.49 (2H, d, phenyl-H2,6), 7.40 (2H, t, phenyl-H3,5), 4.42 (2H, t, NCH2), 2.70 (2H, t, CH2CO).
6k
IR (KBr) νmax (cm− 1) 1682,1665; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.01 (1H, s, CONH), 9.47 (1H, s, NHNHCSNH), 9.44 (1H, s, NHNHCSNH), 8.43 (1H, s, H4), 8.19 (1H,d, H8),7.95-7.83 (3H, m, H5, H6, H7), 7.27 (2H, d, phenyl-H2,6), 6.91 (2H, t, phenyl-H3,5), 4.41 (2H, t, NCH2), 3.76 (3H, s, OCH3), 2.69 (2H, t, CH2CO).
6l
IR (KBr) νmax (cm− 1) 1703, 1633; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 9.99 (1H, s, CONH), 9.74 (1H, s, NHNHCSNH), 9.48 (1H, s, NHNHCSNH), 8.41 (1H, s, H4), 8.17 (1H,d, H8),7.92-7.82 (3H, m, H5, H6, H7), 7.36 (2H, d, phenyl-H2,6), 7.10 (2H, t, phenyl-H3,5), 4.39 (2H, t, NCH2), 2.67 (2H, t, CH2CO), 2.25 (3H, s, CH3).
General procedure for 3-[(1(2H)-phthalazinone-2-yl(methyl]-4-aryl-1,2,4-triazole-5-thiones (7a–f)
To a solution of the [1(2H)-phthalazinone-2-yl]acetylthiosemicarbazide (0.001 mol) in 50 mL methanol was added an aqueous solution of Na2CO3 (10 mL; 10%) and the reaction mixture was refluxed for 4 h, cooled and then neutralized with glacial acetic acid (10%). The precipitate formed was filtered, washed with water and crystallized from an appropriate solvent.
7a
(KBr) νmax (cm− 1) 1650; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.97 (1H, s, NH), 8.34 (1H, s, H4), 8.06 (1H, d, H8), 7.91-7.80 (3H, m, H5,6,7), 7.32-7.29 (5H, m, phenyl-H), 5.26 (2H, s, CH2). Analysis for C17H13N5OS (335.38); Calcd: C; 60.88, H; 3.91, N; 20.88. Found: C; 60.89, H; 3.99, N; 20.71%.
7b
IR (KBr) νmax (cm− 1) 1630; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.97 (1H, s, NH), 8.24 (1H, s, H4), 8.08 (1H, d, H8), 7.86-7.76 (3H, m, H5,6,7), 6.99-6.85 (5H, m, phenyl-H), 5.36 (2H, s, CH2), 5.24 (2H, s, CH2-C6H5). Analysis for C18H15N5OS (349.41); Calcd: C; 61.87, H; 4.33, N; 20.04. Found: C; 61.92, H; 4.33, N; 19.72%.
7c
IR (KBr) νmax (cm− 1) 1652; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.97 (1H, s, NH), 8.49 (1H, s, H4), 8.28 (1H, d, H8), 7.98-7.89 (3H, m, H5,6,7), 7.31-7.23 (3H, m, phenyl-H3,4,5), 7.13 (2H, d, phenyl-H2,6), 5.19 (2H, s, CH2), 4.18 (2H, t, N-CH2-CH2-C6H5), 2.85 (2H, t, N-CH2-CH2-C6H5). Analysis for C19H17N5OS (363.43); Calcd: C; 62.79, H; 4.71, N; 19.27. Found: C; 62.36, H; 4.64, N; 18.97%.
7d
IR (KBr) νmax (cm− 1) 1634; 1H-NMR (400MHz) (DMSO-d6) δ (ppm) 13.97 (1H, s, NH), 8.34 (1H, s, H4), 8.05 (1H, d, H8), 7.92-7.82 (3H, m, H5,6,7), 7.33 (4H, s, phenyl-H), 5.30 (2H, s, CH2). Analysis for C17H12ClN5OS (369.83); Calcd: C; 55.21, H; 3.27, N; 18.94. Found: C; 55.28, H; 3.37, N; 18.87%.
7e
IR (KBr) νmax (cm− 1) 1647; 1H-NMR (400MHz) (DMSO-d6) δ (ppm) 13.86 (1H, s, NH), 8.35 (1H, s, H4), 8.06 (1H, d, H8), 7.92-7.81 (3H, m, H5,6,7), 7.18 (2H, d, phenyl-H3,5), 6.80 (2H, d, phenyl-H2,6), 5.25 (2H, s, CH2), 3.64 (3H, s, OCH3). Analysis for C18H15N5O2S (365.41); Calcd: C; 59.16, H; 4.14, N; 19.17. Found: C; 58.85, H; 4.05, N; 18.80%.
7f
IR (KBr) νmax (cm− 1) 1665; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.87 (1H, s, NH), 8.34 (1H, s, H4), 8.05 (1H, d, H8), 7.93-7.80 (3H, m, H5,6,7), 7.14 (2H, d, phenyl-H3,5), 7.07 (2H, d, phenyl-H2,6), 5.25 (2H, s, CH2), 2.16 (3H, s, CH3). Analysis for C18H15N5OS (349.41); Calcd: C; 61.87, H; 4.33, N; 20.04. Found: C; 62.00, H; 4.32, N; 19.95%.
General procedure for 3-[(1(2H)-phthalazinone-2-yl(ethyl]-4-aryl-1,2,4-triazole-5-thiones (7g–l)
A NaOH pellet (0.01 mol) was added a solution of the [1(2H)-phthalazinone-2-yl]propanoylthiosemicarbazide (0.001 mol) in 50 mL ethanol and the mixture was refluxed until the starting material had been consumed. At the end of this period, the resulting solution was poured into ice water and neutralized with glacial acetic acid (10%). The precipitate formed was filtered, washed with water and crystallized from an appropriate solvent.
7g
IR (KBr) νmax (cm− 1) 1635; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.65 (1H, s, NH), 8.39 (1H, s, H4), 8.19 (1H, d, H8), 7.94-7.85 (3H, m, H5,6,7), 7.52-7.41 (5H, m, phenyl-H), 4.26 (2H, t, phthalazinone-CH2CH2), 2.29 (2H, t, phthalazinone-CH2CH2). Analysis for C18H15N5OS (349.41); Calcd: C; 61.87, H; 4.33, N; 20.04. Found: C; 61.83, H; 4.13, N; 20.22%.
7h
IR (KBr) νmax (cm− 1) 1626; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.68 (1H, s, NH), 8.39 (1H, s, H4), 8.22 (1H, d, H8), 7.94-7.85 (3H, m, H5,6,7), 7.34-7.26 (5H, m, phenyl-H), 5.30 (2H, s, CH2-C6H5), 4.36 (2H, t, phthalazinone-CH2CH2), 3.04 (2H, t, phthalazinone-CH2CH2). Analysis for C19H17N5OS (363.43); Calcd: C; 62.79, H; 4.41, N; 19.27. Found: C; 62.52, H; 4.71, N; 19.23%.
7i
IR (KBr) νmax (cm− 1) 1653; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.53 (1H, s, NH), 8.44 (1H, s, H4), 8.25 (1H, d, H8), 7.95-7.85 (3H, m, H5,6,7), 7.31-7.20 (5H, m, phenyl-H), 4.36 (2H, t, phthalazinone-CH2CH2), 4.14 (2H, t, N-CH2CH2C6H5), 3.02 (2H, t, N-CH2CH2C6H5), 2.87 (2H, t, phthalazinone-CH2CH2). Analysis for C20H19N5OS (377.46); Calcd: C; 63.64, H; 5.07, N; 18.55. Found: C; 63.28, H; 5.07, N; 18.48%.
7j
IR (KBr) νmax (cm− 1) 1634; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.70 (1H, s, NH), 8.39 (1H, s, H4), 8.19 (1H, d, H8), 7.94-7.84 (3H, m, H5,6,7), 7.57 (2H, d, phenyl-H3,5), 7.50 (2H, d, phenyl-H2,6), 4.26 (2H, t, phthalazinone-CH2CH2), 2.98 (2H, t, phthalazinone-CH2CH2). Analysis for C18H14ClN5OS (383.85); Calcd: C; 56.32, H; 3.68, N; 18.24. Found: C; 56.24, H; 3.69, N; 18.12%.
7k
IR (KBr) νmax (cm− 1) 1633; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.60 (1H, s, NH), 8.40 (1H, s, H4), 8.19 (1H, d, H8), 7.94-7.86 (3H, m, H5,6,7), 7.33 (2H, d, phenyl-H3,5), 7.01 (2H, d, phenyl-H2,6), 4.26 (2H, t, phthalazinone-CH2CH2), 3.35 (3H, s, OCH3), 2.94 (2H, t, phthalazinone-CH2CH2). Analysis for C19H17N5OS (363.43); Calcd: C; 62.79, H; 4.71, N; 19.27. Found: C; 62.37, H; 4.79, N; 19.18%.
7l
IR (KBr) νmax (cm− 1) 1634; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 13.62 (1H, s, NH), 8.39 (1H, s, H4), 8.19 (1H, d, H8), 7.94-7.84 (3H, m, H5,6,7), 7.27 (4H, s, phenyl-H), 4.26 (2H, t, phthalazinone-CH2CH2), 2.95 (2H, t, phthalazinone-CH2CH2), 2.34 (3H, s, CH3). Analysis for C19H17N5O2S (379.73); Calcd: C; 60.14, H; 4.52, N; 18.46. Found: C; 60.01, H; 4.55, N; 18.46%.
General procedure for 2-[(phthalazinone-2-yl(methyl]-5-arylamino-1,3,4-thiadiazoles (8a–f)
The appropriate [1(2H)-phthalazinone-2-yl]acetylthiosemicarbazide derivative (0.001 mol) was added portion-wise to H2SO4 (5 mL) cooled in an ice bath with constant stirring. After dissolution, the reaction mixture was further stirred for 30 min, poured over crushed ice and neutralized by saturated Na2CO3 solution at room temperature. Then the solid material was filtered, washed with water and crystallized from an appropriate solvent.
8a
IR (KBr) νmax (cm− 1) 1645; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.32 (1H, s, NH), 8.51 (1H, s, H4), 8.30 (1H, d, H8), 7.98-7.88 (3H, m, H5,6,7), 7.55 (2H, d, phenyl-H2,6), 7.31 (2H, t, phenyl-H3,5), 6.97 (1H, t, phenyl-H4), 5.55 (2H, s, CH2). Analysis for C17H13N5OS (335.38); Calcd: C; 60.88, H; 3.91, N; 20.88. Found: C; 60.85, H; 3.64, N; 21.00%.
8b
IR (KBr) νmax (cm− 1) 1648; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 8.46 (1H, s, H4), 8.25 (1H, d, H8), 8.21 (1H, t, NH),7.94-7.85 (3H, m, H5,6,7), 7.30-7.21 (5H, m, phenyl-H), 5.43 (2H, s, CH2), 4.41 (2H, d, NH-CH2C6H5). Analysis for C18H15N5OS (349.41); Calcd: C; 61.87, H; 4.33, N; 20.04. Found: C; 62.08, H; 4.36, N; 20.10%.
8c
IR (KBr) νmax (cm− 1) 1668; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 8.51 (1H, s, H4), 8.29 (1H, d, H8), 7.98-7.90 (3H, m, H5,6,7), 7.82 (1H, t, NH), 7.29-7.17 (5H, m, phenyl-H), 5.46 (2H, s, CH2), 3.46 (2H, q, NH-CH2CH2C6H5), 2.84 (2H, t, NH-CH2CH2C6H5). Analysis for C19H17N5OS (363.43); Calcd: C; 62.79, H; 4.71, N; 19.27. Found: C; 62.20, H; 4.68, N; 19.05%.
8d
IR (KBr) νmax (cm− 1) 1643; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.46 (1H, s, NH), 8.51 (1H, s, H4), 8.29 (1H, d, H8), 7.98-7.88 (3H, m, H5,6,7), 7.60 (2H, d, phenyl-H2,6), 7.36 (2H, t, phenyl-H3,5), 5.56 (2H, s, CH2). Analysis for C17H12ClN5OS (369.82); Calcd: C; 55.21, H; 3.27, N; 18.94. Found: C; 54.82, H; 3.20, N; 18.69%.
8e
IR (KBr) νmax (cm− 1) 1645; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.10 (1H, s, NH), 8.51 (1H, s, H4), 8.28 (1H, d, H8), 7.97-7.88 (3H, m, H5,6,7), 7.45 (2H, d, phenyl-H2,6), 6.89 (2H, d, phenyl-H3,5), 5.52 (2H, s, CH2), 3.70 (3H, s, OCH3). Analysis for C18H15N5OS (349.41); Calcd: C; 61.87, H; 4.33, N; 20.04. Found: C; 61.39, H; 4.32, N; 19.75%.
8f
IR (KBr) νmax (cm− 1) 1636; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.20 (1H, s, NH), 8.51 (1H, s, H4), 8.28 (1H, d, H8), 7.98-7.89 (3H, m, H5,6,7), 7.42 (2H, d, phenyl-H2,6), 7.11 (2H, d, phenyl-H3,5), 5.54 (2H, s, CH2), 2.22 (3H, s, CH3). Analysis for C18H15N5O2S (365.41); Calcd: C; 59.16, H; 4.14, N; 19.17. Found: C; 59.12, H; 4.10, N; 18.99%.
General procedure for 2-[(phthalazinone-2-yl(ethyl]-5-arylamino-1,3,4-thiadiazoles (8g–l)
[1(2H)-phthalazinone-2-yl]propanoylthiosemicarbazides (0.001 mol) (6) were dissolved in 10 mL toluene and p-toluenesulfonic acid (0.0015 mol) was added and refluxed until the starting material had been consumed. At the end of this period, the reaction mixture was neutralized with 10% ammonium hydroxide. Then the solid material was filtered, washed with water and crystallized from an appropriate solvent.
8g
IR (KBr) νmax (cm− 1) 1651; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.22 (1H, s, NH), 8.46 (1H, s, H4), 8.26 (1H, d, H8), 7.96-7.87 (3H, m, H5,6,7), 7.54 (2H, d, phenyl-H2,6), 7.30 (2H, t, phenyl-H3,5), 6.96 (1H, t, phenyl-H4), 4.49 (2H, t, phthalazinone-CH2CH2), 3.44 (2H, t, phthalazinone-CH2CH2). Analysis for C18H15N5OS (349.41); Calcd: C; 61.87, H; 4.33, N; 20.04. Found: C; 62.09, H; 4.24, N; 19.99%.
8h
IR (KBr) νmax (cm− 1) 1645; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 8.44 (1H, s, H4), 8.25 (1H, d, H8), 8.07 (1H, t, NH), 7.96-7.86 (3H, m, H5,6,7), 7.34-7.25 (5H, m, phenyl-H), 4.43-4.40 (4H, m, phthalazinone-CH2CH2, NH-CH2C6H5), 3.32 (2H, t, phthalazinone-CH2CH2). Analysis for C19H17N5OS (363.43); Calcd: C; 62.79, H; 4.71, N; 19.27. Found: C; 63.12, H; 4.75, N; 19.23%.
8i
IR (KBr) νmax (cm− 1) 1647, 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 8.46 (1H, s, H4), 8.26 (1H, d, H8), 7.96-7.86 (3H, m, H5,6,7), 7.67 (1H, t, NH), 7.30-7.18 (5H, m, phenyl-H), 4.43 (2H, t, phthalazinone-CH2CH2), 3.42 (2H, q, NH-CH2CH2C6H5), 3.33 (2H, t, phthalazinone-CH2CH2), 2.83 (2H, t, NH-CH2CH2C6H5). Analysis for C20H19N5OS (377.46); Calcd: C; 63.64, H; 5.07, N; 18.55. Found: C; 63.59, H; 5.18, N; 18.51%.
8j
IR (KBr) νmax (cm− 1) 1662; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.37 (1H, s, NH), 8.46 (1H, s, H4), 8.22 (1H, d, H8), 7.96-7.88 (3H, m, H5,6,7), 7.59 (2H, d, phenyl-H2,6), 7.35 (2H, d, phenyl-H3,5), 4.49 (2H, t, phthalazinone-CH2CH2), 3.44 (2H, t, phthalazinone-CH2CH2). Analysis for C18H14ClN5OS (383.85); Calcd: C; 56.32, H; 3.68, N; 18.24. Found: C; 55.45, H; 3.53, N; 17.92%.
8k
IR (KBr) νmax (cm− 1) 1652; 1H-NMR (400MHz) (DMSO-d6) δ (ppm) 9.99 (1H, s, NH), 8.44 (1H, s, H4), 8.24 (1H, d, H8), 7.94-7.86 (3H, m, H5,6,7), 7.44 (2H, d, phenyl-H2,6), 6.87 (2H, d, phenyl-H3,5), 4.46 (2H, t, phthalazinone-CH2CH2), 3.70 (3H, s, OCH3), 3.39 (2H, t, phthalazinone-CH2CH2). Analysis for C19H17N5OS (363.43); Calcd: C; 62.79, H; 4.71, N; 19.27. Found: C; 61.91, H; 4.65, N; 18.86%.
8l
IR (KBr) νmax (cm− 1) 1646; 1H-NMR (400 MHz) (DMSO-d6) δ (ppm) 10.10 (1H, s, NH), 8.46 (1H, s, H4), 8.26 (1H, d, H8), 7.96-7.85 (3H, m, H5,6,7), 7.42 (2H, d, phenyl-H2,6), 7.10 (2H, d, phenyl-H3,5), 4.84 (2H, t, phthalazinone-CH2CH2), 3.44 (2H, t, phthalazinone-CH2CH2), 2.24 (3H, s, CH3). Analysis for C19H17N5O2S (379.43); Calcd: C; 60.14, H; 4.52, N; 18.46. Found: C; 60.01, H; 4.55, N; 18.46%.
Antimicrobial activity
Minimum inhibitory concentration (MIC) values for the synthesized compounds were determined by using the broth microdilution method [Citation13,Citation14]. Two Gram-positive (S. aureus ATCC 25923 and B. subtilis ATCC 6633) and two Gram-negative (E. coli ATCC 25922, P. aeruginosa ATCC 27853) bacteria were used as quality control strains. For determining anti-yeast activities of the compounds, the following reference strains were tested: Candida albicans ATCC 10231 and Candida parapsilosis ATCC 90018. Ampicillin trihydrate and fluconazole were used as standard antibacterial and antifungal agents, respectively. Flucanozole was dissolved in sterile distilled water, ampicillin trihydrate in phosphate buffer (pH 8) and the stock solution of the synthesized compounds was dissolved in dimethyl sulfoxide (DMSO) and distilled water (50%) at a concentration of 2048 μg/mL. Twofold dilutions of the synthesized compounds were prepared (1024, 512,……….2 μg/mL), and twofold dilutions of the reference compounds were prepared at 64–0.125 μg/mL. All bacteria were cultivated in Mueller–Hinton Agar (Merck). The bacteria inoculums was prepared in Mueller–Hinton Broth (Merck) which had been kept at 36°C overnight and was diluted with broth to give a final concentration of 5 × 105 cfu/mL. All fungi were cultivated in Sabouraud Dextrose Agar (Merck). The fungi inoculums were prepared in Sabouraud liquid medium (Oxoid) which had been kept at 36°C overnight and was diluted with RPMI-1640 medium with L-glutamine buffered with 3-[N-morpholino]-propansulfonic acid (MOPS) at pH 7 to give a final concentration of 2.5 × 103 cfu/mL. The microplates were incubated at 36°C and read visually after 24 h, except for Candida species when it was at 48 h. The incubation chamber was kept humid. At the end of the incubation period, MIC values were recorded as the lowest concentrations of the substances that gave no visible turbidity. The DMSO diluents at a maximum final concentration of 12.5% had no effect on the microorganism's growth.
Results and discussion
Chemistry
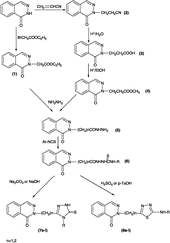
1(2H)-Phthalazinone was used as the starting material. Synthesis of the compounds 1–3 and [1(2H)-phthalazinone-2-yl]acetylhydrazine were accomplished according to previously reported procedures [Citation12,Citation15]. Methyl [1(2H)-phthalazinone-2-yl]propanoate (SciFinder Scholar 2006, 618441-99-9) and 3-[1(2H)-phthalazinone-2-yl]propanoylhydrazine (618442-00-5) were synthesized according to the available procedures. Synthesis of the novel compounds 6–8 is reported in this study. Structures of the title compounds were confirmed by IR, 1H-NMR and elemental analyses. [1(2H)-Phthalazinone-2-yl]acetyl/propanoylthiosemicarbazide derivatives (6a–l) were prepared by the reaction of [1(2H)-phthalazinone-2-yl]acetyl/propanoylhydrazine with the appropriate isothiocyanate derivative in methanol or ethanol. [1(2H)-Phthalazinone-2-yl]acetyl/propanoylthiosemicarbazide derivatives (6a–l) were cyclized to the corresponding 3-[(1(2H)-phthalazinone-2-yl(methyl/ethyl]-4-aryl-1,2,4-triazole-5-thione derivatives (7a–l) under alkaline conditions. The other title compounds, 2-[(phthalazinone-2-yl(methyl/ethyl]-5-arylamino-1,3,4-thiadiazole derivatives (8a–l) were obtained by the cyclization of [1(2H)-phthalazinone-2-yl]-acetyl/propanoylthiosemicarbazide derivatives (7a–l) under acidic conditions.
In this study, we selected aromatic isothiocyanate derivatives to synthesize the acyl thiosemicarbazide derivatives, since the acyl thiosemicarbazides carrying an aromatic substituents gave good yield when cyclized to 1,2,4-triazole and 1,3,4-thiadiazole derivatives. In addition, we preferred to synthesize 1,2,4-triazole and 1,3,4-thiadiazole derivatives which were attached at position-2 of the phthalazinone ring through methylene or ethylene bridges, since many compounds that carry 1,2,4-triazole and 1,3,4-thiadiazole rings have been reported to be attached to other rings through methylene or ethylene bridges in the literature [Citation5,Citation16].
Physicochemical data for the [1(2H)-Phthalazinone-2-yl]acetyl/propanoylthiosemicarbazide derivatives (6a–l) is given in whereas that for (7a–l) and (8a–l) is given in . The synthetic route for the title compounds is shown in Scheme .
Table I. Physicochemical data of [1(2H)-Phthalazinone-2-yl]acetyl/propanoylthiosemicarbazide derivatives (6a–l).
Table II. Physicochemical data for the title compounds (7a–l, 8a–l).
Antimicrobial activity
The synthesized compounds were tested against two Gram (+) bacteria (S. aureus, B. subtilis), two Gram ( − ) bacteria (P. aeruginosa, E.coli) and two yeast-like fungi C. albicans and C. parapsilosis using the broth microdilution method. Ampicillin and fluconazole were used as standard antibacterial and standard antifungal agents, respectively.
As shown in , none of the title compounds had activity against S. aureus, P. aeruginosa and E.coli but, generally, the title compounds were found to be active against B. subtilis and the fungi. Derivatives carrying 1,3,4-thiadiazole ring, generally, showed higher antimicrobial activity against B. subtilis and the fungi compared to the other compounds. The antibacterial activity of 8a was 50% of that of ampicillin against B. subtilis. This derivative was found to be more active against B. subtilis when compared to the other synthesized compounds. The antibacterial activity of compounds 7b, 7e, 7i, 8b, 8g, 8i, 8k and 8l was 25% of that of ampicillin against B. subtilis. Therefore it can be suggested that these compounds show promise as antibacterials and worth further work on their derivatives. The antifungal activity of compounds 7c, 8c–8e and 8i was 25% of that of fluconazole against C. albicans. The MIC values of 7k against C. albicans and C. parapsilosis were determined as 64 μg/mL and 32 μg/mL, respectively. Compound 7i was active against C. parapsilosis and its MIC value was 32 μg/mL. Compounds 8g and 8l exhibited antifungal activity with a MIC value of 16 μg/mL against C. parapsilosis and these values for 8c–8f against C. parapsilosis were 32 μg/mL. Therefore, compounds 7c, 7i, 7k, 8c–8e, 8g, 8i and 8l could be good starting points for developing better antifungal agents against yeast-like fungi. Chain elongation between the two heterocyclic rings may be a good choice for further studies. Additionally, further substitution on the phthalazinone moiety should be studied.
Table III. Minimum inhibitory concentrations (MICs, μg/mL) for the title compounds.
Acknowledgements
This study was supported by a grant from the Research Foundation of Gazi University (EF 02/2006-01).
References
- Gold HS, Moellering RC, Engl N. J Med Chem 1996; 335: 1445–1453
- Bossche VH, Marichal P, Odds FC. Trends Microbiol 1994; 10: 393–400
- Cohen ML. Science 1992; 257: 1050–1055
- Terzioğlu N, Karalı N, Gürsoy A, Kiraz M, Erturan Z. Acta Pharmaceutica Turcica 1998; 2: 77–82, XXXX
- Varvaresou A, Kakolidou AT, Papastaikoudi TS, Tiligada E. Arzneim-Forsch/Drug Res 2000; 50(I)48–54
- Gülerman NN, Doğan HN, Rollas S, Johansson C, Çelik C. Farmaco 2001; 56: 953–958
- Servi S, Genç M, Gür S, Koca M. Eur J Med Chem 2005; 40: 687–693
- Dogan HN, Duran A, Rollas S, Sener G, Uysal MK, Gulen D. Bioorg Med Chem 2002; 10: 2893–2898
- Kassim EM, Abd-El-Nour KN, El-Diwany AI, Abou-Aiad TH. Ind J Chem 1996; 35B: 692–696
- Kassem EM, Kamel MM, El-Zahar M. Pharmazie 1990; 45: 215–216
- Demirayak S, Karaburun AC, Beis R. Eur J Med Chem 2004; 39: 1089–1095
- Doğruer DS, Küpeli E, Yeşilada E, Şahin MF. Arch Pharm Pharm Med Chem 2004; 337: 303–310
- National Committee for Clinical Laboratory Standards. Method for broth dilution antifungal susceptibility testing yeast; approved standard, M27-A, 15, 10, NCCLS, VA Medical Center, Tuscon, 1996.
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, third ed. Approved standard. NCCLS document M100-S12, NCCLS, Wayne, PA, USA, 2002.
- Hassanien AZ Abd El-Baset. Phosphorus, Sulfur and Silicon and the Related Elements 2003; 178: 1987–1997
- El-Masry AH, Fahmy HH, Abdelwahed SHA. Molecules 2000; 5: 1429–1438