Abstract
Biocatalysis, the conversion of substrates into valuable products by the use of enzymes, has some striking advantages in comparison to standard organic chemistry for drug synthesis. By biocatalysis, substrates that contain several identical reactive groups at different positions can be converted with high regio-selectivity and enantio-selectivity. In this study, an E. coli isolate (E132) was identified which was able to convert the steroid desoxycorticosterone into the product 4-pregnen-20,21-diol-3-one in real terms. The product was purified from the cell culture supernatant by HPLC and its structure was demonstrated by mass spectrometry and NMR spectroscopy. It was tested on inhibition of human 5α-reductases type I and type II. At a concentration of 10 μM, inhibition was 49.0% for type I and 81.8% for type II, whereas there was no inhibition of human aromatase (CYP19) at 20 μM and human 17α-hydroxylase-C17,20-lyase (CYP17) at 2.5 μM detectable. The IC50 value of 4-pregnen-20,21-diol-3-one for human 5α-reductase type II was determined to be 1.56 μM.
Introduction
The use of enzymes for oxidation or reduction of substrates in organic synthesis is an attractive strategy, especially in cases the substrate to be converted contains several identical reactive groups at different positions [Citation1]. Enzymes are so-called biocatalysts and have the advantage of converting a substrate with strong regio-selectivity and enantio-selectivity in most cases. They are applied usually in single step reactions and they can be used for conversion steps under mild conditions concerning temperature, pressure and solvent needs. Their use significantly reduces substrate input as well as waste production in synthesis reactions, because of a standard efficiency beyond 90% as are normally obtained and is therefore ecologically preferable. The major advantage of biocatalysis, however, relates to a significant enlargement of the accessible spectrum of products. Enzyme reactions usually result in the formation of enatiomerically pure products many of which are not accessible by classical chemical synthesis [Citation2]. More recently we reported on the development of an Escherichia coli whole cell biocatalyst for the efficient synthesis of various steroids [Citation3] (). In whole cell biocatalysts the converting enzymatic activity is connected to an intact, non-disrupted cellular system, which has some advantages in comparison to purified or crude enzyme preparations. First, the cell, as a natural kind of immobilization device, provides a stabilizing and protecting environment for the damageable enzyme molecule. Second, cells containing the enzyme can be removed from a synthesis reaction by a simple centrifugation step and - if required - transferred to another synthesis reaction, which is usually not possible for pure enzymes. And third, for the preparation of whole cell biocatalysts, not such high sophisticated and multi-step protocols as are essential for the preparation of pure enzymes are needed [Citation4]. The whole cell biocatalysts for steroid biosynthesis was obtained by the surface display of bovine adrenodoxin [Citation5] using the Autodisplay system in E. coli [Citation6] and the subsequent addition of different P450 enzymes and adrenodoxin reductase (AdR) (). By the application of CYP11A1, efficient conversion of cholesterol to pregnenolone was achieved while by the application of CYP11B1, 11-deoxycortiocosterone was efficiently converted to corticosterone using the whole cell biocatalyst [Citation3].
Figure 1 Whole cell biocatalysts for steroid biotransformation as obtained by Autodisplay of bovine adrenodoxin (according to references [Citation3] and [Citation6]). In case that the P450 constituent consist of the enyzme CYP11B1, the whole cell biocatalysts efficiently converts the substrate 11-deoxycorticosterone (DOC) to the product corticosterone (B).
![Figure 1 Whole cell biocatalysts for steroid biotransformation as obtained by Autodisplay of bovine adrenodoxin (according to references [Citation3] and [Citation6]). In case that the P450 constituent consist of the enyzme CYP11B1, the whole cell biocatalysts efficiently converts the substrate 11-deoxycorticosterone (DOC) to the product corticosterone (B).](/cms/asset/5c007a27-16ec-42d5-a670-8b2dc0a59461/ienz_a_242415_f0001_b.gif)
Human 5α-reductase catalyses the last step in androgen biosynthesis, namely the conversion of testosterone to the more potent androgen dihydrotestosterone. Because there are a number of diseases where the 5α-reductase enzyme is of therapeutic interest, it appears to be an important drug target. Inhibitors of the enzyme can provide a new pharmacological treatment for severe diseases such as benign prostate hyperplasia (BPH) and prostatic cancer or for skin disorders, such as acne, male pattern baldness and hirsutism. Two distinct isoforms of the enzyme are known, which are denoted type I and type II [Citation7]. They are encoded by separate genes, show different biochemical characteristics, such as pH optimum, and different tissue distribution patterns, as well as they respond unequal to pharmaceutical agents [Citation8, Citation9]. Since the main isoform present in human prostate is 5α-reductase type II, selective inhibitors might be promising drug candidates for the treatment of BPH [Citation10]. 5α-Reductase type I is mainly expressed in the scalp skin and epithelial cells and could therefore be a target for selective inhibitors in the treatment of baldness, acne and hirsutism, but it should not be concealed that both isoforms can be found in lower levels in other tissues such as liver and embryonic kidney [Citation11]. Reports are available on selective and dual inhibitors of both isoenzymes [Citation12, Citation13], either with a steroidal [Citation14] or a non-steroidal scaffold [Citation15], as well as promising prodrug concepts [Citation16] and pharmacophore models have been developed [Citation17]. More recently an investigation on the synthesis of some epimeric 20-hydoxy, 20-oxime, and 20,21-aziridine derivatives of progesterone and their evaluation as inhibitors of 17α-hydroxylase-C17,20-lyase (CYP17) and 5α-reductase was reported [Citation18]. It turned out, that the 20-oximes were potent dual inhibitors of 5α-reductase and CYP17.
In the presented study, we report on the identification of an E. coli whole cell biocatalyst for the efficient synthesis of 4-pregnen-20,21-diol-3-one by conversion of 11-deoxycortico-sterone (21-hydroxypregn-4-ene-3,20-dione). Based on the structural similarities of the new compound 4-pregnen-20,21-diol-3-one with the reported enzyme inhibitors based on the progesterone scaffold [Citation18], it was tested on inhibition of both 5α-reductase isoforms as well as on inhibition of CYP17 and CYP19. It turned out that 4-pregnen-20,21-diol-3-one is a new, selective inhibitor of human 5α-reductase type II with an IC50 value of 1.56 μM.
Material and methods
HPTLC of steroids
DOC conversion assays contained 30 μM 11-deoxycorticosterone and 0.6 μCi 3H-labelled 11-deoxycorticosterone. Concentrated E. coli cells were incubated for 30 min. Steroids were extracted twice from the cell culture with 50 μL chloroform and the organic phase was dried by evaporation in a fume hood. The residues were dissolved in 10 μL methanol and spotted onto glass-baked silica-coated high performance thin layer chromatography (HPTLC) plates (Merck, Darmstadt, Germany). The HPTLC plates were developed twice in methylene chloride/methanol/water (300:20:1). The reaction products were analyzed after 2 days exposure on a bioimaging analyzer (BAS-2500, Fuji Photo Film Co., Ltd). CYP11B1-dependent activity assays were performed as controls and contained 0.6 μM adrenodoxin reductase, 1.6 μM adrenodoxin, 3 μM CYP11B1 and 100 μM NADPH.
Steroid preparation
E. coli E132 cells which were grown overnight in 100 ml NB medium were concentrated to 20 mL. The concentrated culture was supplemented with DOC to a concentration of 5 mM and incubated for 24 h. Steroids were extracted trebly with chloroform and dried in a rotary evaporator. Purification of the biotransformation product was performed on a reversed phase HPLC system (Jasco 887 PU) on a C18 column (Nucleosil 120-5) with an isocratic mobile phase consisting of isopropanol and acetonitrile (30:1) by the use of a RI detector (Bischoff 8110, Leonberg, Germany). The purity of the product was analyzed using HPTLC with DOC as control steroid.
Spectroscopic analysis
Analysis was performed on a Reflex III TF mass spectrometer (Bruker Daltronics) equipped with the SCOUT 384 probe ion source. The system uses a pulsed nitrogen laser (337 nm, model VSL-337ND, Laser Science) with 500 μJ/pulse energy. 5-Aminochinoline served as matrix.The ions were accelerated under delayed extraction conditions in the negative mode with an acceleration voltage of 20 kV and a reflector voltage of 225 kV. A 69 kV potential difference between the target and the extraction lens was applied with a delay of 6 μs. A Leroy 9384C 1 GHz digital storage oscilloscope (Chestnut Ridge) was used for data acquisition. The data were further processed with the program XMASS 5.15 (Bruker Daltronics).
13C NMR (500 Mhz) spectra of the steroids in CDCl3 were recorded on a Bruker DRX 500 NMR spectrometer.
Inhibition assays
5α-reductase
As a source for human 5α-reductase enzyme type I, the epithelial-like human prostatic carcinoma cell line DU145 was used in 24-well plates as described previously [Citation19]. In brief, cells were grown overnight in RPMI-1640 medium including 10% FCS (fetal calf serum) to become adherent. An appropriate dilution of the inhibitor dissolved in DMSO was prepared as a stock solution in order to achieve a final concentration of 10 μM within the test assay. The cell culture medium was replaced by fresh medium containing 5 μL of the inhibitor solution and 5 nM [3H] androstendione as a substrate in addition. As a control, 5 μL DMSO was added without inhibitor to the fresh medium and treated identically. After incubation for 6 h with 5% CO2 in a humidified atmosphere at 37°C, the cell culture medium was completely evacuated and subsequently extracted with 800 μL diethyl ether. Steroids were obtained by evaporation of the organic phase in a fume hood and the sediment was suspended in 50 μL EtOH for HPLC analysis.
For human 5α-reductase type II and for rat 5α-reductase type I testing, enzymes were prepared as described by Hartmann and co-workers [Citation20]. Briefly, for human type II enzyme, prostatic tissue obtained by dissection from BPH patients was freed from fat and connective tissue and cut into small pieces. 3 Ml of 20 mM phosphate buffer (pH 6.5) containing 0.32 mM sucrose and 1 mM DTT was added per gram of tissue. Homogenization was performed with an Ultrathurrax (IKA Labortechnik, Staufen, Germany) with 10 strokes at 20 500 rpm in 60 s intervals on ice. After filtration the liquid suspension was centrifuged at 105 000 × g at 4°C for 60 min in a Beckman ultracentrifuge. The supernatant was discarded and the pellet was resuspended in phosphate buffer and the centrifugation step was repeated. Finally the pellet was suspended in a minimum volume of phosphate buffer and stored in 300 μL aliquots at − 70°C and thawed prior to use for testing. For rat type I enzyme, rat prostates obtained within 5 min from sacrificed male rats were treated similarly. All preparation steps were performed on ice. For inhibitor tests with the human type II enzyme preparation, 40 mM citrate buffer was used at pH 5.5. The test mixture had a final volume of 250 mL and contained 100 μM NADPH, 0.2 μM testosterone, including 45 nCi [1,2-3H]-testosterone and 2% DMSO with or without inhibitor in a final concentration of 10 μM. The reaction was started by addition of the enzyme preparation and stopped by adding 50 μL of 5 mM HgCl2. Subsequently, steroids were extracted with 500 μL diethyl ether and collected by separately evaporating the organic phase. Prior to HPLC analysis the samples were suspended in 50 μL EtOH. The testing with rat type I enzyme preparation was performed accordingly, however it was performed in 40 mM phosphate buffer at pH 6.6. Compounds showing an inhibition of more than 70% at the initial concentration of 10 μM in comparison to the corresponding control were subsequently tested three different concentrations in order to determine the IC50 value.
HPLC analyses were performed using a high pressure delivery pump (Waters M6000A, Milford, USA), a radioactivity detector (LB506C, Berthold, Wildbad, Germany) and an autosampler system (851-AS, Jasco, Tokyo, Japan). Nucleosil 120-3-C8 was applied as stationary phase in prepacked columns (125 × 4 mm, Macherey-Nagel, Düren, Germany). Methanol/water (1:1) was used as mobile phase for the separation of steroidal metabolites. Data acquisition and integration were carried out by the use of the HALABE 1.6.5 software (Berthold, Wildbad, Germany).
Human placental P450 arom (CYP19)
The enzyme was prepared according to the method of Hartmann and Batzl [Citation21] and the inhibition assay was performed as described therein.
Human 17α-hydroxylase-C17,20-lyase (CYP17)
For this purpose, cells of E. coli XL1 blue containing a plasmid for the expression of human CYP17 together with rat NADPH-P450 reductase within the inner membrane were grown overnight and protein expression was induced by the addition of 1 mM IPTG (isopropyl thiogalactoside) as described earlier [Citation22]. From these cells the inner membrane fraction was prepared according to Schultheiss et al. [Citation23] and this preparation was used for inhibitor testing. Progesterone (1.25 mM containing 600 nCi of [3H]progesterone) and 10 μL of an appropriate dilution of the inhibitor dissolved in 96% EtOH were transferred to 1.5 mL reaction tubes and dried by evaporation. 400 μL of sodium phosphate (0.1 M, pH 7,4) containing 12.5 mM glucose were added and the mixture was incubated for 10 min at 37°C. Reaction was started by the addition of 100 μL membrane preparation and the tubes were horizontally shaken for 45 min at 37°C and 200 rpm. After stopping the reaction by heating the tubes to 95°C for 5 min, steroids were extracted for 5 min with an equal volume of ice cold ethyl acetate. Samples were evaporated, dissolved in 50 μL methanol and subjected to HPLC analysis as described [Citation22].
Results
Whole cell biocatalysis for steroid bioconversion
We recently described the development of a whole cell biocatalyst in E. coli for the synthesis of steroids[Citation5]. Using the P450 enzyme CYP11B1 within this system efficient biotransformation of 11-desoxycorticosterone to corticosterone by oxidation at C-11 of the steroid was achieved. In order to improve the whole cell system in terms of obtained amounts of product, we decided to screen the in house strain collection for an optimal E. coli host background for this conversion. As a first step in this screening, we incubated the different strains of E. coli available in the strain collection with the radioactive labeled substrate 11-DOC. This was a control experiments to be able to exclude any host mediated background activity which could not have been distinguished anymore from CYP11B1 after the whole system had been setup for steroid conversion using the Autodisplay system. For this purpose the substrate DOC was incubated in small scale reactions with different strains of E. coli and subsequently analyzed by HPTLC. An excerpt of the performed experiments is shown in . Most of the strains analyzed were not able to convert the substrate DOC to any detectable product. To our surprise, however, within all strains analyzed there was a single strain of E. coli (E132), that was able to convert 11-DOC to a radioactive labeled product in significant amounts (, lane 1). This product was clearly different from corticosterone obtained by the whole cell biocatalyst system as described in , and which was set up as a control in these experiments (, lane 10). In order to verify the enzymatic conversion of DOC to the unknown product by E. coli strain E132, several control reactions were performed and analyzed by HPTLC (). The results of these experiments clearly indicated that the conversion of DOC to the product different from corticosterone was specific for strain E132, and not due to a cross contamination with any of the constituents used in control experiments.
Figure 2 HPTLC screening of steroid converting enzyme activity in strains of the E. coli strain collection and subsequent phosphoimager analysis using 3H-labeled DOC as a substrate. DOC = 11-deoxycorticosterone, B = corticosterone, P = product. lane 1: E. coli strain E132, 2: E133; 3: 134; 4: E135; 5: E144; 6: E151; 7: E153; 8: E152; 9: negative control without cells; 10: positive control containing all the constituents as described in Figure 1, products of the biotransformation of 3H-DOC with CYP11B1.
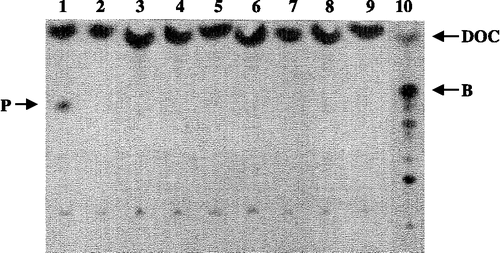
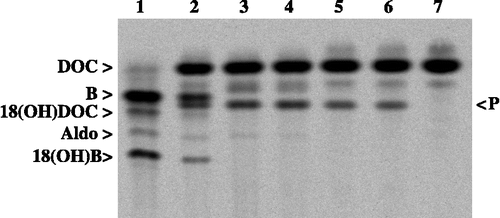
Figure 3 HPTLC and phosphoimager analysis of conversions using 3H-labeled DOC as a substrate in order to verify the biotransformation reaction of E. coli strain E132. The lanes contain the following samples: 1: control reaction of the mitochondrial CYP11B1 system, 2: E. coli E132 combined with the control reaction of 1), 3: components of 2) without adrenodoxin, 4: components of 2) without adrenodoxin reductase, 5: components of 2) without adrenodoxin and adrenodoxin reductase, 6: components of 2) without adrenodoxin and CYP11B1, 7: substrate conversion with E. coli E127 (negative control). The product of the biotransformation is marked on the right with a P and the products of the mitochondrial CYP11B1 system are designated on the left as B (corticosterone), aldo (aldosterone), 18(OH)B and 18(OH)DOC.
Identification of 4-pregnen-20,21-diol-3-one as the product of DOC biotransformation
For the identification of the product produced by E. coli E132 from the substrate DOC, cells were grown overnight in 100 ml NB medium, harvested by centrifugation and resuspended into 20 ml of fresh medium, corresponding to an increase in concentration of 5:1. The cells were supplemented with 10 mg of non-radioactive DOC and incubated for another 16 h at 30°C. After the incubation, steroids were collected by chloroform extraction and subjected to a reversed phase HPLC on a C18 column with an isocratic mobile phase consisting of isopropanol and acetonitrile. The analyte was devoid any detectable amounts of DOC, which was controlled by the determination of the retention time of DOC under similar conditions. Therefore the biotransformation of DOC to the unknown steroidal product was complete under the biotransformation conditions applied, which could be confirmed by HPTLC analysis. The HPLC fractions containing the product of biotransformation were collected, combined and subjected to subsequent MS and NMR analysis.
Mass analysis of the bioconversion product was performed with MALDI MS in negative mode, which results in values reduced by 1 (). The mass of the substrate DOC and the product were determined to be 330 and 332. The difference of 2 pointed to a reduction reaction of a ketogroup. In principle, this reduction step could have taken place either at the ketogroup at C-3 or C-20 of the steroid skeleton. Therefore 13C NMR measurements were performed in order to specify the reduction position. 13C NMR data of the product obtained by biotransformation in CDCl3 are listed in in comparison to signals produced by the steroid containing enone at position C-3 (the substrate DOC) as reported by a previous study [Citation24]. The signals assigned to C-3 of both steroids are nearly identical, whereas the absorption assigned to C-20 of the substrate DOC is typical for a carbonyl functional group and the signal assigned to C-20 of the product clearly points on a hydroxy functional group. Moreover by the 13C NMR data as given in , a possible enone reduction at position C-4/C-5 could be excluded. The MS and NMR data indicated that the substrate DOC was reduced in a biocatalytic reaction by E. coli E132 at position C-20 to produce the product 4-pregnen-20,21-diol-3-one (20-dihydro DOC) (Scheme ).
Figure 4 MALDI spectrometry analysis of the product 4-pregnen-20,21-diol-3-one (P) and the substrate DOC (inset) of the biocatalytic reaction as described. Signals at 143.11 and 284.04 correspond to the matrix 5-aminochinolin, whereas the signal at 315.06 represents an unknown impurity caused during the MS sample preparation.
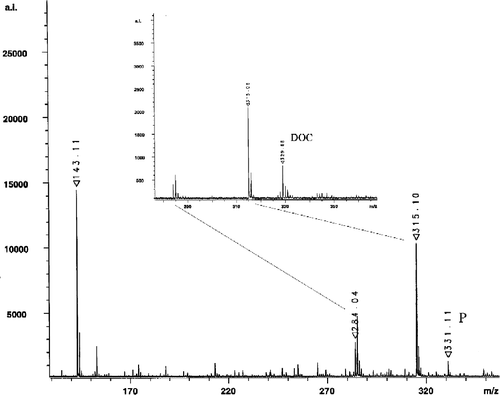
Table I. 13C NMR data in CDCl3 for substrate DOC and the product of biotransformation by E. coli E132, 4-Pregnen-20, 21-diol-3-one.
Scheme 1 (A): converting DOC to 20-dihydro-DOC (4-pregnen-20,21-diol-3-one), a whole cell biotransformation performed by E. coli E132. (B): Progesterone and the 20-oxime derivatives thereof as potent dual inhibitor of 5α-reductase types I and II and 17α-hydroxylase-C17,20-lyase (according to Ling et al. [Citation18]).
![Scheme 1 (A): converting DOC to 20-dihydro-DOC (4-pregnen-20,21-diol-3-one), a whole cell biotransformation performed by E. coli E132. (B): Progesterone and the 20-oxime derivatives thereof as potent dual inhibitor of 5α-reductase types I and II and 17α-hydroxylase-C17,20-lyase (according to Ling et al. [Citation18]).](/cms/asset/7261b176-b96f-4f52-9b09-9c3bbf6d7f75/ienz_a_242415_f0005_b.gif)
Inhibition studies
As the product obtained in the biotransformation described here (4-pregnen-20,21-diol-3-one) has striking structural similarities to the 20-oxime derivatives of progesterone recently described as potent inhibitors of 5α-reductase and CYP17, we decided to test the purified compound obtained by HPLC on enzyme inhibition. As shown in , at an initial concentration of 10 μM, inhibition of human 5α-reductase type I was 49.0%, whereas inhibition for human 5α-reductase type II was 81.8%. The IC50 value determined subsequently for human 5α-reductase type II turned out to be 1.56 μM. No inhibition was found for human CYP17 at an concentration of 2.5 μM and human aromatase at an concentration of 20 μM. Therefore we concluded that 4-pregnen-20,21-diol-3-one is a selective inhibitor of human 5α-reductase type II. Interestingly the compound appeared also to inhibit the rat type I 5α-reductase (82.5% at 10 μM).
Table II. Inhibition of human and rat 5α-reductases by 4-pregnen-20,21-diol-3-one (20-dihydro-DOC).
Discussion
The present study describes the biocatalytic access to 4-pregnen-20,21-diol-3-one by biotransformation of 11-deoxycorticosterone using whole cells of E. coli E132. Amounts in the mg range could easily be prepared from an overnight 20 ml badge culture of the cells with the substrate. The product obtained by chloroform extraction turned out to be pure and free of any contamination with the substrate or any other steroid as turned out by HPLC and HPTLC analysis. This is a clear advantage of using cells of E. coli for steroid biotransformation, which are, at least under these conditions, not able to produce any other type of steroid in addition. One can speculate on the enzyme that could be responsible for the biotransformation of DOC to 4-pregnen-20,21-diol-3-one by E. coli E132. In accordance to similar enzymatic activities described before for eucaryotic cells (e.g. the reduction of the ketogroup is the first step in biotransformation of progesterone and related steroids in the placenta and other tissues [Citation25]) and for some procaryotes (e.g. Mycobacterium bovis [Citation26] or Streptomyces exfoliates [Citation27]), it should be called a steroid C-20 dehydrogenase. This is the first report on such an enzyme activity in E. coli. Whether it is rather a 20α- or a 20β-dehydrogenase needs to be determined in further experiments on the stereoselectivity of the reaction. Another interesting question is that on substrate specificity of the biotransformation conferred by E. coli E132 and whether other steroids containing a ketogroup at position C-20 could be converted to the reduced product in a similar way as found for DOC. In first preliminary experiments on the structural nature of the enzyme, we added metyrapon and 5-aminolevulinic acid to the biotransformation reaction of DOC by E. coli E132 in similar experiments as described in . Metyrapon is a known inhibitor of P450 mediated steroid conversion and 5-aminolevulinic acid serves as a building block for porphyrin biosynthesis. None of these assay alterations had any effect of the amount of 4-pregnen-20,21-diol-3-one that was obtained by biotransformation of DOC performed by E. coli E132 (data not shown). This indicates that the underlying enzymatic activity is rather not due to a P450 enzyme. Differential cell fractionation as described earlier [Citation23] revealed, that the enzyme responsible for DOC conversion in E. coli E132 is not located within the outer or inner membrane fraction but appears to be located within the soluble cell fraction. Further experiments need to be performed in order to elucidate the molecular structure of the enzyme responsible for the observed steroid conversion in E. coli E132. However at this point it needs to be emphasized, that from the synthesis point of view, the substrate conversion using whole cells of E. coli appears to be much more attractive, than using a purified enzyme preparation. The use of whole cells for the conversion of DOC into 4-pregnen-20,21-diol-3-one proved to be simple, efficient and robust, without needing any sophisticated, laborious or time consuming preparation or purification steps.
The compound obtained here by biotransformation of DOC, 4-pregnen-20,21-diol-3-one, turned out to be a selective inhibitor of human 5α-reductase type II. No indication was found by different HPLC approaches, that 4-pregnen-20,21-diol-3-one could serve as a substrate of human 5α-reductase. It inhibited also the type I rat enzyme to a similar degree, but showed no inhibition of human 17α-hydroxylase-C17,20-lyase at all. This appears to be interesting, because the 20-oxime derivatives of progesterone described before as inhibitors of 5α-reductase (Scheme ) inhibited 17α-hydroxylase-C17,20-lyase too at a nanomolar range [Citation18].
Although the isoform of the 5α-reductase was not specified within those studies, the source of the enzyme, which was prostate tissue points on type II enzyme. The IC50 values determined for 5α-reductase were 0.06 μM (17a) and 0.12 μM (17b), and the IC50 values for CYP17 were 0.04 μM (17a) and 0.03 μM. As 4-pregnen-20,21-diol-3-one showed no inhibition of CYP17 in our studies, this could be an indication that specificity of inhibition lies within the functional groups substituting the C-20 and C-21 position in the steroidal inhibitors with an identical scaffold available here. However, this hypothesis needs confirmation by future experiments on structure activity relationships. Whereas biotransformation of steroids by a recombinant yeast strains expressing cytochrome P-450 enzymes or other naturally available steroid converting enzymes is a common tool for the production of specified steroids, this is the first report on the production 4-pregnen-20,21-diol-3-one by biotransformation providing a simple and efficient access. A chemical route of synthesis for this compound with interesting features has not yet been described.
Acknowledgements
The authors thank A. Palusczak for excellent technical assistance and K. Hollemeyer and J. Zapp for MS and NMR support, respectively.
References
- Mertens R, Liese A. Curr Opin Biotechnol 2004; 15: 343–348
- Liese A, Filho MV. Curr Opin Biotechnol 1999; 10: 595–603
- Jose J, Bernhardt R, Hannemann F. J Biotechnol 2002; 95: 257–268
- Jose J, von Schwichow S. ChemBioChem 2004; 5: 100–108
- Jose J, Hannemann F, Bernhardt R. ChemBioChem 2001; 2: 695–701
- Jose J. Appl Microbiol Biotechnol 2006; 69: 607–614
- Russell DW, Wilson JD. Annu Rev Biochem 1994; 63: 25–61
- Andersson S, Berman DM, Jenkins EP, Russell DW. Nature 1991; 354: 159–161
- Andersson S, Russell DW. Proc Natl Acad Sci USA 1990; 87: 3640–3644
- Reichert W, Michel A, Hartmann RW, Jose J. J Steroid Biochem Mol Biol 2001; 78: 275–284
- Panter BU, Jose J, Hartmann RW. Mol Cell Biochem 2005; 270: 201–208
- Salem OI, Frotscher M, Scherer C, Neugebauer A, Biemel K, Streiber M, Maas R, Hartmann RW. J Med Chem 2006; 49: 748–759
- Reichert W, Hartmann RW, Jose J. J Enz Inhib 2001; 16: 47–53
- Hartmann RW, Hector M, Haidar S, Ehmer PB, Reichert W, Jose J. J Med Chem 2000; 43: 4266–4277
- Picard F, Barassin S, Mokhtarian A, Hartmann RW. J Med Chem 2002; 45: 3406–3417
- Streiber M, Picard F, Scherer C, Seidel SB, Hartmann RW. J Pharm Sci 2005; 94: 473–480
- Faragalla J, Bremner J, Brown D, Griffith R, Heaton A. J Mol Graphics Modell 2003; 22: 83–92
- Ling YZ, Li JS, Kato K, Liu Y, Wang X, Klus GT, Marat K, Nnane IP, Brodie AM. Bioorg Med Chem 1998; 6: 1683–1693
- Reichert W, Jose J, Hartmann RW. Arch Pharm (Weinheim) 2000; 333: 201–204
- Kattner L, Gohring S, Hartmann RW. Arch Pharm (Weinheim) 1995; 328: 239–245
- Hartmann RW, Batzl C. J Med Chem 1986; 29: 1362–1369
- Ehmer PB, Jose J, Hartmann RW. J Steroid Biochem Mol Biol 2000; 75: 57–63
- Schultheiss E, Paar C, Schwab H, Jose J. J Mol Catal B: Enzym 2002; 18: 89–97
- Frimer AA, Aljadeff G, Rubinstein S, Breitbart H. Arch Pharm (Weinheim) 1997; 330: 117–121
- Couture JF, Cantin L, Legrand P, Luu-The V, Labrie F, Breton R. Acta Crystallogr D Biol Crystallogr 2002; 58: 135–139
- Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, Simon S, Harris B, Atkin R, Doggett J, Mayes R, Keating L, Wheeler PR, Parkhill J, Barrell BG, Cole ST, Gordon SV, Hewinson RG. Proc Natl Acad Sci USA 2003; 100, 7877–7782
- Marekov L, Krook M, Jornvall H. FEBS Lett 1990; 266: 51–54