Abstract
Fatty aldehyde dehydrogenase (FALDH) is an NAD+-dependent oxidoreductase involved in the metabolism of fatty alcohols. Enzyme activity has been implicated in the pathology of diabetes and cancer. Mutations in the human gene inactivate the enzyme and cause accumulation of fatty alcohols in Sjögren-Larsson syndrome, a neurological disorder resulting in physical and mental handicaps. Microsomal FALDH was expressed in E. coli and purified. Using an in vitro activity assay an optimum pH of ∼9.5 and temperature of ∼35°C were determined. Medium- and long-chain fatty aldehydes were converted to the corresponding acids and kinetic parameters determined. The enzyme showed high activity with heptanal, tetradecanal, hexadecanal and octadecanal with lower activities for the other tested substrates. The enzyme was also able to convert some fatty alcohol substrates to their corresponding aldehydes and acids, at 25–30% the rate of aldehyde oxidation. A structural model of FALDH has been constructed, and catalytically important residues have been proposed to be involved in alcohol and aldehyde oxidation: Gln-120, Glu-207, Cys-241, Phe-333, Tyr-410 and His-411. These results place FALDH in a central role in the fatty alcohol/acid interconversion cycle, and provide a direct link between enzyme inactivation and disease pathology caused by accumulation of alcohols.
Introduction
Sjögren-Larsson syndrome (SLS) is an inherited error in fatty alcohol metabolism [Citation1]. It is caused by a defect in the aldehyde dehydrogenase 3A2 gene, which codes for the NAD+-dependent oxidoreductase, FALDH (also known as ALDH10 or ALDH3A2; Swissprot P51648 and P51648-2). A large number of different mutations causing SLS are known, including those causing premature truncation or complex rearrangements [Citation2,Citation3], and inactivating point mutations Citation4-7. In the majority of cases, the effects on the function of the enzyme are unknown. The K266N mutation is known to lead to instability of the mRNA resulting in low levels of translation of a moderately active protein [Citation2,Citation4]. Patients with SLS experience a number of symptoms arising from accumulation of long-chain fatty alcohols in the central and peripheral nervous systems and other tissues, giving rise to intense itching of the skin, and mental and physical handicap. Although the incidence of SLS in most parts of the world is low (around 6 per million in the whole of Sweden), it is increased in small, immobile populations such as those found in Northern Sweden (around 164 per million) [Citation6]. There is no treatment for SLS since many of the accumulating alcohols are produced endogenously, although alleviation of some symptoms is possible [Citation8,Citation9].
The role of FALDH is not well defined and most of the known functions have been elucidated from studying accumulation of fatty alcohols in SLS patients. Rizzo et al. proposed a fatty alcohol cycle [Citation1,Citation10], which allows inter-conversion between fatty alcohols and fatty acids. Fatty aldehydes are produced as intermediates in the oxidation of fatty alcohols and in the reduction of fatty acyl-CoA esters to the alcohols [Citation1]. Aldehydes can also be directly produced as a result of oxidative damage to ether glycerolipids [Citation11] or protein deprenylation [Citation12]. The aldehydes involved include long-chain fatty aldehydes such as hexadecanal (C16) and octadecanal (C18) [Citation1] and the aldehyde derived from leukotriene B4 [Citation13]. Most recently phytenal, an intermediate in the conversion of phytol to phytanic acid, has been identified as a substrate for FALDH [Citation14,Citation15]. High levels of phytanic acid in the diet are a known risk factor for prostate cancer in men [Citation16], but it is unknown whether the levels of conversion of phytol to phytanic acid by FALDH give rise to a significant risk. Abnormal regulation of FALDH has also been implicated in diabetes [Citation17].
The ALDH3A2 gene is expressed in humans [Citation18] and mice [Citation19] as two different FALDH proteins, which arise from alternative gene splicing. The major isoform (human variant I; Swissprot P51648) uses a short exon 10 comprising four amino acids and a stop codon, and accounts for ∼90% of the expressed protein and is localized in microsomes. The minor form (human variant II; Swissprot P51648-2) is derived from exon 9' which encodes for an extended C-terminus of hydrophobic amino acids, and accounts for ∼10% of the expressed protein with an unknown sub-cellular localization. Despite a large number of studies on mutations within the ALDH3A2 gene, little attention has given to studying the properties of these enzyme isoforms. Characterisation of the native human liver FALDH has been reported [Citation20], but this was done before the existence of the two variants was known. Here, we report the recombinant expression in E. coli, purification and characterisation of the most highly expressed form of FALDH (variant I), and report in vitro oxidation of alcohol substrates to aldehydes. This new activity supports the link between loss of FALDH function and accumulation of alcohols in SLS patients.
Methods
Sources of materials
All chemicals were obtained from the Sigma-Aldrich Chemical Co. or Fisher Ltd, and were of analytical grade or higher. Buffers were made using Milli-Q water and were pH adjusted at ambient temperature. Molecular biology reagents were obtained from New England BioLabs, Stratagene, Promega or Novagen. The ALDH3A2 variant II gene was purchased from the IMAGE consortium. Solutions for molecular biology were sterilised by autoclaving or filtration. Plastic-ware was sterilised by autoclaving or was purchased sterile directly from the manufacturer. Protein columns and chromatography systems were obtained from GE Healthcare.
Sub-cloning and expression of FALDH variant I
The following reagents were mixed in a sterile 0.2 mL PCR tube: forward primer (1 μL, 100 pmol: CACACATATGATGAGCTCGAAGTCCGGC); reverse primer (1 μL, 100 pmol: ACACGGATCCTCAGTAATATTCTGCCTTGACAAGCACAGCGGCTAC); dNTP mix (2 μL, 100 mM); Pfu polymerase buffer (2 μL, 10 × ); Pfu DNA polymerase (0.5 μL); MgCl2 (0.8 μL, 100 mM); DNA template purchased from the IMAGE consortium (2 μL, 100 μg/mL). The mixture was made up to a final volume of 20 μL with sterile Milli-Q water. Reactions were run at the following conditions: step 1 94 °C for 4 min; step 2, 94°C for 30 s; step 3, 58°C for 45 seconds; step 4, 72°C for 1.5 min. Steps 2–4 were repeated for 35 cycles followed by a final extension step at 72°C for 7 min. Agarose gel electrophoresis of samples was performed using a 1% (w/v) gel and run alongside a 1 Kb DNA ladder. Bands were excised from the gel and purified before inserting into the pGEM-T vector system (Promega). DNA was prepared using the Wizard miniprep system (Promega) and clones were analysed by 0.8% agarose gel electrophoresis and sequenced. The required clone and pET28a(+) vector (Novagen) were digested with Nde1 and BamH1, and ligated. Clones were analysed by agarose gel electrophoresis and resequenced. Recombinant His6-FALDH was expressed in E. coli BL21 (DE3) using Lennox LB media supplemented with 50 μg/mL kanamycin sulfate at 37°C, inducing with 0.1 mM IPTG for 4 hours.
Purification of recombinant His6-FALDH
All manipulations were performed at 4°C unless otherwise specified. Fractions were analysed by SDS-PAGE [Citation21] using a 10% running gel and protein concentrations were determined by the method of Bradford [Citation22]. E. coli cells (∼ 2 g) were suspended in 50 mM Tris-HCl, pH 8.0, 5 mM 2-mercaptoethanol, 0.1 mM PMSF, 1 mM benzamidine-HCl and lysed using a ‘One-shot’ cell disruptor (Constant systems) set at 144.8 KPa (21 lbs/in2). N-lauroylsarcosine was added to 1.5% (w/v) and the lysate was stirred for 20 min before centrifugation (Beckman JA14 rotor, 8 000 r.p.m., 6164 × g, 20 min). Polyethyleneimine was added to 0.1% with stirring and after 20 min the sample was centrifuged as before, to produce the crude extract.
A HiTrap Q sepharose column (5 mL) was equilibrated at 2 mL/min. with 50 mM Tris-HCl, pH 8.0. Crude extract (20 mL, ∼134 mg) containing His6-FALDH was loaded at a rate of 2 mL/min. The column was washed at 5 mL/min with the same buffer and protein eluted with 0-1 M NaCl in buffer over 100 mL with 3 mL fractions collected. Fractions #19–23 (15 mL, ∼15 mg) were pooled for further purification using HisTrap columns (1 mL) pre-charged with NiSO4. The column was equilibrated with 20 mM NaH2PO4-NaOH, 0.5 M NaCl, pH 7.4 at 1 mL/min, and the anion-exchange pool loaded. The column was washed with 10 mL of the same buffer, and protein eluted with a 0–0.5 M imidazole gradient over 10 mL at 0.5 mL/min, with fractions (0.5 mL) collected. Fractions #12–15 (2 mL, ∼1.2 mg) were desalted using a PD-10 column (BioRad) into 50 mM Na4P2O7-NaOH, pH 9.5. Fractions from the anion-exchange column containing partly-purified His6-FALDH were analysed by Western blotting using an anti-His antibody in order to confirm the size of the expressed protein.
Synthesis of aldehyde substrates
Tetradecanal, hexadecanal and octadecanal were synthesised [Citation23] from the corresponding alcohols (8.3 mmol) by oxidation with pyridinium chlorochromate (PCC) (12.3 mmol) in dichloromethane (∼30 mL). Tetradecanol was added as a solution in dichloromethane on ice to PCC, with the other alcohols added slowly as solids. After 1 h; the reaction mixture was allowed to warm to ambient temperature and stirring continued for 6–18 h. Aldehyde products were purified by silica gel chromatography using ethyl acetate: hexane (1:9). 1H NMR spectra were consistent with those reported for tetradecanal (49% yield) [Citation24], hexadecanal (78% yield) [Citation25] and octadecanal (78% yield) [Citation26].
Enzyme assays
FALDH assays were based on the production of NADH in Na4P2O7-NaOH, pH 9.5, in the presence of 50 μM decanal unless otherwise stated. Reactions (2.4 mL) consisted of enzyme (5 μg), substrate (with solubilising agent), 1.5 mM NAD+ in buffer at 37°C. Substrates were solubilised in 1.5% N-lauroylsarcosine (C5–C12) or 0.1% Triton X-100 (C14–C18, phytol, all-trans-farnasal). Assays were followed for 10 min and initiated by addition of substrate in isopropanol (50 μL). Each condition was measured at least three times and initial rates converted to nmol/min/mg protein using the reported extinction coefficient (ϵ = 6.023 mM− 1 cm− 1 at 340 nm) [Citation27]. Protein was quantified by Bradford analyses [Citation22]. Negative controls with boiled enzyme, or lacking enzyme or substrate were performed as necessary. Kinetic parameters were derived with Leonora [Citation28] using the direct linear plot [Citation29,Citation30]. Values for kcat were calculated assuming a mass for His6-FALDH of 57142.3 Da (calculated by protparam: http://www.expasy.ch/ tools/protparam.html).
Identification of reaction products
Purified FALDH (5 μg) was mixed with following reagents in a sterile 15 mL test tube: 50 mM Na4P2O7-NaOH buffer pH 9.5; 1.5 mM NAD+; 1.5% (w/v) N-lauroylsarcosine (C12 aldehyde) or 0.1% (w/v) Triton X-100 (for C16 aldehyde, phytol and farnesal); 100 μM Substrate; Milli-Q water to a final volume of 2.4 mL. The reaction with phytol was performed in the dark. The reactions were mixed and incubated at 37°C for 4 h, and organic products extracted 4 times with 2.4 mL of CDCl3: CD3OD and the organic phase was dried in vacuo. 1H NMR spectra were consistent with reported spectra for dodecanoic acid [Citation31], hexadecanoic acid [Citation31] and phytenal [Citation32].
Modelling of FALDH structure
The FALDH homology model was constructed using ALDH3 crystal structure [Citation33]. Following alignment of the primary sequence(s) using ClustalX (66.1% sequence homology), the homology model was then constructed using the What If package [Citation34]. The proposed model was viewed and refined using the YASARA dynamics molecular modelling package Citation35-37. Ramachandran Plots and were analysed by Procheck [Citation38]. The resultant model was compared against the FALDH model in the Swiss-Model Repository (http://swissmodel.expasy.org/repository; accession # P51648).
Results and discussion
Expression and purification of FALDH variant I
FALDH variant II was obtained for use as template DNA in the PCR cloning. Variant II has an extended C-terminal region compared to variant I due to the presence of exon 9'. Amplification of the gene for FALDH variant I was achieved using a reverse primer incorporating the common sequence found at the 3' end of exon 9 of the template with additional codons incorporating the final four amino-acids and a stop codon found in exon 10 of variant I. Following sub-cloning of the variant I gene into the expression vector, a recombinant protein of the predicted size (∼57 KDa) was observed by SDS-PAGE analyses. DNA sequencing confirmed the presence of the expected His-tag sequence; an unexpected Q445R substitution was present in the recombinant protein, located in a C-terminal loop region (Supplementary Materials, ).
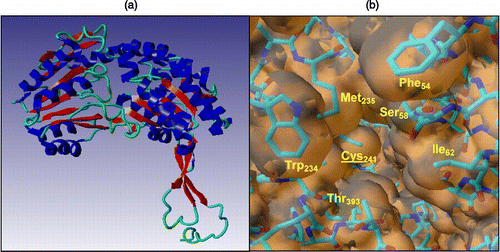
Figure 1 a) Model of the secondary structure of FALDH highlighting the presence of the Rossmann fold; b) The hydrophobic pocket of FALDH generated from the molecular graphics package, YASARA Citation35-37. The substrate has been proposed to insert into the cleft to aid catalysis by association with the active site residue, Cys-241 (underlined, centre).
Crude cell extracts were produced by cell lysis followed by solubilisation with the detergent, N-lauroylsarcosine. Recombinant FALDH was purified to 90–95% purity (as judged by SDS-PAGE analyses) using a combination of anion-exchange and metal-chelate chromatographies (Supplementary Materials, ). Western blot analyses of partly-purified FALDH protein present in anion-exchange fractions confirmed the presence of the His-tag in the purified protein at ∼57 KDa (results not shown).
Table I. 1: FALDH (4 μg) activity with straight-chain substrates (50 μM), in the presence of N-lauroylsarcosine (C5-C12) or Triton X-100 (C14–C18).
Conversion of substrates by recombinant FALDH
FALDH catalytic activity was measured in a continuous spectrophotometric assay based on the increasing absorbance resulting from reduction of NAD+ to NADH. Initially the effects of various cofactors and solubilising agents on FALDH activity with aldehyde substrates were determined (Supplementary Materials, ). In the case of hexadecanal the best solubilising agent was Triton X-100, and this was also observed when tetradecanal and octadecanal were used as substrates. For short-chain substrates (C5–C12) N-lauroylsarcosine proved to be a more effective solubilising agent. Other solubilising agents, e.g. β-cyclodextrin, were less effective for all tested substrates. Further experiments suggested an optimum pH of ∼9.5 and an optimum temperature of ∼35°C (Supplementary Materials), consistent with previous reports on the purified native enzyme [Citation20]. In contrast to previous reports [Citation20], amine-based buffers, such as glycine or Tris, were avoided in our assays. 1H NMR experiments indicated rapid reaction of these buffers with the substrate aldehyde group, presumably due to formation of an imine (Supplementary materials, Figure 4). Control experiments showed that no reaction occurred in the presence of pyrophosphate-NaOH buffer (data not shown), which was used in the majority of our experiments since its pKa value is close to the previously reported optimum pH of FALDH [Citation20].
Table II. 2: Kinetic parameters (Km, Vmax and kcat) for various straight- and branched-chain aldehyde substrates using 0.5 μg FALDH.
A series of straight-chain and branch-chain aldehyde substrates were assayed under the optimised conditions (Table I). As expected, high levels of activity were observed with long-chain fatty aldehydes, tetradecanal, hexadecanal and octadecanal. Surprisingly, the highest activity was observed when heptanal was used as substrate (C7). The activities observed with pentanal (C5) and octanal (C8) were significantly lower. Presumably this phenomenon reflects a balance between solubilisation of the substrate and catalytic efficiency of the enzyme. Finally, incubation of substrates followed by 1H NMR analyses of the products confirmed that the aldehydes (dodecanal) were been oxidised to their corresponding fatty acids.
Kinetic parameters for selected aldehyde substrates were determined (). All of the Km values were in the 4–35 μM range, although, in the cases of the longer-chained substrates, marked deviation from Michaelis-Menten behaviour was observed at higher substrate concentrations, presumably due to improper substrate solubilisation. These Km values are of similar magnitude to those previously observed [Citation20]. However, the observed Vmax values are significantly lower than those previously reported [Citation20]. This could be due to differences in the assay system or it may be a consequence of assaying a single isoform of the enzyme. Moreover, recombinant FALDH can be difficult to solubilise efficiently during purification, and it is likely that a significant proportion of mis-folded protein is present within the soluble fraction.
The observation that farnesal can be converted to farnesoic acid (; entry 6) is interesting, as very little appears to be known about the metabolism of farnesol. In our assays farnesal was converted at about 50% of the efficiency (as judged by kcat/Km) of octadecanal (, entry 5), a known substrate of FALDH and about 10% of the efficiency as the best substrate, decanal (, entry 1). FALDH is known to be able to oxidise branched-chain aldehydes such as phytenal [Citation14,Citation15], which are structurally related to farnesal. Farnesal is known to be produced during protein deprenylation [Citation12], and as an unsaturated aldehyde is likely to be highly reactive.
Patients with Sjögren-Larsson syndrome accumulate long-chain fatty alcohols in various tissues. The ability of recombinant ALDH10 to oxidise fatty alcohol substrates (decanol, C10 and hexadecanol, C16) was therefore tested. Under identical conditions to those used for the corresponding aldehyde substrates, rates of 44 ± 3 and 64 ± 3 nmol/min/mg protein were observed for decanol and hexadecanol, respectively. These rates are ca. 25–30% of the rates observed for the corresponding aldehyde substrates (151 ± 30 and 136 ± 35 nmol/min/mg protein, respectively). Assays using the branch-chain isoprenoid lipid, phytol, showed that a high rate was observed, but control reactions lacking enzyme suggest that this was due to a photo-chemical reaction [Citation32].
Catalytic mechanism for FALDH
No crystal structure of either FALDH variant has been reported in the literature. However, FALDH shows high levels of primary sequence homology to aldehyde dehydrogenase 3 (ALDH3), for which a structure has been determined [Citation33] upon which our model was based. This model suggests that FALDH has a very similar structure to ALDH3, with the catalytic residues in the Rossman fold (). FALDH has a longer C-terminal sequence than ALDH3, which is thought to be a trans-membrane domain. This model allows prediction of the catalytic residues involved in aldehyde oxidation.
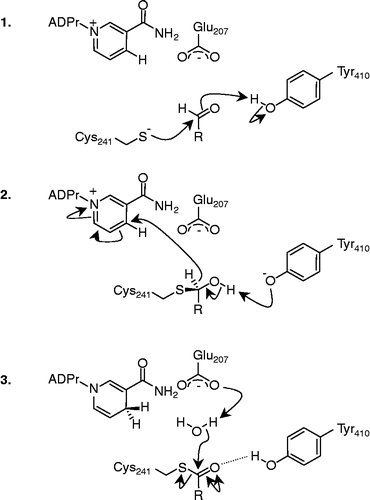
A mechanism for the oxidation of aldehyde substrates by FALDH was previously proposed, based on mechanistic studies of other NAD(P)+-dependent oxidoreductases [Citation39]. Our structural modelling studies support this general mechanism, in which an ionised active site cysteine residue (Cys-241) reacts with the aldehyde substrate to form a thiohemiacetal (; 1). In our model this process is facilitated by transfer of a proton from the side chain of Tyr-410 onto the oxygen of the aldehyde carbonyl group. Tyr-410 is also implicated in the oxidation step (; 2) and is reprotonated during hydride transfer. Our model also suggests that hydrolysis of the thioester product (; 3) is probably mediated by Glu-207. Although none of these catalytic residues involved in aldehyde oxidation have been reported to be mutated in SLS patients, it is possible that the proximal mutations H411Y and G412R exert their effects on aldehyde oxidation by displacing the side-chain of Tyr-410.
Figure 2 The three stages of the proposed mechanism for aldehyde oxidation: 1. attack of the Cys-241 thiolate anion on the aldehyde carbonyl. 2. Rearrangement of the thiohemiacetal intermediate and hydride transfer to NAD+. 3. Hydrolysis of the thioester to regenerate Cys241 and generate the respective acid. R = fatty acid side-chain. ADPr, Adenosine diphosphate ribose;
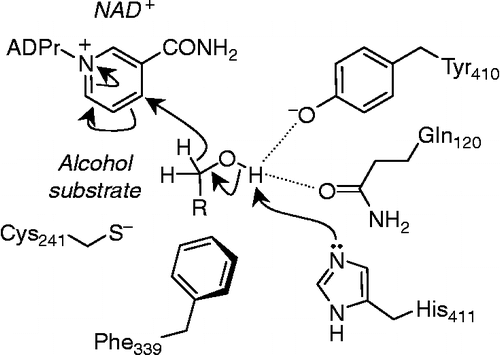
The new alcohol to aldehyde oxidation activity reported in this paper requires that a new mechanism for this reaction is invoked (). The analogous reaction catalysed by lactate dehydrogenase requires an active site histidine [Citation40], believed to be His-411 in FALDH. The proton of the alcohol substrate is also hydrogen bonded to the side-chains of Tyr-410 and Gln-120. Abstraction of the alcohol OH proton by His-411 (which is unprotonated under the alkaline assay conditions favoured by FALDH) drives transfer of a hydride ion to the NAD+ cosubstrate. The side-chain of Cys-241 is not involved in this reaction but is poised to capture the aldehyde product to form a thiohemiacetal (Figure 2; 1). Thus, toxic and reactive aldehydes are prevented from escaping from the enzyme active site and can be further oxidised to fatty acids upon binding of a second molecule of NAD+.
Molecular basis of mutations leading to Sjögren-Larsson syndrome
A large number of inactivating mutations in FALDH leading to SLS have been reported. In many cases, these involve frame-shift mutations and premature truncation of the protein and result in loss of the catalytic core of the enzyme. A number of mis-sense mutations causing inactivation are also known. Of these, the molecular basis of only the K266N mutation has been determined, which is reduced stability of the mRNA and hence reduced expression of the protein [Citation2,Citation4].
Our model suggests the molecular effects of six mis-sense mutations that apparently affect catalysis by FALDH: H411Y, G412R, T184R, T184M and G185A and D245N. His-411 is proposed to be involved in oxidation of alcohol substrates, and the H411Y mutation will disrupt the key abstraction of the hydroxyl hydrogen. Gly-412 is proximal to proposed catalytic residues (Tyr-410, His-411), and the G412R mutation may exert its effects by disrupting interaction of their side-chains with substrate. The T184R, T184M and G185A and D245N mutations all appear to exert their effects by disrupting NAD+ binding or utilisation. Thr-184 and Gly-185 residues appear to be proximal with the nicotinamide ring or ribose moiety, and mutation of these side-chains presumably causes conformation changes resulting in reduced or loss of NAD+ binding. In the final case, D245N, Asp-245 appears to hydrogen bond to Ile-332, which is between Phe-333 and Glu-331. The latter two residues bind to the nicotinamide ring or ribose hydroxyl groups, respectively. The corresponding D247N mutation in ALDH3, which has high levels of sequence homology to FALDH, has been shown to reduce Vmax values [Citation41] suggesting that NAD+ co-substrate binding is occurring but catalytic reduction is diminished.
Conclusions
This paper reports the first study of recombinant FALDH. Our results confirm that FALDH is able to oxidise a wide range of aldehyde substrates [Citation20], and indeed this range is potentially much wider than was previously thought. Our results hint that FALDH could be involved more generally in the metabolism of isoprenoid branched-chain lipids, such as farnesal. FALDH may be involved in oxidation of (some) alcohols to aldehydes and the accumulation of long-chain fatty alcohols in SLS patients supports a primary role for FALDH in their conversion. It has been reported that separation of microsomal alcohol and aldehyde dehydrogenase activities and reconstitution of the complex is possible [Citation42], but these experiments utilised dodecanol as a substrate. Thus, it is not clear whether this reported fatty alcohol oxidase is responsible for conversion of long-chain fatty alcohols such as hexadecanol to their corresponding aldehydes.
| Abbreviations | ||
| ALDH3A2: | = | aldehyde dehydrogenase 3A2a.k.a. FALDH |
| ALDH10: | = | aldehyde dehydrogenase 10a.k.a. FALDH |
| FALDH: | = | fatty aldehyde dehydrogenase |
| IPTG: | = | isopropyl-β-D-thiogalactopyranoside |
| SDS-PAGE: | = | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SEM: | = | standard error of the mean |
| SLS: | = | Sjögren-Larsson syndrome |
Supplementary Material
Download PDF (589.6 KB)Acknowledgements
We thank Professor C. J. Schofield and Dr. D. Butler (University of Oxford) for helpful discussions. This work was funded by a University of Bath Departmental studentship for KDEB and by E.U. grant Refsum's Disease: Diagnosis, Pathology & Treatment (QLG3-CT-2002-00696) to MDL.
References
- Rizzo WB. Mol Genet Metab 1998; 65: 63–73
- Rizzo WB, Carney G, Lin ZL. Am J Hum Genet 1999; 65: 1547–1560
- Ijlst L, Oostheim W, van Werkhoven M, Willemsen M, Wanders RJA. J Inherit Metab Dis 1999; 22: 319–321
- Rizzo WB, Lin Z, Carney G. Chem-Biol Interact 2001; 130: 297–307
- Willemsen M, Ijlst L, Steijlen PM, Rotteveel JJ, de Jong JGN, van Domburg P, Mayatepek E, Gabreels FJM, Wanders RJA. Brain 2001; 124: 1426–1437
- Sillen A, Jagell S, Wadelius C. Human Genetics 1997; 100: 201–203
- Rizzo WB, Carney G. Hum Mutat 2005; 26: 1–10
- Fernandez-Vozmediano JM, Armario-Hita JC, Gonzalez-Cabrerizo A. Pediat Dermatol 2003; 20: 179–180
- Willemsen M, Lutt MAJ, Steijlen PM, Cruysberg JRM, van der Graaf M, Nijhuis-van der Sanden MWG, Pasman JW, Mayatepek E, Rotteveel JJ. Euro J Pediat 2001; 160: 711–717
- Rizzo WB, Craft DA, Dammann AL, Phillips MW. J Biol Chem 1987; 262: 17412–17419
- Rizzo WB, Heinz E, Simon M, Craft DA. Biochim Biophys Acta-Mol Basis Dis 2000; 1535: 1–9
- Tschantz WR, Digits JA, Pyun HJ, Coates RM, Casey PJ. J Biol Chem 2001; 276: 2321–2324
- Willemsen M, Rotteveel JJ, de Jong JGN, Wanders RJA, Ijlst L, Hoffmann GF, Mayatepek E. J Neurol Sci 2001; 183: 61–67
- van den Brink DM, van Miert JM, Wanders RJA. Clin Chem 2005; 51: 240–242
- van den Brink DM, van Miert JNI, Dacremont G, Rontani JF, Jansen GA, Wanders RJA. Mol Genet Metab 2004; 82: 33–37
- Xu JF, Thornburg T, Turner AR, Vitolins M, Case D, Shadle J, Hinson L, Sun JL, Liu WN, Chang BL, Adams TS, Zheng SL, Torti FM. Prostate 2005; 63: 209–214
- Demozay D, Rocchi S, Mas JC, Grillo S, Pirola L, Chavey C, Van Obberghen E. J Biol Chem 2004; 279: 6261–6270
- Rogers GR, Markova NG, DeLaurenzi V, Rizzo WB, Compton JG. Genomics 1997; 39: 127–135
- Lin ZL, Carney G, Rizzo WB. Mol Genet Metabol 2000; 71: 496–505
- Kelson TL, McVoy JRS, Rizzo WB. Biochim Biophys Acta 1997; 1335: 99–110
- Laemmli UK. Nature 1970; 227: 680–685
- Bradford MM. Anal Biochem 1976; 72: 248–254
- Singh RS, Mukherjee K, Banerjee R, Chaudhuri A, Hait SK, Moulik SP, Ramadas Y, Vijayalakshmi A, Rao NM. Chem-Euro J 2002; 8: 900–909
- Aurell MJ, Ceita L, Mestres R, Tortajada A. Tetrahedron 1997; 53: 10883–10898
- Taber DF, Amedio JC, Jung KY. J Org Chem 1987; 52: 5621–5622
- Easton CJ, Xia L, Pitt MJ, Ferrante A, Poulos A, Rathjen DA. Synthesis 2001; 451–457
- Smith TE. Biochemistry 1966; 5: 2919–2926
- Cornish-Bowden A. Analysis of enzyme kinetic data. Oxford University Press, Oxford 1995
- Cornish-Bowden A, Eisenthal R. Biochem J 1974; 139: 721–730
- Eisenthal R, Cornish-Bowden A. Biochem J 1974; 139: 715–720
- Bochet CG. Angewandte Chemie-Int Ed 2001; 40: 2071–2073
- Mihara S, Tateba H. J Org Chem 1986; 51: 1142–1144
- Liu ZJ, Sun YJ, Rose J, Chung YJ, Hsiao CD, Chang WR, Kuo I, Perozich J, Lindahl R, Hempel J, Wang BC. Nat Struct Biol 1997; 4: 317–326
- Vriend G. J Mol Graphics 1990; 8: 52
- Krieger E, Darden T, Nabuurs SB, Finkelstein A, Vriend G. Proteins-Structure Funct Bioinform 2004; 57: 678–683
- Krieger E, Koraimann G, Vriend G. Proteins-Structure Funct and Genet 2002; 47: 393–402
- Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. J Am Chem Soc 1995; 117: 5179–5197
- Laskowski RA, Macarthur MW, Moss DS, Thornton JM. J Applied Cryst 1993; 26: 283–291
- Mukherji M, Schofield CJ, Wierzbicki AS, Jansen GA, Wanders RJA, Lloyd MD. Prog Lipid Res 2003; 42: 359–376
- Millar DBS, Schwert GW. J Biol Chem 1963; 238: 3249–3255
- Hempel J, Kuo I, Perozich J, Wang BC, Lindahl R, Nicholas H. Eur J Biochem 2001; 268: 722–726
- Ichihara K, Kusunose E, Noda Y, Kusunose M. Biochim Biophys Acta 1986; 878: 412–418