Abstract
In an attempt to combine the anti-HIV inhibitory capacity of reverse transcriptase (RT) inhibitors (NRTIs) and integrase (IN) inhibitors (INIs), several heterodimer analogues of the previously reported [d4T]-PABC-[INI] and [d4T]-OABC-[INI] prototypes have been prepared. In these novel series, we wished to extend our results to conjugates which incorporated an enzymatically labile aminoacid unit (L-alanine) connected to d4T through a self-immolative para- or ortho-aminobenzyl carbonate (PABC or OABC) spacer. Among the novel heterodimers, several derivatives show a potent anti-HIV-1 activity, which proved comparable to that of the [L-708,906]-PABC-[d4T] Heterodimer A prototype. However, although the compounds proved inhibitory to HIV-1, they were less potent than the parent compounds from which they were derived.
Introduction
In recent years, significant advances have been made in the development of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase and protease (PR) inhibitors for the chemotherapy of HIV infections (AIDS / acquired immunodeficiency syndrome) Citation1-4. Currently, the standard treatment strategy for AIDS is referred to the highly active antiretroviral therapy (HAART) which is a combination of three or more anti-HIV drugs inhibiting two viral enzymes (RT and PR) and virus fusion (interaction with glycoprotein 41) Citation5-7. Unfortunately, for all classes the emergence of resistance drastically reduces the efficacy of current therapies Citation8-10. Encouragingly, a new drug class that inhibits the HIV-1 integrase (IN) is in development and may soon be available in the clinic Citation11-15. IN catalyzes an essential step in the viral replication cycle and it has been validated as an attractive target since it is an essential, there is no mammalian homologue of IN. Inhibitors of integrase enzyme block the integration of viral double-stranded DNA into the host cell's chromosomal DNA. The diketo acid (DKA) derivatives were reported as first class of HIV integrase selective inhibitors Citation16-23. The story of DKA derivatives started with the reports of the selective inhibitory properties of 4-(3,5-dibenzyloxyphenyl)-2-hydroxy-4-oxobut-2-enoic acid (L-708,906 / Merck) and 4-[1-(4-fluorobenzyl)-1H-pyrrol-2-yl]-2-hydroxy-4-oxobut-2-enoic acid (L-731,988 / Merck) toward strand transfer (). Not only these compounds potently blocked integration in extracellular assays but they exhibited good antiviral effects against HIV-infected cells [Citation17,Citation18].
Figure 1 Chemical Structures of Representative α,γ-Diketo-Based Integrase and the Nucleoside Reverse Transcriptase Inhibitor (the 2′,3′-didehydro-2′,3′-dideoxythymidine).
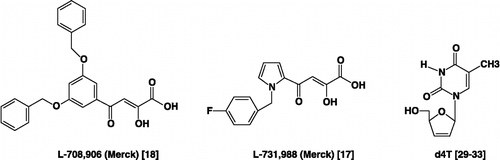
We have an ongoing program based on the “double-drug” strategy and we had previously developed potent hybrid-type anti-HIV agents that combined an effective anti-HIV agent such as a nucleoside RT inhibitor (NRTI) used for masking the free carboxylic acid of the HIV IN inhibitor in a single molecule [Citation24]. In fact, this combination approach has been advocated for three main reasons: (1) the low membrane permeability of INIs would be improved as the cell membrane has affinity for nucleosides [Citation25,Citation26]; (2) the conjugation of HIV INI with the NRTI would facilitate the penetration through the biological membrane mediated by the nucleoside transporter [Citation27,Citation28]; (3) once the prodrugs avoided the extracellular hydrolysis, the intracellular hydrolysis would regenerate the parent inhibitors, which could act on two separate targets and exhibit synergistic anti-HIV efficacy. In our previous paper, the synthesized hybrid-type prodrugs contained a DKA in which the carboxyl group was linked to the 5′-hydroxyl group of the NRTI (the 2′,3′-didehydro-2′,3′-dideoxythymidine, d4T, Stavudine, Zerit®) Citation29-33 using spontaneously self-immolative 1,6- or 1,4-elimination spacers (). Conjugates of the general formula [INI]-PABC-[d4T] revealed to display significant anti-HIV activity at submicromolar concentrations, while OABC spacers dramatically diminished activity. However, they were less potent inhibitors than the parent compounds from which they derived [Citation24]. Particularly remarkable, the Heterodimer A prototype [L-708,906]-PABC-[d4T] showed good antiviral activity (IC50 0.43 μM against HIV-1LAI in CEM-SS cells).
Therefore, to obtain better insights in the feasibility of these heterodimer approach to increase the inhibitory efficacy of the test compounds against HIV, this paper describes the synthesis and anti-HIV evaluation of a novel series of [INI]-spacer-[d4T] heterodimers which incorporated an enzymatically labile aminoacid unit (L-alanine) connected to d4T through a self-cleavable para- or ortho-aminobenzyl carbonate (PABC or OABC) spacer. The mechanism of conversion of double-drugs to d4T and IN inhibitor that could be proposed involved the hydrolysis of the peptide linkage between either the DKA and the amino acid residue (L-alanine) () or the alanyl unit and the PABC moiety ().
Materials and methods
Chemistry
Instrumentation
Commercial reagent (N,N-dimethylformamide/Aldrich) was used as received without additional purification. Tetrahydrofuran (THF) was distilled from sodium/benzophenone immediately prior to use. Reagent grade dichloromethane (CH2Cl2) was refluxed and distilled from phosphorus pentoxide. Unless otherwise stated, reactions were run under argon and monitored by thin-layer chromatography (TLC) using precoated silica gel 60 F254 sheets (0.2 mm layer) purchased from Macherey-Nagel, and compounds were detected by UV absorption at 254 nm. Column chromatography was achieved by using Merck silica gel 60 (0.063–0.200 nm). All samples were kept in a drying oven at 30°C over P4O10 for at least 24 h prior to analysis.
Melting points were determined using a Kofler melting point apparatus and are uncorrected. IR spectra were recorded on a Perkin Elmer Spectrum BX FT-IR and only noteworthy absorptions are listed. 1H and 13C-NMR spectra were recorded on a JEOL NMR LA 400 (400 MHz) spectrometer using TMS as an internal standard. Chemical shifts were reported as δ values in parts per million units, downfield from TMS. The splitting pattern abbreviations are as follows: s = singlet, d = doublet, t = triplet, m = multiplet. Coupling constants J are given in hertz (Hz). NH and OH signals appeared as broad singlets exchangeable with D2O.
4-(3,5-Dibenzyloxyphenyl)-2-hydroxy-4-oxobut-2-enoic acid (1a) [Citation24]
Methyl N-[4-(3,5-dibenzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]alanylate (2a) method 1
To a stirred solution of 1a (1.05 g, 2.60 mmol) in anhydrous tetrahydrofuran (60 mL) under an argon atmosphere were added L-alanine methyl ester hydrochloride (432 mg, 3.12 mmol, 1.2 equiv.) and 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ, 707 mg, 2.86 mmol, 1.1 equiv.), and the mixture was stirred at room temperature for 20 h. After removal of the solvent in vacuo, the residue was dissolved in ethyl acetate (50 mL), washed with 10% citric acid / H2O (50 mL), and water (50 mL), dried over MgSO4, filtered and concentrated in vacuo. The residue was crystallized from diethyl ether to give the title compound 2a as yellow crystals (880 mg); Yield: 69%; TLC Rf (n-hexane: acetone = 60: 40) 0.52.
Method 2. To a stirred solution of 1a (352 mg, 0.87 mmol) in anhydrous N,N-dimethylformamide (5 mL) under an argon atmosphere were added respectively 1-hydroxy-benzotriazole hydrate (HOBT, 294 mg, 2.17 mmol, 2.5 equiv.), benzotriazol-1-yl-oxytris(dimethylamino)phosphonium hexafluorophosphate (BOP, 577 mg, 1.30 mmol, 1.5 equiv.), L-alanine methyl ester hydrochloride (146 mg, 1.04 mmol, 1.2 equiv.) and triethylamine (0.24 mL, 1.74 mmol, 2 equiv.) and the mixture was stirred at room temperature for 20 h [the reaction was monitored by TLC in cyclohexane: ethyl acetate = 50: 50 until no starting material remained]. After removal of the solvent in vacuo, the residue was dissolved in ethyl acetate (50 mL), washed with 10% citric acid / H2O (50 mL), 5% NaHCO3 / H2O (50 mL) and water (50 mL), brine / H2O (50 mL), dried over MgSO4, filtered and concentrated in vacuo. The residue was crystallized from diethyl ether to give the title compound 2a as yellow crystals (350 mg); Yield: 83%; TLC Rf (cyclohexane: ethyl acetate = 50: 50) 0.56; mp = 104°C; IR υmax (KBr)/cm-1 3375 (NH), 1750 (C = O), 1682 (C = O), 1594, 1380, 1350, 1166, 1057, 838, 734, 706; 1H-NMR δ (d6-DMSO) 1.80 (d, J 7.3, 3H, Ala-CH3), 3.63 (s, 3H, Ala-OCH3), 4.46 (m, 1H, Ala-CH), 5.18 (s, 4H, Bn-CH2), 7.25 (s, 1H, CH = C(OH)), 7.28–7.46 (m, 13H, Bn-H+ Ph-H), 9.19 (d, J 7.3, 1H, Ala-NH); 13C-NMR δ (d6-DMSO) 16.5 (Ala-CH3), 47.9 (Ala-CH), 52.1 (Ala-OCH3), 69.7 (Bn-CH2), 95.2 (CH = C(OH)), 106.3 (Ph-C2+ Ph-C6), 107.5 (Ph-C4), 127.8, 127.9, 128.5 (Bn-C), 135.8 (Ph-C1), 136.6 (Bn-C1), 159.8 (Ph-C3+ Ph-C5), 161.0 (C-2), 172.1 (C-1), 177.3 (Ala-CO), 186.2 (C-4).
Methyl N-[4-(3-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]alanylate (2b)
Compound 2b was prepared from precursor 1b (1 g, 3.35 mmol) by the same procedure as that described for compound 2a. Yield: 30%, orange oil (390 mg); TLC Rf (cyclohexane: ethyl acetate = 50: 50) 0.64; IR υmax (KBr)/cm− 1 3395 (NH), 1745 (CO), 1682 (CO), 1580, 1515, 1454, 1262, 1216, 1163, 1026; 1H-NMR δ (d6-DMSO) δ 1.38 (d, J 7.1, 3H, Ala-CH3), 3.64 (s, 3H, Ala-OCH3), 4.45 (m, 1H, Ala-CH), 5.20 (s, 2H, Bn-CH2) 7.09 (s, 1H, CH = C(OH)), 7.31–7.65 (m, 9H, Bn-H, Ph-H), 9.16 (d, J 7.6, 1H, Ala-NH); 13C-NMR δ (d6-DMSO) 16.5 (Ala-CH3), 47.9 (Ala-CH), 52.1 (Ala-OCH3), 69.5 (Bn-CH2), 94.9 (CH = C(OH)), 113.0 (Ph-C2), 120.3 (Ph-C4), 120.7 (Ph-C6), 127.7, 127.9, 128.5 (Bn-C), 130.4 (Ph-C5), 135.2 (Bn-C1), 136.7 (Ph-C1), 158.7 (Ph-C3), 161.1 (C-2), 172.1 (C-1), 177.3 (Ala-CO), 186.2 (C-4).
Methyl N-[4-(4-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]alanylate (2c)
Compound 2c was prepared from precursor 1c (501 mg, 1.68 mmol) by the same procedure as that described for compound 2a. Yield: 51%, yellow solid (510 mg); TLC Rf (cyclohexane: ethyl acetate = 50: 50) 0.59; mp = 92°C; IR υmax (KBr)/cm− 1 3395 (NH), 1747 (CO), 1681 (CO), 1600, 1505, 1381, 1254, 1172, 1120, 1005, 829; 1H-NMR δ (d6-DMSO) δ 1.38 (d, J 7.3, 3H, Ala-CH3), 3.64 (s, 3H, Ala-OCH3), 4.44 (m, 1H, Ala-CH), 5.23 (s, 2H, Bn-CH2), 7.05 (s, 1H, CH = C(OH)), 7.18 (d, J 8.8, 2H, Ph-H), 7.34–7.42 (m, 5H, Bn-H), 8.05 (d, J 8.8, 2H, Ph-H), 9.13 (d, J 7.6, 1H, Ala-NH); 13C-NMR δ (d6-DMSO) 16.5 (Ala-CH3), 47.9 (Ala-CH), 52.1 (Ala-OCH3), 69.7 (Bn-CH2), 94.1 (CH = C(OH)), 115.3 (Ph-C), 127.8, 128.0, 128.5 (Bn-C), 130.1 (Ph-C), 136.3 (Bn-C1), 161.1 (C-2), 163.1 (Ph-C4), 170.6 (C-1), 175.6 (Ala-CO), 186.9 (C-4).
N-[4-(3,5-Dibenzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]alanine (3a)
To a solution of β-diketo acid methyl ester 2a (250 mg, 0.51 mmol) in THF/methanol 1/1 (10 mL) was added 1N aqueous LiOH solution (2 mL). The reaction mixture was stirred for 2 h at room temperature and concentrated under reduced pressure. The residue was dissolved in water (50 mL), carefully acidified by addition of 1N HCl/ice water 1/1 until moistened pH paper indicated pH = 1 and then extracted twice with ethyl acetate (2 × 50 mL). The combined organic layers were washed twice with ice water/brine 1/1, dried over MgSO4, filtered and concentrated in vacuo to give the title compound 3a as brown crystals (197 mg); Yield: 81%; TLC Rf (cyclohexane: ethyl acetate = 40: 60) 0.19; mp = 126°C; IR υmax (KBr)/cm− 1 3405 (NH), 1751 (CO), 1735(CO), 1655 (CO), 1578, 1528, 1453, 1293, 1262, 1164, 1048; 1H-NMR δ (d6-DMSO) δ 1.45 (d, J 7.1, 3H, Ala-CH3), 4.43 (m, 1H, Ala-CH), 5.27 (s, 4H, Bn-CH2), 7.32 (s, 1H, CH = C(OH)), 7.39–7.55 (m, 13H, Bn-H+ Ph-H), 9.08 (d, J 7.6, 1H, Ala-NH); 13C-NMR δ (d6-DMSO) 16.6 (Ala-CH3), 47.9 (Ala-CH), 69.7 (Bn-CH2), 95.2 (CH = C(OH)), 106.3 (Ph-C), 127.8, 127.9, 128.4 (Bn-C), 135.8 (Bn-C1), 136.6 (Ph-C1), 159.6, 159.8 (Ph-C3+ Ph-C5), 160.9 (C-2), 171.9 (C-1), 177.6 (Ala-CO), 186.0 (C-4).
N-[4-(3-Benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]alanine (3b)
Compound 3b was prepared from precursor 2b (349 mg, 0.91 mmol) by the same procedure as that described for compound 3a. Yield: 71%, brown oil (240 mg); TLC Rf (cyclohexane: ethyl acetate = 40: 60) 0.13; IR υmax (KBr)/cm− 1 3392 (NH), 1746 (CO), 1727 (CO), 1658 (CO), 1576, 1527, 1453, 1262, 1195, 1096, 1025, 804; 1H-NMR δ (d6-DMSO) δ 1.58 (d, J 7.1, 3H, Ala-CH3), 4.71 (m, 1H, Ala-CH), 5.13 (s, 2H, Bn-CH2), 7.17 (s, 1H, CH = C(OH)), 7.19–7.64 (m, 9H, Ph-H, Bn-H), 7.64 (d, J 7.3, 1H, NH-Ala); 13C-NMR δ (CDCl3) 17.9 (Ala-CH3), 48.1 (Ala-CH), 70.3 (Bn-CH2), 94.6 (CH = C(OH)), 113.2 (Ph-C2), 120.6 (Ph-C4), 120.8 (Ph-C6), 127.5, 128.2, 128.7 (Bn-C), 130.0 (Ph-C5), 135.4 (Bn-C1), 136.4 (Ph-C1), 159.1 (Ph-C3), 161.2 (C-2), 175.3 (C-1), 176.8 (Ala-CO), 186.9 (C-4).
N-[4-(4-Benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]alanine (3c)
Compound 3c was prepared from precursor 2c (598 mg, 1.56 mmol) by the same procedure as that described for compound 3a. Yield: 38%, white crystals (220 mg); TLC Rf (cyclohexane: ethyl acetate = 40: 60) 0.16; IR υmax (KBr)/cm− 1 3383 (NH), 1743 (CO), 1726 (CO), 1661 (CO), 1604, 1533, 1507, 1455, 1261, 1205, 1171, 1110; 1H-NMR δ (d6-DMSO) 1.37 (d, J 7.3, 3H, Ala-CH3), 4.35 (m, 1H, Ala-CH), 5.23 (s, 2H, Bn-CH2), 7.05 (s, 1H, CH = C(OH)), 7.18 (d, J 8.8, 2H, Ph-H), 7.34–7.47 (m, 5H, Bn-H), 8.04 (d, J 8.8, 2H, Ph-H), 8.89 (d, J 7.6, 1H, Ala-NH); 13C-NMR δ (d6-DMSO) 16.9 (Ala-CH3), 47.8 (Ala-CH), 69.6 (Bn-CH2), 94.0 (CH = C(OH)), 115.3 (Ph-C), 127.8, 128.1, 128.5 (Bn-C), 130.1 (Ph-C), 136.3 (Bn-C1), 160.9 (C-2), 163.0 (Ph-C4), 173.2 (C-1), 175.8 (Ala-CO).
N-[4-(3,5-dibenzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-pab-oh (4A)
To a stirred and cooled (0°C) solution of 3a (880 mg, 1.85 mmol) in anhydrous tetrahydrofuran (8 mL) under an argon atmosphere were added 4-aminobenzyl alcohol (273 mg, 2.22 mmol, 1.2 equiv.) and 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ, 480mg, 1.94 mmol, 1.05 equiv.), and the mixture was stirred at room temperature for 2 days [the reaction was monitored by TLC in cyclohexane: ethyl acetate = 20: 80 until no starting material remained]. After removal of the solvent in vacuo, the residue was dissolved in ethyl acetate (50 mL), washed with 10% citric acid / H2O (40 mL), and water (40 mL), dried over MgSO4, filtered and concentrated in vacuo. The residue was crystallized from diethyl ether to give the title compound 4a as yellow crystals (610 mg); Yield: 57%; TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.47; mp = 192°C; IR υmax (KBr)/cm− 1 3411 (OH), 3270 (NH), 1666 (CO), 1638 (CO), 1595, 1529, 1382, 1297, 1261, 1167, 1096, 1025, 800; 1H-NMR δ (d6-DMSO) 1.42 (d, J 7.1, 3H, Ala-CH3), 4.42 (d, J 5.6, 2H, PAB-CH2OH), 4.53 (m, 1H, Ala-CH), 5.11 (t, J 5.6, 1H, PAB-OH), 5.18 (s, 4H, Bn-CH2), 7.24 (s, 1H, CH = C(OH)), 7.32–7.46 (m, 15H, Bn-H+ Ph-H+ PAB-H), 7.53 (d, J 8.3, 2H, PAB-H), 8.82 (d, J 7.3, 1H, Ala-NH), 10.04 (s, 1H, PAB-NH); 13C-NMR δ (d6-DMSO) 17.9 (Ala-CH3), 49.5 (Ala-CH), 62.5 (PAB-CH2OH), 69.6 (Bn-CH2), 95.1 (CH = C(OH)), 106.3 (Ph-C), 119.1 (PAB-C2+ PAB-C6), 126.9 (PAB-C3+ PAB-C5), 127.8, 127.9, 128.5 (Bn-C), 136.6 (Ph-C1), 159.8 (Ph-C3+ Ph-C5), 170.1 (C-1), 177.6 (Ala-CO), 184.5 (C-4).
N-[4-(3-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-pab-oh (4B)
Compound 4b was prepared from precursor 3b (240 mg, 0.65 mmol) by the same procedure as that described for compound 4a, except that the reaction mixture was stirred for 5 days. Yield: 49%, brown crystals (150 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.45; mp = 162°C; IR υmax (KBr)/cm− 1 3461 (OH), 3399 (NH), 3268 (NH), 1665 (CO), 1635 (CO), 1598, 1531, 1455, 1415, 1291, 1259, 1208, 1005; 1H-NMR δ (d6-DMSO) 1.42 (d, J 7.1, 3H, Ala-CH3), 4.42 (d, J 5.1, 2H, PAB-CH2OH), 4.54 (m, 1H, Ala-CH), 5.09 (s, 1H, PAB-OH), 5.20 (s, 2H, Bn-CH2), 7.10 (s, 1H, CH = C(OH)), 7.24 (d, J 8.5, 2H, PAB-H), 7.31–7.65 (m, 11H, Bn-H+ Ph-H+ PAB-H), 8.79 (d, J 7.6, 1H, Ala-NH), 10.02 (s, 1H, PAB-NH).
N-[4-(4-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-pab-oh (4C)
Compound 4c was prepared from precursor 3c (222 mg, 0.60 mmol) by the same procedure as that described for compound 4a, except that the reaction mixture was stirred for 3 days. Yield: 74%, brown crystals (210 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.50; mp = 182°C; IR υmax (KBr)/cm− 1 3353 (OH), 3262 (NH), 1670 (CO), 1605 (CO), 1510, 1455, 1260, 1173, 1097, 1015, 801; 1H-NMR δ (d6-DMSO) 1.42 (d, J 7.1, 3H, Ala-CH3), 4.42 (d, J 5.6, 2H, PAB-CH2OH), 4.53 (m, 1H, Ala-CH), 5.09 (t, J 5.6, 1H, PAB-OH), 5.23 (s, 2H, Bn-CH2), 7.05 (s, 1H, CH = C(OH)), 7.17 (d, J 8.8, 2H, Ph-H), 7.24 (d, J 8.3, 2H, PAB-H), 7.34–7.47 (m, 5H, Bn-H), 7.53 (d, J 8.5, 2H, PAB-H), 8.04 (d, J 9.0, 2H, Ph-H), 8.72 (d, J 7.1, 1H, Ala-NH), 10.02 (s, 1H, PAB-NH); 13C-NMR δ (d6-DMSO) 18.0 (Ala-CH3), 49.4 (Ala-CH), 62.6 (PAB-CH2OH), 69.7 (Bn-CH2), 94.0 (CH = C(OH)), 115.3 (Ph-C), 119.1, 121.5 (PAB-C), 126.3, 126.5, 126.9, 127.8, 128.1, 128.5, 128.9, 129.5, 130.1, 136.0, 136.3, 137.6, 160.9 (C-2), 163.1 (Ph-C4), 170.2 (C-1), 175.9 (Ala-CO).
N-[4-(3,5-dibenzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-oab-oh (5A)
Compound 5a was prepared from precursor 3a (998 mg, 2.1 mmol) by the same procedure as that described for compound 4a, except that the reaction mixture was added with 2-aminobenzyl alcohol (310 mg, 2.52 mmol, 1.2 equiv.) and stirred for 5 days. Yield: 30%, brown crystals (360 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.64; mp = 96°C; IR υmax (KBr)/cm− 1 3260 (NH + OH), 1698 (CO), 1667 (CO), 1591, 1519, 1453, 1347, 1294, 1164; 1H-NMR δ (d6-DMSO) 1.45 (d, J 7.1, 3H, Ala-CH3), 4.47 (s, 2H, OAB-CH2OH), 4.53 (m, 1H, Ala-CH), 5.18 (s, 4H, Bn-CH2), 5.36 (s, 1H, OAB-OH), 6.98 (s, 1H, CH = C(OH)), 7.12–7.60 (m, 17H, Bn-H+ Ph-H+ OAB-H), 9.00 (d, J 7.1, 1H, Ala-NH), 9.59 (s, 1H, OAB-NH); 13C-NMR δ (d6-DMSO) 17.5 (Ala-CH3), 60.5 (OAB-CH2OH), 69.7 (Bn-CH2), 95.2 (CH = C(OH)), 106.3 (Ph-C), 122.1 (OAB-C6), 123.4 (OAB-C4), 127.1, 127.3, 127.7, 127.9, 128.4, 131.3, 136.6 (Ph-C1), 143.6, 159.8 (Ph-C3+ Ph-C5), 168.4 (C-1), 177.8 (Ala-CO), 185.2 (C-4).
N-[4-(3-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-oab-oh (5B)
Compound 5b was prepared from precursor 3b (199 mg, 0.54 mmol) by the same procedure as that described for compound 4a, except that the reaction mixture was added with 2-aminobenzyl alcohol (80 mg, 0.65 mmol, 1.2 equiv.) and stirred for 5 days. Yield: 78%, brown oil (200 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.59; IR υmax (KBr)/cm− 1 3308 (OH + NH), 1674 (CO), 1592, 1516, 1453, 1262, 1259, 1096, 1020, 798; 1H-NMR δ (d6-DMSO) 1.46 (d, J 7.1, 3H, Ala-CH3), 4.46 (s, 2H, OAB-CH2OH), 4.54 (m, 1H, Ala-CH), 5.21 (s, 2H, Bn-CH2), 5.35 (s, 1H, OAB-OH), 7.12 (s, 1H, CH = C(OH)), 7.22–7.66 (m, 13H, Bn-H+ Ph-H+ OAB-H), 8.99 (d, J 7.3, 1H, Ala-NH), 9.59 (s, 1H, OAB-NH).
N-[4-(4-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-oab-oh (5C)
Compound 5c was prepared from precursor 3c (222 mg, 0.60 mmol) by the same procedure as that described for compound 4a, except that the reaction mixture was added with 2-aminobenzyl alcohol (89 mg, 0.72 mmol, 1.2 equiv.) and stirred for 5 days. Yield: 60%, brown crystals (170 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.66; mp = 174°C; IR υmax (KBr)/cm− 1 3467 (OH), 3254 (NH), 1667 (CO), 1603(CO), 1533, 1509, 1453, 1250, 1176, 1006, 998, 747; 1H-NMR δ (d6-DMSO) 1.45 (d, J 7.1, 3H, Ala-CH3), 4.47 (s, 2H, OAB-CH2OH), 4.54 (m, 1H, Ala-CH), 5.23 (s, 4H, Bn-CH2), 5.34 (s, 1H, OAB-OH), 7.07 (s, 1H, CH = C(OH)), 7.13–7.47 (m, 10H, Bn-H+ Ph-H+ OAB-H), 7.59 (d, J 7.8, 1H, OAB-H), 8.05 (d, J 8.8, 2H, Ph-H), 8.91 (d, J 7.8, 1H, Ala-NH), 9.59 (s, 1H, OAB-NH).
5′-PNPC-2′,3′-didehydro-2′,3′-dieoxythymidine (6)
To a stirred and cooled (0°C) solution of d4T (500 mg, 2.23 mmol) in anhydrous tetrahydrofuran (30 mL) under an argon atmosphere was added respectively: pyridine (0.27 mL, 3.34 mmol) and a solution of 4-nitrophenyl chloroformate (675 mg, 3.34 mmol) in anhydrous tetrahydrofuran (8 mL). The reaction mixture was stirred at room temperature and after 2 h TLC (silica, chloroform: methanol = 97: 3) indicated completion. The mixture was diluted with ethyl acetate (100 mL) and washed respectively with water (100 mL), 5% NaHCO3 / H2O (200 mL), dried over MgSO4, filtered and concentrated in vacuo. The residue was crystallized from diethyl ether to give the title compound 6 as white crystals (770 mg); Yield: 89%; TLC Rf (chloroform: methanol = 97: 3) 0.57; mp = 190°C; IR υmax (KBr)/cm− 1 3039 (NH), 1770 (C = O), 1694 (C = O), 1524, 1466, 1351, 1270, 1216, 1084, 859; 1H-NMR δ (CDCl3) 8.77 (s, 1H, NH), 8.30 (d, J 8.5, 2H, H-III:PNP and H-V:PNP), 7.39 (s, 1H, H-6), 7.35 (d, J 8.5, 2H, H-II:PNP and H-VI:PNP), 7.10 (s, 1H, H-1′), 6.38 (d, J 6.1,1H, H-2′), 5.98 (d, J 6.1, 1H, H-3′), 5.13 (s, 1H, H-4′), 4.54 (s, 2H, H-5′a and H-5′b), 1.87 (s, 3H, CH3); 13C-NMR δ (CDCl3) 163.6 (C-4), 155.0 (C-I:PNP), 152.3 (OC = O), 150.1 (C-2), 145.7 (C-IV:PNP), 135.9 (C-6), 132.7 (C-2′), 128.0 (C-3′), 125.5 (C-II and C-V:PNP), 121.7 (C-II and C-VI:PNP), 111.3 (C-5), 89.6 (C-1′), 83.5 (C-4′), 68.8 (C-5′), 12.4 (CH3).
Heterodimer [4-(3,5-Dibenzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-pabc-c5′[d4t] (7A)
To a stirred and cooled (0°C) solution of 4a (372 mg, 0.64 mmol) in anhydrous dichloromethane (15 mL) under an argon atmosphere was added respectively: 4-dimethylaminopyridine (1.3 equiv., 102 mg, 0.83 mmol), 5′-PNPC-d4T (6, 1 equiv.) and a catalytic amount of N,N-diisopropylethylamine. The reaction mixture was stirred at room temperature and after 5 days TLC (silica, cyclohexane: ethyl acetate = 20: 80) indicated completion. The mixture was diluted with ethyl acetate: water (1/1, 200 mL). The organic layer was separated, washed with a solution of 10% citric acid/H2O (200 mL), then water (200 mL), dried over MgSO4 and concentrated to dryness under reduced pressure. The residue was purified by silica-gel chromatography using ethyl acetate in cyclohexane (from 50% to 100%) as eluents to yield the title compound 7a as yellow crystals (200 mg); Yield: 38%, mp = 122°C; TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.18; IR υmax (KBr)/cm− 1 3321 (NH), 1751 (CO), 1693 (CO), 1594, 1516, 1262, 1157, 1087, 1052, 1025, 799; 1H-NMR δ (CDCl3) 1.48 (d, J 6.8, 3H, Ala-CH3), 1.57 (s, 3H, d4T-CH3), 4.23 (d, J 10.5, 1H, d4T-H5′a), 4.33 (d, J 10.5, 1H, d4T-H5′b, 4.65 (m, 1H, Ala-CH), 4.88 (s, 1H, d4T-H4′), 4.99 (s, 6H, Bn-CH2 + CH2OCO), 5.71 (d, J 6.1, 1H, H-3′), 6.14 (d, J 6.1, 1H, H-2′), 6.89 (s, 1H, d4T-H1′), 7.07 (s, 1H, CH = C(OH), 7.14-7.33 (m, 16H, Bn-H+ Ph-H+ PAB-H + d4T-H6), 7.48 (d, J 8.1, 2H, PAB-H) 7.74 (d, J 7.3, 1H, Ala-NH), 8.72 (s, 1H, PAB-NH), 9.00 (s, 1H, d4T-NH) 13C-NMR δ (CDCl3) 12.3 (d4T-CH3), 17.7 (Ala-CH3), 50.2 (Ala-CH), 67.6 (d4T-C5′), 70.1 (CH2OCO), 70.6 (Bn-CH2), 84.1 (d4T-C4′), 89.6 (d4T-C1′), 95.1 (CH = C(OH)), 106.9 (Ph-C), 111.3 (d4T-C5), 120.2 (PAB-C2+ PAB-C6), 127.7, 127.8, 128.4, 128.9, 129.9, 130.7, 133.1 (d4T-C3′), 136.2, 136.5, 136.6, 138.6, 151.0 (OCO), 154.9 (d4T-C2), 160.4 (Ph-C3+ PAB-C5), 162.0 (d4T-C4), 164.2 (C-2), 169.7 (C-1), 176.5 (Ala-CO), 187.2 (C-4).
Heterodimer N-[4-(3-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-pabc-c5′[d4t] (7B)
Compound 7b was prepared from precursor 4b (119 mg, 0.25 mmol) by the same procedure as that described for compound 7a, except that the reaction mixture was stirred for 6 days. Yield: 37%, brown crystals (50 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.07; mp = 116°C; IR υmax (KBr)/cm− 1 3402 (NH), 1693 (CO), 1519, 1451, 1414, 1335, 1262, 1097, 1021, 862, 800; 1H-NMR δ (CDCl3) 1.49 (d, J 7.1, 3H, Ala-CH3), 1.71 (s, 3H, d4T-CH3), 4.28 (dd, J 2.1, J 13.2, 1H, d4T-H5′a), 4.39 (dd, J 2.1, J 13.2, 1H, d4T-H5′b), 4.75 (m, 1H, Ala-CH), 4.96–5.16 (m, 5H, d4T-H4′+ Bn-CH2 + CH2OCO), 5.78 (d, J 5.5, 1H, d4T-H3′), 6.19 (d, J 5.5, 1H, d4T-H2′), 6.82 (s, 1H, d4T-H1′), 7.26–7.38 (m, 15H, Bn-H+ Ph-H+ PAB-H + d4T-H6+ CH = C(OH)), 7.81 (d, J 8.1, 1H, Ala-NH), 8.07 (s, 1H, PAB-NH), 8.63 (s, 1H, d4T-NH).
Heterodimer N-[4-(4-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-pabc-c5′[d4t] (7C)
Compound 7c was prepared from precursor 4c (351 mg, 0.74 mmol) by the same procedure as that described for compound 7a, except that the reaction mixture was stirred for 6 days. Yield: 28%, brown crystals (150 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.17; mp = 110°C; IR υmax (KBr)/cm− 1 3386 (NH), 1748 (CO), 1702 (CO), 1612 (CO), 1508, 1452, 1416, 1262, 1172, 1092, 1021, 800; 1H-NMR δ (CDCl3) 1.50 (d, J 7.1, 3H, Ala-CH3), 1.63 (s, 3H, d4T-CH3), 4.26 (dd, J 2.2, J 12.2, 1H, d4T-H5′a), 4.38 (dd, J 2.2, J 12.2, 1H, d4T-H5′b), 4.61 (m, 1H, Ala-CH), 4.95 (s, 1H, d4T-H4′), 5.05 (s, 2H, CH2OCO), 5.09 (s, 2H, Bn-CH2), 5.76 (d, J 5.9, 1H, d4T-H3′), 6.19 (d, J 5.9, 1H, d4T-H2′), 6.94–7.56 (m, 13H, Ph-H+ Bn-H+ PAB-H+ CH = C(OH) + d4T-H6), 7.55 (d, J 7.1, 1H, Ala-NH), 7.93 (d, J 8.8, 2H, Ph-H), 8.08 (s, 1H, PAB-NH), 8.42 (s, 1H, d4T-NH).
Heterodimer N-[4-(3,5-dibenzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-oabc-c5′[d4t] (8A)
Compound 8a was prepared from precursor 5a (279 mg, 0.48 mmol) by the same procedure as that described for compound 7a, except that the reaction mixture was stirred for 6 days. Yield: 20%, brown crystals (80 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.14; mp = 118°C; IR υmax (KBr)/cm− 1 3294 (NH), 1754 (CO), 1692 (CO), 1592, 1500, 1453, 1346, 1262, 1153, 1095, 1021, 800; 1H-NMR δ (CDCl3) 1.55 (d, J 7.1, 3H, Ala-CH3), 1.71 (s, 3H, d4T-CH3), 4.19 (dd, J 2.0, J 12.0, 1H, d4T-H5′a), 4.28 (dd, J 2.0, J 12.0, 1H, d4T-H5′b), 4.66 (m, 1H, Ala-CH), 4.82 (s, 1H, d4T-H4′), 5.02 (s, 6H, Bn-CH2 + CH2OCO), 5.71 (d, J 5.7, 1H, H-3′), 6.10 (d, J 5.7, 1H, H-2′), 6.88 (s, 1H, d4T-H1′), 7.10 (s, 1H, CH = C(OH) 7.19–7.36 (m, 17H, Bn-H+ Ph-H+ OAB-H + d4T-H6), 7.62 (d, J 7.6, 1H, Ala-NH), 7.81 (d, J 8.1, 1H, OAB-H), 7.97 (s, 1H, OAB-NH); 9.06 (s, 1H, d4T-NH).
Heterodimer N-[4-(3-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-oabc-c5′[d4t] (8B)
Compound 8b was prepared from precursor 5b (251 mg, 0.53 mmol) by the same procedure as that described for compound 7a. Yield: 13%, brown crystals (60 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.09; mp = 118°C; IR υmax (KBr)/cm− 1 3264 (NH), 1759 (CO), 1693 (CO), 1530, 1453, 1396, 1262, 1089, 1022, 801; 1H-NMR δ (CDCl3) 1.45 (d, J 7.1, 3H, Ala-CH3), 1.80 (s, 3H, d4T-CH3), 4.27 (dd, J 2.7, J 12.7, 1H, d4T-H5′a), 4.35 (dd, J 2.7, J 12.7, 1H, d4T-H5′b), 4.73 (m, 1H, Ala-CH), 4.92 (s, 1H, d4T-H4′), 5.14 (s, 4H, Bn-CH2+CH2OCO), 5.81 (d, J 5.1, 1H, d4T-H3′), 6.19 (d, J 5.1, 1H, d4T-H2′), 6.97 (s, 1H, d4T-H1′), 7.19–7.47 (m, 15H, Bn-H+ Ph-H+ OAB-H + d4T-H6+ CH = C(OH)), 7.72 (d, J 8.3, 1H, Ala-NH), 7.88 (d, J 7.6, 1H, OAB-H), 8.01 (s, 1H, OAB-NH), 9.14 (s, 1H, d4T-NH).
Heterodimer N-[4-(4-benzyloxyphenyl)-2-hydroxyenol-4-oxobutyryl]-alanyl-oabc-c5′[d4t] (8C)
Compound 8c was prepared from precursor 5c (152 mg, 0.32 mmol) by the same procedure as that described for compound 7a. Yield: 35%, brown crystals (80 mg); TLC Rf (cyclohexane: ethyl acetate = 20: 80) 0.15; mp = 134°C; IR υmax (KBr)/cm− 1 3269 (NH), 1756 (CO), 1694 (CO), 1601, 1506, 1455, 1397, 1258, 1173, 1119, 1072, 759; 1H-NMR δ (CDCl3) 1.56 (d, J 7.1, 3H, Ala-CH3), 1.73 (s, 3H, d4T-CH3), 4.18 (dd, J 2.8, J 12.2, 1H, d4T-H5′a), 4.27 (dd, J 2.8, J 12.2, 1H, d4T-H5′b), 4.66 (m, 1H, Ala-CH), 4.84 (s, 1H, d4T-H4′), 5.06 (s, 2H, CH2OCO)), 5.09 (s, 2H, Bn-CH2), 5.73 (d, J 6.1, 1H, d4T-H3′), 6.12 (d, J 6.1, 1H, d4T-H2′), 6.89 (s, 1H, d4T-H1′), 6.98 (d, J 9.0, 2H, Ph-H), 7.07 (s, 1H, CH = C(OH)), 7.11–7.38 (m, 10H, Bn-H+ OAB-H + d4T-H6), 7.64 (d, J 8.3, 1H, Ala-NH), 7.80 (d, J 7.8, 1H, OAB-H), 792 (d, J 9.0, 2H, Ph-H), 8.15 (s, 1H, OAB-NH), 9.08 (s, 1H, d4T-NH); 13C-NMR δ (CDCl3) 12.2 (d4T-CH3), 17,8 (Ala-CH3), 50.0 (Ala-CH), 67.0 (d4T-C5′), 68.1 (CH2OCO), 70.3 (Bn-CH2), 83.6 (d4T-C4′), 89.4 (d4T-C1′), 93.8 (CH = C(OH)), 111.1 (d4T-C5), 115.2 (Ph-C3 et Ph-C5), 124.6, 125.7, 126.6, 127.5, 127.6, 128.4, 128.8, 130.1, 130.5, 131.8, 132.7, 135.9, 136.0, 136.2, 150.6 (OCO), 155.8 (d4T-C2), 162.0 (d4T-C4), 163.4 and 163.5 (C-2+ Ph-C4), 169.9 (C-1), 175.7 (Ala-CO), 186.9 (C-4).
Antiviral test procedures
The cultures of CEM-SS and MT4 cells were maintained at 37°C in a 5% CO2 atmosphere in RPMI-1640 medium supplemented with 10% complement-depleted foetal bovine serum (FBS). The antiviral HIV-1 activity of a given compound in CEM-SS cells was measured by quantification of the Reverse Transcriptase activity (RT) associated with particles released from HIV-1LAI-infected cells in the culture medium. CEM-SS cells were infected with 100 TCDI50 (the virus stock was titrated under the same experimental conditions); after 30 min. adsorption, free virus particles were washed out and cells were re-suspended in RPMI-1640 plus 10% calf foetal serum at a final concentration of 105 cells mL− 1 in the presence of different concentrations of test compounds. After 5 days, virus production was measured by RT assay as already described. The 50% inhibitory concentration (IC50) was derived from the computer generated median effect plot of the dose-effect [program: therapeutic and safety indexes, 35]. The cytotoxicity of the drugs was evaluated in parallel by incubating uninfected cells in the presence of different concentrations of antiviral products. The cell viability was determined by a measure of mitochondrial dehydrogenase activity, enzymes reducing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into formazan (whose quantity was measured by the absorbance at 540 nm) [Citation36]. The 50% cytotoxic concentration (CC50) is the concentration of drug which reduces cell viability by 50% and was calculated with the program used in the determination of the IC50. The assays using different cells and virus isolates were done according to previously published protocols [Citation34,Citation37]; virus production was quantified by the RT activity associated to virus particles released from the cells in the culture medium. Conditions in which the inhibitory properties were measured on HIV-1 Reverse Transcriptase in vitro has also been described [Citation34]. The CEM-SS cells were obtained from P. Nara through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (Bethesda, Md., USA).
Results and discussion
Chemistry
The target prodrugs of the general formula [DKA]-Alanyl-PABC-[d4T] (7a–c) and [DKA]-Alanyl-OABC-[d4T] (8a–c) described in the present study were prepared by attaching the nucleoside inhibitor moiety to the spacer end of the IN inhibitor-spacer moieties. The synthetic method for these target compounds involved the formation of the key precursors: the NRTI (d4T) [Citation38] and the DKAs synthesized according to the method described in our previous paper [Citation24].
The synthesis was started by the coupling of the ester-protected amino acid residue (L-alanine) to the benzylated DKA 1a–c with benzotriazol-1-yloxytris(dimethylamino)-phosphonium hexafluorophosphate (BOP) and 1-hydroxybenzotriazole (HOBT) in the presence of triethylamine (Scheme ). A first attempt was achieved for the formation of peptide linkage between the DKA 1a and L-alanine methyl ester hydrochloride in the presence of EDDQ (2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline) used as coupling agent and the expected product 2a was isolated in moderate yield (69%). The resulting intermediate esters 2a–c were hydrolyzed under mild basic conditions (LiOH in THF/methanol) to give the free acids 3a–c in 38 to 81% yields.
Then, the selective insertion of a p- or o-aminobenzyl alcohol (PAB-OH and OAB-OH) spacer between the [DKA]-Alanine 3a-c and the NRTI (d4T) was carried out in anhydrous THF under an argon atmosphere in the presence of EEDQ inducing the formation of peptide linkage and the expected products 4a–c and 5a–c were isolated (30 to 78%) [Citation39].
Finally, the activated carbonate d4T 6 was linked directly to the DKAs functionalized with respectively the p- and o-aminobenzyl alcohol spacers 4a–c and 5a–c. Thus, the desired activated d4T-carbonate 6 was prepared by treatment of d4T with 1.5 equiv. of 4-nitrophenyl chloroformate in the presence of pyridine in a reasonable yield (79%) [Citation24,Citation40]. The final coupling step was performed subsequently by addition of activated d4T-carbonate 6 to a solution of the intermediates 4a–c and 5a–c in anhydrous dichloromethane in the presence of DMAP and a catalytic amount of N,N-diisopropylethylamine to yield the expected target prodrugs 7a–c and 8a–c isolated after purification by silica-gel chromatography (13 to 38%).
Biological
The synthesized target bis-substrate molecules [DKA]-Alanyl-PABC-[d4T] (7a–c) and [DKA]-Alanyl-OABC-[d4T] (8a–c) were evaluated in vitro for their ability to inhibit replication of HIV-1 in two human T-4 lymphoblastoid cell lines CEM-SS and MT-4 (). RT inhibition was also evaluated in peripheral blood mononuclear (PBM) cells infected with HIV-2 D194 strains. The clinically used nucleoside analogues AZT and d4T were included as reference materials. For the purpose of reference, data for precursors methyl [DKA]-alanylate (2a–c), the free acids [DKA]-alanine (3a–c) are also displayed. We also include corresponding data for the [DKA]-alanyl-PAB-OH (4a–c) and [DKA]-alanyl-OAB-OH (5a–c) precursors. As the data displayed in , all active compounds proved to be more potent in the CEM model system; also in order to rationalize the analysis, we concentrated on these results.
Table I. Antiviral and Cytotoxicity Evaluation of Precursors 2a–c, 3a–c, 4a–c and 5a–c and the conjugates [INI]-spacer-[d4T] (7a–c and 8a–c) against Selected HIV Strains.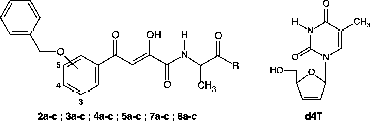
Surprisingly, all the bis-substrate molecules [DKA]-Alanyl-PABC-[d4T] (7a–c) (IC50 0.52 to 1.48 μM in CEM-SS cells) and [DKA]-Alanyl-OABC-[d4T] (8a–c) (IC50 1.95 to 5.05 μM) showed no notable elevation in antiviral potency against HIV-1 compared to the corresponding prototype heterodimers [DKA]-PABC-[d4T] (IC50 0.43 to 1.60 μM) and [DKA]-OABC-[d4T] (IC50 1.47 to 4.67 μM) published in a previous paper [Citation24]. In fact, the prototype heterodimer A [DKA]-PABC-[d4T] showed a marked anti-HIV activity of 0.43 μM in CEM-SS cells.
As the data in showed, relative to d4T (IC50 0.26 μM against HIV-1LAI in CEM-SS cells), only the heterodimer 7b still remained active at submicromolar concentration (IC50 0.52 μM against HIV-1LAI in CEM-SS cells). The heterodimers 7a,c and 8a–c displayed a weak activity in the CEM-SS cells (IC50 1.24 μM to 5.05 μM). Moreover, all prodrugs 7a–c and 8a–c showed a much higher cytotoxicity in CEM-SS cells in comparison with the corresponding free parent drugs 1a–c [Citation24].
Furthermore, it is noteworthy that all the precursors: the methyl [DKA]-alanylate (2a–c), the free acid [DKA]-alanine (3c), the [DKA]-alanyl-PAB-OH (4a–c) and [DKA]-alanyl-OAB-OH (5a–c) were unfortunately devoid of anti-HIV activity in the CEM-SS and MT-4 cells (IC50>10 μM) or displayed a weak activity depending on the nature of the cells, at least in the CEM-SS cells (IC50 6.4 μM for compound [DKA]-alanyl-OAB-OH (5a). Particularly striking were the free acids [DKA]-alanine (3a,b) since they displayed IC50 respectively 11.4 μM and 13.1 μM in CEM-SS cells by comparison to the free drugs: the DKAs 1a,b (IC50 0.58 μM and 1.49 μM against HIV-1LAI in CEM-SS cells) [Citation24].
In conclusion, these results were disappointing since the novel bipharmacophore drugs associating in a single molecule a NRTI covalently linked to an INI through a self-cleavable (PABC or OABC) spacer incorporating an enzymatically labile aminoacid unit (L-alanine) did not increase the inhibitory efficacy. Although the results were not as great as expected, they could nevertheless be useful to draw at least some preliminary structure-activity relationships (SARs). The first interesting fact that emerged was that in the final compounds (7a–c and 8a–c), the 7 series displayed better activities. These results were clearly related to the position of the substitution on the d4T side of the spacer. On the other hand, in both family of prodrugs, the b series exhibited remarkable anti-HIV activity (IC50 1.52 and 1.95 μM in CEM-SS cells) that was nearly three times more potent than those of the 7 and 8 a and c series. This result led us to presume that the meta-benzyloxyphenyl was the best substitution on the DKA moiety of the prodrugs.
However, considering that these prodrugs revealed more cytotoxic than d4T, the results suggested an effective intracellular hydrolysis of the prodrugs as their cytotoxicity might be due to the partial release of the [DKA]-Alanyl-PAB-OH or [DKA]-Alanyl-OAB-OH moieties and d4T inside the cells.
Conclusion
In summary, we report in this paper on novel derivatives of the prototype Heterodimer A [L-708,906]-PABC-[d4T] by modifying the spacer. Such compounds were designed as membrane-soluble prodrugs. Several members of this class of compounds show potent anti-HIV-1 activity comparable to those of the prototype heterodimer accompanied unfortunately by an increase in cytotoxicity.
Acknowledgements
We thank “Ensemble Contre le Sida” (Sidaction) for financial support.
References
- De Clercq E. J Biochem Cell Biol 2004; 36: 1800–1822
- De Clercq E. J Med Chem 1995; 38: 2491–2517
- De Clercq E. Nat Rev Drug Discovery 2002; 1: 13–25
- Imamichi T. Curr Pharm Des 2004; 10: 4039–4053
- Richman DD. Nature 2001; 410: 995–1001
- Dayam R, Neamati N. Biochemistry of HIV therapeutics. Encyclopedia of molecular cell biology and molecular medicine. Wiley-VCH, Weinheim, Germany 2004; 155–178
- Barbaro G, Scozzafava A, Mastrolorenzo A, Supuran CT. Curr Pharm Des 2005; 11: 1805–1843
- Cohen J. Science 2002; 296: 2320–2324
- Mocroft A, Lundgren JD. Antimicrob Chemother 2004; 54: 10–13
- Shehu-Xhilaga M, Tachedjian G, Crowe SM, Kedzierska K. Curr Med Chem 2005; 12: 1705–1719
- Pommier Y, Neamati N. Adv Virus Res 1999; 52: 427–458
- Asante-Appiah E, Skalka AM. Adv Virus Res 1999; 52: 351–369
- Nair V. Rev Med Virol 2002; 12: 179–193
- Hazuda DJ, Young SD. Adv Antiviral Drug Des 2004; 4: 63–77
- Pommier Y, Johnson AA, Marchand C. Nat Rev Drug Disc 2005; 4: 236–248
- Goldgur Y, Craigie R, Cohen GH, Fujiwara T, Yoshinaga T, Fujishita T, Sugimoto H, Endo T, Murai H, Davies DR. Proc Natl Acad Sci U.S.A 1999; 96: 13040–13043
- Hazuda DJ, Felock P, Witmer M, Wolfe A, Stillmock K, Grobler JA, Espeseth A, Gabryelski L, Schleif W, Blau C, Miller MD. Science 2000; 287: 646–650
- Espeseth AS, Felock P, Wolfe A, Witmer M, Grobler J, Anthony N, Egbertson M, Melamed JY, Young S, Hamill T, Cole JL, Hazuda DJ. Proc Natl Acad Sci U.S.A 2000; 97: 11244–11249
- Fujishita T, Yoshinaga T. WO 9950245. 1999
- Wai JS, Egbertson MS, Payne LS, Fisher TE, Embrey MW, Tran LO, Melamed JY, Langford HM, Guare JP, Jr, Zhuang L, Grey VE, Vacca JP, Holloway MK, Naylor-Olsen AM, Hazuda DJ, Felock PJ, Wolfe AL, Stillmock KA, Schleif WA, Gabryelski LJ, Young SD. J Med Chem 2000; 43: 4923–4926
- Grobler JA, Stillmock K, Hu B, Witmer M, Felock P, Espeseth AS, Wolfe A, Egbertson M, Bourgeois M, Melamed J, Wai JS, Young S, Vacca J, Hazuda DJ. Proc Natl Acad Sci U.S.A 2002; 99: 6661–6666
- Pais GCG, Zhang X, Marchand C, Neamati N, Cowansage K, Svarovskaia ES, Pathak VK, Tang Y, Nicklaus M, Pommier Y, Burke TR, Jr. J Med Chem 2002; 45: 3184–3194
- Zhang X, Pais GCG, Svarovskaia ES, Marchand C, Johnson AA, Karki RG, Nicklaus MC, Pathak VK, Pommier Y, Burke TR. Bioorg Med Chem Lett 2003; 13: 1215–1219
- Fossey C, Vu A, Zarafu I, Ladurée D, Schmidt S, Laumond G, Aubertin AM. J Enz Inhib Med Chem 2006
- Cass CE, Young JD, Baldwin SA. Biochem Cell Biol 1998; 76: 761
- Ralevic V, Burnstock G. Pharmacol Rev 1998; 50: 413
- Chan TCK, Shaffer L, Redmond R, Pennington KL. Biochem Pharmacol 1993; 46: 273
- Domin BA, Mahony WB, Zimmerman TP. Biochem Pharmacol 1993; 46: 725
- Mansuri MM, Starrett JE, Ghazzouli I, Hitchcock MJM, Sterzycki RZ, Brankovan V, Lin T, August EM, Prusoff WH, Sommadossi J, Martin JC. J Med Chem 1989; 32: 461–466
- Shiragami H, Uchida Y, Izaw K. Nucleosides & Nucleotides 1996; 15: 47–58
- Lin T, Schinazi RF, Prusoff WH. Biochem Pharmacol 1987; 36: 2713–2718
- Balzarini J, Kang GJ, Dalal M, Herdewijn P, Broder DeClercqE, Johns DG. Mol Pharmacol 1987; 32: 162–167
- Browne MJ, Mayer KH, Chafee SB, Dudley MN, Posner MR, Steinberg SM, Graham KK, Geletko SM, Zinner SH, Denman SL, Dunkle LM, Kaul S, Mc Laren C, Skowron G, Kontlab NM, Kennedy TA, Weitberg AB, Curt GA. J Infect Dis 1993; 167: 21–29
- Moog C, Wick A, Le Ber P, Kirn A, Aubertin AM. Antiviral Res 1994; 24: 275–288
- Chou J, Chou TC. Computer Software for Apple II Series and IBM-PC and Instruction Manualp (Elsevier-Biosoft, Cambridge): 19–28
- Mosmann T. J Immunol Meth 1983; 65: 55–63
- Lefebvre I, Perigaud C, Pompon A, Aubertin AM, Girardet JL, Kirn A, Gosselin G, Imbach JL. J Med Chem 1995; 38: 3941–3950
- Joshi BV, Rao TS, Reese CB. J Chem Soc Perkin Trans 1992; I: 2537–2544
- Belleau B, Malek GA. J Am Chem Soc 1968; 90: 1651–1652
- Hay MP, Wilson WR, Denny WA. Tetrahedron 2000; 56(4)645–657
![Figure 2 Modifications carried out on the [L-708,906]-PABC-[d4T] heterodimer prototype.](/cms/asset/b8f26943-5ac3-4509-951f-20848c1176ab/ienz_a_242424_f0002_b.gif)
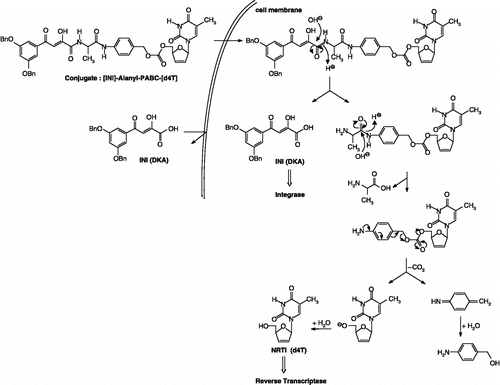
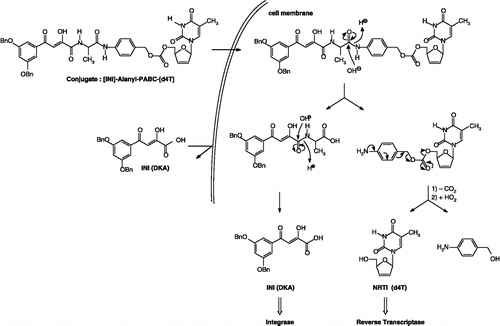
![Scheme 1 Synthesis of [DKA]-Alanyl-PABC-[d4T] (7a–c) and [DKA]-Alanyl-OABC-[d4T] (8a–c) conjugates.](/cms/asset/9b6d743a-c0d2-4f90-9ff0-ee8e292d7993/ienz_a_242424_f0005_b.gif)