Abstract
The Schiff base ligand, oxalic bis[(2-hydroxybenzylidene)hydrazide], H2L, and its Cu(II), Ni(II), Co(II), UO2(VI) and Fe(III) complexes were prepared and tested as antibacterial agents. The Schiff base acts as a dibasic tetra- or hexadentate ligand with metal cations in molar ratio 1:1 or 2:1 (M:L) to yield either mono- or binuclear complexes, respectively. The ligand and its metal complexes were characterized by elemental analyses, IR, 1H NMR, Mass, and UV-Visible spectra and the magnetic moments and electrical conductance of the complexes were also determined. For binuclear complexes, the magnetic moments are quite low compared to the calculated value for two metal ions complexes and this shows antiferromagnetic interactions between the two adjacent metal ions. The ligand and its metal complexes were tested against a Gram + ve bacteria (Staphylococcus aureus), a Gram -ve bacteria (Escherichia coli), and a fungi (Candida albicans). The tested compounds exhibited high antibacterial activities.
Introduction
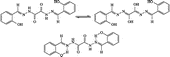
Considering the constant emergence of antibiotic resistance to clinically used compounds, it is of critical importance to develop novel antibiotic classes, which eventually would target the lipoid layer of the organisms and other aspects of the pathogen life cycle. Metal complexes constitute one such possible class with biological activity [Citation1,Citation2]. Schiff bases play an important role in inorganic chemistry as they easily form stable complexes with most transition metal ions. The development of the field of bioinorganic chemistry has increased the interest in Schiff base complexes, since it has been recognized that many of these complexes may serve as models for biologically important species Citation2-7. The remarkable biological activity of acid hydrazides, a class of Schiff base, their corresponding aroylhydrazones, and the dependence of their mode of chelation with transition metal ions present in the living system have been of significant interest Citation7-14. The coordination compounds of hydrazide and aroylhydrazones have been reported to act as enzyme inhibitors and are useful due to their pharmacological applications.Citation6-13Citation14. Isonicotinic acid hydrazide (INH) is a drug of proven therapeutic importance and is used as in bacterial diseases, e.g., tuberculosis [Citation15]. Hydrazones derived from condensation of isonicotinic acid hydrazide with pyridine aldehydes have been found to show better antitubercular activity than INH.[Citation13]. Tri- and terdentate Shiff bases may contain ONO or ONS donor atoms and their metal complexes may be monomeric, dimeric, trimeric or tetrameric with abnormal magnetic properties and characteristic structures Citation8-16. The synthesis and biological activity of the Zn(II)-complex of the Schiff base, oxalic bis[(2-hydroxybenzylidene)-hydrazide], has been previously studied [Citation16]. The complex has the formula [Zn(L)Cl2] () in which the Schiff base functions as a tetradentate ligand [Citation16]. The present work was undertaken in order to throw light on the ligational behaviour of the Schiff base derived from oxalylhydrazide towards other metal ions such as Cu(II), Ni(II), Co(II), UO2(VI) and Fe(III) as well as their biological activity in inhibiting the growth of some pathogenic bacteria.
Experimental
Materials
CuCl2.2H2O, NiCl2.6H2O, CoCl2.6H2O, FeCl3.6H2O, UO2(OCOCH3)2.2H2O were obtained from BDH. Oxaloyldihydrazide, salicylaldehyde were either BDH or Merck products and were used without further purification. Organic solvents used were reagent grade.
Preparation of the schiff base ligand, H2L
The ligand was prepared according to the previous reported method [Citation14]. Salicylaldehyde (2.4 g., 20 mmol, dissolved in 10 mL absolute ethanol) was added to a stirred hot ethanolic solution of oxaloyldihydrazide (1.2 g, 10 mmol dissolved in 30 mL absolute ethanol). Then 2–3 drops of conc. H2SO4 were added and the reaction mixture was refluxed for 8 h. The white solid precipitated was filter off, wash with 5 mL ethanol, and then recrystallized from ethanol (yield 70%, m.p. 169°C).
Preparations of the metal complexes
Preparation of the mononuclear metal complexes
Metal salt (3 mmol in 10 mL ethanol)) [CuCl2.2H2O, NiCl2.6H2O, CoCl2.6H2O, FeCl3.6H2O or UO2(acetate).2H2O] was heated on a water bath to ensure complete dissolution of the metal salt. To this solution, the ligand (0.978 g, 3 mmol in 10 mL ethanol) was added gradually. The reaction mixture was refluxed for 3 h with constant stirring. The precipitated coloured solid complexes were filtered, washed several times with 50% (v/v) ethanol-water mixture to remove any traces of the unreacted starting materials, then washed with diethyl ether and dried in a vacuum desiccator over CaCl2. The obtained solid metal complexes and their colours are shown in . The complexes are stable solids, which decomposed above 250°C without melting and they are insoluble in common organic solvents such as ethanol, methanol, chloroform acetone but soluble in DMSO and DMF.
Table I. Characteristic of H2L and its corresponding mono- and binuclear metal complexes.
Preparation of the binuclear metal complexes
Metal salt (6 mmol in 20 mL ethanol)) [CuCl2.2H2O, NiCl2.6H2O, CoCl2.6H2O, FeCl3.6H2O or UO2(acetate).2H2O] was heated on a water bath to ensure complete dissolution of the metal salt. The metal salt solution was added gradually to a stirred hot solution of the ligand (0.978 g, 3 mmol in 10 mL ethanol). The reaction mixture was refluxed for 12 h under constant stirring. The precipitated solid complexes were filtered, washed several times with 50% (v/v) ethanol-water mixture to remove any traces of the unreacted starting materials, then washed with diethyl ether and dried in air. The obtained metal complexes and their colours are shown in . The complexes are stable solids, which decomposed above 250°C. They are insoluble in common organic solvents such as ethanol, methanol, chloroform and acetone but soluble in DMSO and DMF.
Physical measurements and analyses
Reflectance spectra of the ligand and its metal complexes were recorded as BaSO4 discs using a model 1601 Shimadzu UV-Visible in the range 190–1100 nm. The solution of the ligand, in ethanol was recorded on a Jasco V-550 UV-Visible spectrometer in the range 200–900 nm. IR spectra were recorded as CsI discs using a FT-IR 4000 Perkin Elmer Spectrometer. 1H NMR spectra were carried out in DMSO-d6 at room temperature using TMS as internal standard on a Brucker 250 MHz spectrophotometer. Magnetic susceptibilities of the complexes were measured by the Gouy method at room temperature using a model MK1 Johnson Matthey Alpha products magnetic susceptibility balance. The effective magnetic moments were calculated using the relation (μeff = 2.828 (χm T)½ B.M. where χm is the molar susceptibility corrected using Pascal's constants for diamagnetism of all atoms in the compounds. The TG-DTA measurements were carried out on a Shimadzu thermo gravimetric analyzer in dry nitrogen atmosphere and a heating rate of 10°C/min using the TA-50 WS1 program. Mass spectra were recorded at 70 eV and 300°C on an MS 5988 Hewlett-Packard mass spectrometer. Conductivities were measured in DMF solutions of the complexes (10− 3 M) using a model LBR, WTWD-812 Wilhelm Conductivity meter fitted with a model LTA100 cell. Analyses of the metals followed decomposition of their complexes with concentrated nitric acid. The resultant solution was diluted with distilled water, filtered to remove the precipitated ligand. The solution was then neutralized with aqueous ammonia solution and the metal ions titrated with EDTA. Analysis of the uranyl complex was carried out at the Central Laboratory for Environmental Quality Monitoring, CLQM, Kalubia, Cairo, Egypt. The complex was first dried and ground followed by digestion by nitric-HF digestion mixture using Milestone Microwave Digester Model MLS 1200 Mega. The digestible uranium metal was analyzed using a Perkin Elmer ICP OES, Model Optima-3000 coupled with an Ultra Sonic Nebulizer, USN. Microanalyses of carbon, hydrogen, nitrogen and chlorine were carried out at the Micro analytical Center, Cairo University, Giza, Egypt. Chlorine in the Fe(III) complexes was determined by ion chromatography using a (1C) Dionex 500 instrument for anions at the Central Laboratory for Environmental Quality Monitoring, El-Kanater, Cairo, Egypt.
Pharmacology
The in vitro evaluation of antimicrobial activity was carried out at Saudi Pharmaceutical industries and Medical Appliance Corporation. The purpose of the screening program was to provide the antimicrobial activity and bacteriostatic and fungistatic efficiency of the investigated metal complexes. The prepared compounds were tested against one strain of Gram + ve bacteria (Staphylococcus aureus), Gram − ve bacteria (Escherichia coli) and fungi (Candida albicans) to provide the MIC's (Minimum inhibitory concentration) for each complex. Bacteriostatic and fungistatic efficiency is the lowest concentration of solution which inhibits the growth of a test organism.
Results and discussion
Characterization of the ligand
The Schiff base () was prepared by the reaction of salicylaldehyde with oxaloyldihydrazide in a 2:1 molar ratio [Citation14]. Elemental analysis data shared that the ligand has the molecular formula given in . The 1H NMR spectrum () of the ligand in deuterated DMSO-d6 showed that the aromatic proton signals appeared at δ 6.8–7.3 ppm and the CH = N proton signals at δ 7.9 ppm as expected Citation16-21. The signals of the NH and the phenolic OH protons appeared at δ 16.1 and 11.1 ppm, respectively [Citation16,Citation20,Citation21].
Table II. 1H NMR data for the ligand HL in DMSO-d6.
The IR spectrum of the ligand () shows a weak broad absorption band at 3530 cm− 1 assigned for νOH of the phenolic groups. The stretching vibration [Citation20,Citation21] of NH and the enolic groups originating through tautomerism (Figure 2) appeared as weak bands at 3176 and 3107 cm− 1, repectively. The low value of the latter bands and their weak intensities are due to H-bonding, which also indicates the tautomerism between the keto and enol forms leading to a conjugated system. H-Bonding appears as a series of weak absorption bands in the range 2414-2330 cm− 1. Intramolecular H-bonding occurs between the hydrogen atoms of the phenolic OH groups, the imine NH and the oxygen atom of the ketonic groups. The conjugation system of the ligand leads to a decrease in the absorption frequencies of both the ketonic and the azomethine groups. This may be attributed to a decrease of their band orders, and they are better represented as ,
and
bonds. The stretching vibrations of these bands appear at 1677, 1558 and 1541 cm− 1, respectively [Citation20,Citation21]. ν(C–O) of the phenol moiety appears at 1386 cm− 1 and ν(C–N) appears at 1336 cm− 1. The deformation vibration [Citation20,Citation21], δ, of the phenolic OH group appears at 1228 cm− 1.
Table III. Infrared frequencies of the ligand and its metal complexes.
The UV-VIS spectrum of the solid ligand showed two bands at 226 and 365 nm and a shoulder at 422 nm. Its ethanolic solution spectrum showed three absorption bands at 244, 360 and 394 nm. The differences between the solid and the solution spectra of the ligand are due to the solvent effect. It is also noted that as the extent of conjugation increases, the wavelength of the maximum absorption encroaches on the visible region [Citation22,Citation23]. The first band in the solution spectrum would be assigned to transition of the aromatic rings, the second and third bands would be due to
transition within the C = O and C = N groups [Citation20,Citation21] ().
Table IV. Magnetic moment, electronic and conductance measurements spectral data (nm) for H2L and its metal complexes.
The mass spectrum of the ligand showed its molecular ion at m/e = 326 which coincides with formula weight. Metastable ion(s) were not observed [Citation20,Citation21].
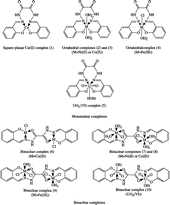
Elemental analyses and IR spectral data of the ligand and its mononuclear (1–5) and binuclear metal complexes (6–10) are collected in Table. 1. The results showed that the H2L coordinated with the metal ions as dibasic tetra- or hexadentate depending upon the molar ratios of M:L.
Metal complexes
The Schiff base ligand H2L is dibasic and behaves as tetradentate or hexadendate containing O2N2 or O4N2 coordination sites depending upon the molar ratios of M:L. In the molar ratio of 1:1 (M:L), it behaves as dibasic tetradentate yielding mononuclear metal complexes as shown in . On the other hand, binuclear metal complexes were obtained using molar ratios of 2:1 (M:L) in which the ligand acts as dibasic hexadentate ().
All metal complexes have octahedral configuration around the metal ions except the Cu(II) and UO2(IV) complexes. The Cu(II)-complex has a square-planar configuration distorted toward tetrahedral while the UO2(IV)-complex has its favour hepta-coordination.
IR spectra of the metal complexes
Mononuclear metal complexes
The IR spectra of the mononuclear complexes (1–5) (Table 3) showed that the band due to the phenolic OH group that appeared in the spectrum of the ligand at 3530 cm− 1, disappeared in the spectra of these complexes. This may be due to the displacement of its proton by the metal ion. Moreover, the spectra showed that the vibrations of the –C = N group were shifted to a lower frequency due to the coordination of the nitrogen atom of the azomethine group. The bands due to the imine, NH and C = O groups which appeared at 3176 and 1677 cm− 1 in the IR spectrum of the parent ligand, respectively, remained in the IR spectrum of the complexes. These bands were not significantly affected by complex formation, indicating the non-participation of these groups in complex formation. As a result, the Schiff base hydrazone ligand, H2L, coordinates in these complexes as monobasic tetradentate with O2N2 coordination sites via the oxygen atoms of the phenolic OH and the nitrogen atoms of the azomethine groups [Citation24].
Binuclear metal complexes
The IR spectra of the binuclear metal complexes (6–10) (Table III) showed a broad band at ≈ 3440– 4000 cm− 1 due to v(OH) of the coordinated water or alcohol molecules which replaced the band of the phenolic OH groups observed in the spectrum of the parent ligand. Moreover, the bands due to the imine NH groups remained. The bands due the C = N and C = O groups are shifted to lower frequencies, indicating the participation of these groups in complexation. As a result, the Schiff base ligand, H2L, coordinates in these complexes as dibasic hexadentate with O4N2 coordination sites via the oxygen atoms of the phenolic OH, the nitrogen atoms of the azomethine and the oxygen atoms of the carbonyl groups as shown in . In all spectra, new bands appeared, for all types of the complexes, at 515–540 and 410–465 cm− 1 that would be assigned to νM–O and νM–N, respectively [Citation24].
The IR spectra of the mono- and binuclear UO2(VI) complexes, Table 3, showed a broad band at 3440 cm− 1 assigned to ν(OH) of the coordinated ethanol group. ν(NH) of the uncoordinated NH groups appeared as a shoulder at 3176 cm− 1, exactly at the same frequency as for the parent ligand. However, no splitting of this band was observed, which may be due to the larger separation of the two ligand moieties, due to the larger volume of the U02(VI) cation in the complex molecules. Well-characterized bands appeared at 1517 and 1654 cm− 1 assigned to the coordinated C = N and C = O groups. The bands occur at a lower frequency compared to that of the parent ligand indicating its involvement in coordinating the UO2(VI) cation in addition to the phenolic oxygen atoms after replacing their hydrogen ions by the uranyl(VI) cation. The ν3(O = U = O) appeared as a strong band at 901 cm− 1 overlapping with another band already present in the spectrum of the parent ligand and thus gaining higher intensity [Citation23,Citation24].
The IR spectrum of the binuclear Fe(III) complex, Table 3, showed a broad band at 3400 cm− 1 due to ν (OH) of the outer-sphere ethanol molecules. Another band appeared at 1515 and 1649 cm− 1 due to the C = N and C = O groups, respectively. These bands are shifted to lower frequency compared to that of the parent ligand. This shift would be due to the effect of the tripositive ferric ion on decreasing the force constant of the C = N and C = O bonds.
Magnetic moments and electronic spectral data of the metal complexes
The reflectance spectra and magnetic moments data of the metal complexes are listed in Table 4. Generally, in all spectra of the metal complexes, the absorption bands due to π–π* and n–π* transitions observed in the spectrum of the free ligand at higher energy have shifted to lower frequencies due to coordination of the ligand with metal ions.
The reflectance spectra of the Cu(II) complexes (1 and 6) (Table 4) showed absorption bands at > 345, > 311, > 330, > 358 and > 665 nm. The first four bands are due to the ligand absorption which are shifted from those of the parent ligand due to complex formation. The bands at > 665 nm are due to attributed to the
transitions characterized by Cu(II) ion in a square-planar geometry [Citation24,Citation25,Citation26]. The shift of the
absorption band to lower energy than that expected for square-planar geometry, at 550 nm for square-planar N,N′-ethylene bis-(salicylideneimine)copper(II), Cu(acacen) [Citation24] may be due to the distortion of the square-planar geometry towards tetrahedral.[Citation24,Citation25,Citation26]. The square-planar geometry of Cu(II) ions in the complexes 1 and 6 are confirmed by the measured magnetic moments values, 1.76 and 2.82 B.M. respectively. The value of 2.8 B.M. for the binuclear complex, 6, lower than the expected value of two Cu(II) ions, may be due to antiferromagnetic interactions between the adjacent Cu(II) ions in the complex. The square-planar geometry, in complex 1, is achieved by the coordination of Cu(II) ion with one molecule H2L through O2N2 coordination sites. However, in complex 6, two Cu(II) ions coordinated to one molecule of the ligand each through O2N coordination sites complete its square-planar geometry by the coordination of a chloride ion [Citation24,Citation25,Citation26].
The reflectance spectrum of the mononuclear Ni(II) complex (2), , showed a main band at 768 nm and a shoulder at 670 nm. The main band may be due to electronic transition and may be overlapped [Citation25,Citation26] by the ligand transitions, which appeared at 355 nm. The third transition,
is out of the scale of the spectrophotometer used. The magnetic moment of the complex is 3.19 B.M. which agrees with the presence of Ni(II) ion in an octahedral geometry [Citation22,Citation25,Citation26] This indicates that the Ni(II) ion is coordinated to O2N2 sites in an octahedral geometry [Citation25,Citation26,Citation27]. The Ni(II) ion completes its six-coordination sphere by two water molecules.
The reflectance spectrum of the binuclear Ni(II) complex (7) showed a diffuse band in the range 659–837 nm. This indicates that each Ni(II) ion is coordinated to the ligand through O2N sites in an octahedral geometry [Citation24,Citation25,Citation26]. Each Ni(II) ion completes its six-coordination sphere by two water molecules and chloride ion. The magnetic moment of this complex is 4.44, which is smaller than the calculated value for two Ni(II) ions in octahedral geometries and may indicate antiferromagnetic interactions between adjacent Ni(II) ions in the complex [24.15].
Octahedral, tetrahedral and square-planar cobalt (II) complexes show magnetic moments between 4.7–5.2, 4.2–4.8 and 2.2–2.9 B.M., respectively [Citation25,Citation26]. The μeff. values for the present Co(II) complexes (3 and 8), , are 5.2 and 7.52 B.M. The magnetic moment of binuclear complex (8) is 7.52 B.M., which is smaller than the calculated value for two Co(II) ions in octahedral geometries and may indicate antiferromagnetic interactions between adjacent Co(II) ions in the complex. The reflectance spectra of complexes 3 and 8 showed the d-d transition, at 656 and 675 nm, respectively. The two shoulders observed in both spectra at 477 and 414 nm may be due to
transition indicating that Co(II) ion is coordinated to O2N2 (complex 1) or O2N (complex 8) coordination sites of the ligand. In complex 8, each Co(II) ion completes its six-coordination sphere with two water molecules and chloride ion.
On the other hand, the reflectance spectra of Fe(II) complexes (4 and 9) showed broad bands at 552 and 392 nm. These bands may be due to the spin forbidden transition and
, 4E(G) electronic transitions [Citation24] which may gain their intensity as a result of the vibronic mechanism in the octahedral field around ferric ion. The magnetic moment of complex 9 is 5.94 B.M. and that of mononuclear Fe(III) complex 4, is 4.20.B.M. These values indicate the presence of antiferromagnetic interactions between adjacent Fe(III) ions in complex 9 [Citation25,Citation26].
The reflectance spectra of the diamagnetic uranyl complexes 5 and 10, in addition to the ligand bands, showed bands at 580 and 576 nm, respectively, imparting the complexes their colours. The bands observed at 523 and 530 nm are due to the electronic transitions from apical oxygen atom to the f-orbitals of the uranyl atom characteristic of the uranyl moiety. Both complexes are diamagnetic [Citation25,Citation26].
Molar conductance of the metal complexes
The conductance measurements, recorded for 10− 3 M solutions of the metal complexes in DMF, are listed in . All complexes are non-conducting indicating their neutrality and that the divalent cations have replaced the phenolic and protons. However, the Fe(III) complexes (4 and 9) showed an appreciable amount of conductance which may be through the replacement of a part of the coordinated chloride ions by solvent molecules as previously reported [Citation24]. A phenomenon usually encountered in complexes containing chloride ions.
1H NMR spectrum of the uranyl complexes
The uranyl complexes (5 and 10) were selected as they are diamagnetic. Their 1H NMR spectra in DMSO-d6 and after deuteration are discussed. The spectra of the complexes differ from that of the free ligand in the following aspects:
The disappearance of the signal due to the phenolic OH groups, in both spectra, is attributed to its involvement in coordinating the uranyl cation, while the signals due to the NH and –C = H groups remained in both spectra, however, with an upfield shift. This may be due to the metal ion indicating that the ligand H2L acts as a dibasic ligand.
The signals due to the aromatic ring showed fine structure and appeared as four separate signals at δ = 7.14, 7.53 and 7.6 ppm.
The NH group which did not take part in coordinating the uranyl cation disappeared on deuteration.
Thermal analyses
The TG-DTA results for the solid complexes 1–10 are listed in . The results show good agreement with the formulae suggested from the analytical data, Table 1. A general decomposition patterns was concluded in which the complexes decomposed in three stages. Beside these three stages, complexes, which have coordinated water, ethanol or chloride ion exhibited an additional stage and were decomposed in four stages (Table 5). Complexes with outer sphere ethanol molecules loose these at 61–110°C. The second stage is the loss of the coordinated water or solvent molecules at 130 to 250°C. The third to four stages represented the loss of the rest of the organic moiety and, finally, the formation of metal oxides.
Table V. Thermal analyses data for some metal complexes of H2L.
Antimicrobial activity and minimum inhibitory concentration, MIC
The tests were performed according to the method previously reported [Citation29]. 0.02 g of each complex was dissolved in 100 mL dimethylsulfoxide, DMSO, to produce 0.02% solutions. To a series of culture tubes containing sterile 5 mL double strength solution of Soyabean Casein Digest Medium (Tryptic Soy Broth), 5 mL of the 0.02% DMSO solution was added and mixed. To determine the bacteriostatic efficiency towards Gram + ve bacterium (Staphylococcus aureus) and the Gram − ve bacterium (Escherichia coli), 1 mL of a 1:10 diluted solution of Tryptic Soy Broth (TSB) (prepared by pipetting 1 mL of bacterial) was added to each culture tube cultures incubated at 37°C for 24 h into 9 mL of sterile Tryptic Soy Broth. For the test for fungistatic efficiency, 0.1 mL of undiluted sample was incubated for 72 h at 37°C with a TSB cultivation of Candida albicans. All inoculated culture tubes were incubated at 30–35°C for 18–24 h. After that, the “MIC” level was assessed visually. MIC was recorded as the first clear tubes after turbidity, starting with the blank broth i.e. the highest dilution of the antiseptic/disinfectant preventing growth is taken as the “MIC” of the test organism. Citation8-10Citation28Citation29.
The tests were conducted for a series of concentrations of 1, 25, 50, 100% DMSO solutions obtained by diluting the stock 0.02% DMSO of each tested compound. The inhibition zones caused by the various compounds on the microorganisms were examined. The results of the preliminary screening test are listed in . Generally, the results showed that all tested compounds exhibited MIC at a concentration of 25%. However, only two complexes, 4 and 9, exhibited MIC at a concentration of 1% reflecting a higher cytotoxic effect. The Schiff base H2L was found to be biologically active and its metal complexes showed significantly enhanced antibacterial activity against one or more bacterial strains, in comparison to the parent ligand from which they were derived. All metal complexes were found to be the highly active against all organisms and in all concentration while the ligand, H2L, exhibited its activity at 50 and 100% conc. for S. aureus and at 25, 50 and 100% for E. coli and did not affect the other tested strain organism, Candida albicans, at any concentration tested. A remarkable result is that the complexes, 1 and 2, were found to have high activity against all strains at most of the concentrations used. On the other hand, complexes 5, 8, and 10 were found to have a high activity against S. aureus and C. albicans at 25 and 50% concentrations.
Table VI. Anti-microbial activity of the tested complexes.
References
- Rice SA, Givskov M, Steinberg P, Kjelleberg S. Bacterial signals and antagonists: The interaction between bacteria and higher organisms. J Mol Microbiol Biotechnol 1999; 1(1)23–31
- Chohan ZH, Supuran CT. In-vitro antibacterial and cytotoxic activity of cobalt (ii), copper (ii), nickel (ii) and zinc (ii) complexes of the antibiotic drug cephalothin (Keflin). J Enz Inhib Med Chem 2005; 20(5)463–468
- Jayabalakrishnan C, Natarajan K. Synthesis, characterization and biological activities of ruthenium(II) carbonyl complexes containing bifunctional tridentate Schiff bases. Synth React Inorg Met -Org Chem 2001; 31(6)983–995
- Chohan ZH, Humayun P, Abdul Rauf, Khalid MK, Supuran CT. Antibacterial cobalt (II), copper (II), nickel (II) and zinc (II) complexes of mercaptothiadiazole-derived furanyl, thienyl, pyrrolyl, salicylyl and pyridinyl Schiff bases. J Enz Inhib Med Chem 2006; 21(2)193–201
- Chohan ZH, Humayun P, Khalid MK, Supuran CT. Organometallic-based antibacterial and antifungal compounds; trantion metal complexes of 1,1′-diacetylferrocene-derived thio-carbohydrazonee, carbohydrazone, thiosemicarbazone and semicarbazone. J Enz Inhib Med Chem 2006; 21(2)193–201
- Jeeworth T, Wah HLK, Bhowon MG, Ghoorhoo D, Babooram K. Synthesis and antibacterial/catalytic properties of schiff bases and Schiff base metal complexes derived from 2,3-diaminopyridine.Synth Read Inorg Met.-Org. Chem 2000; 30(6)1023–1038
- Dharmaraj N, Viswanalhamurthi P, Natarajan K. Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transition Met Chem 2001; 26: 105–109
- Colins CH, Lyne PM. In Microhiul Melfwds. University Park Press 1970; Baltimore, 422
- Ochiai Ei-ichiro. Bioinorganic chemistry. Allyn and Bacon, Boston 1977
- Chohan ZH, Iqbal HS, Scozzafava A, Supuran CT, Iqbal MS. Transition metal acetylsalicylates and their anti-inflammatory activity. 2002; 17(2,1), 87-9(15)
- Albertini R, Pinelli S, Lunghi P. Synthesis, structural characterization and biological activity of helicin thiosemicarbazone monohydrate and a copper(II) complex of salicylaldehyde thiosemicarbazone. Inorg Chim Acta 1999; 286: 134–141
- Elo H, Lumme P. Studies on the acute toxicity of antineoplastic metal chelate. Trans-bis(salicyaldoximato) copper(II) in rats. Inorg Chim Acta 1987; 136(3)149–153
- Elo H, Lumme P. Trans-bis(salicyaldoximato) copper(II) and its derivatives as antipyroliterative and antineoplastic agents. Inorg Chim Acta 1987; 36(1)61–63
- Ali MA, Kabir MH, Nazimuddin M, Majumder SMH, Tarafder MTH, Akhair M. Synthesis, characterization and antiiungal properties of some four-coordinate nickel(II) and four- and five-coordinate copper(II) complexes containing tridentate thiosemicarbazones and heterocyclic. Ind J Chem 1988; 27A: 1064–1067
- Agarwal RK, Singh L, Sharma DK, Singh R. Synthesis, spectral and thermal investigations of some oxovanadium(IV) complexes of isonicotinic acid hydrazide. Turk J Chem 2005; 29: 309–310
- Chohan ZH, Farooq MA, Scozzafava A, Stupuran CT. Antibacterial Schiff bases of oxalyl-hydrazine/diamide incorporating pyrrol and salicylyl moieties and their zinc(II) complexes. (2002). J Enz Inhib Med Chem 2002; 17: 1–7
- Hill HAO, Zarb-Adami N. Some nickel(II) complexes of n-hydroxyethyl and n-hydroxypropylsalicyaldimines. J Inorg Nucl Chem 1975; 37: 2443–2447
- Salib KAR, Iskak MF, El-Behairy M, Abd El-Halim HF. Synthesis and spectroscopic studies of a novel Schiff base derived from o-acetoacetylphenol and ethylamine and its metal complexes. Synth React Inorg Met -Org Chem 2003; 33: 1667–1687
- Miners JO, Sinn E. Alkoxy and phenoxy dimeric copper (II) complexes with salicylaldimine ligands. Bull Chem Soc Jpn 1973; 46: 1461–1547
- Williams DH, Fleming I. Spectroscopic methods in organic chemistry. McGraw-Hill, New York 1966
- Silverstein RM, Bassler GC, Morrill TC. Spectrometric identification of organic compounds. John Wiley & Sons, New York 1991
- Dyer JR. Applications of absorption spectroscope of organic compounds. Prentice-Hall, London 1965
- Ueno K, Martel AE. Ultraviolet and visible absorption spectra of metal.chelates of bis-acetylacetoneethylenediimine and related compounds. J Phys Chem 1957; 61: 257–261
- Bailer JC, Emeleus HJ, Nyholm R, Trotman-Dickinson AF. Comprehensive inorganic chemistry. Pergamon Press, Oxford 1975; 3, 517, 1153, 1088, 1048
- Figgs BN. Introduction to ligand field. Wiley, New York 1966
- Lever ABP. Inorganic electronic spectroscopy2nd Ed. Elsevier, Amsterdam 1984
- Baldwin ME. Sulphitobis(ethylenediamine)cobalt(III) complexes. J Chem Socl 961; 3123–3128
- Gruber SJ, Harris CM, Sinn E. Metal complexes as ligands. IV. Bi-and tri-nuclear complexes derived from metal complexes of tetradentate salicylaldimines. J Inorg Nucl Chem 1968; 30: 1805–1830
- William H, Stephen V. Theory and Application of Microbiological Assay. Academic Press, San Diego 1989

![Figure 1 Proposed structure of Zn(II) complex, from Ref. [16].](/cms/asset/84599788-08e8-4e1a-b191-3cf93bbc3853/ienz_a_244688_f0001_b.gif)