Abstract
The tyrosine kinase inhibitory action of the derivatives of N-Phenyl-N′-{4-(4-quinolyloxy)phenyl}urea is quantitatively analyzed using multiple regression analysis. The analysis has helped to ascertain the role of different substituents in explaining the observed inhibitory actions of these compounds for two receptors, namely vascular endothelial growth factor receptor 2 (VEGFR-2) and platelet-derived growth factor receptor α (PDGFRα). From a derived significant correlation equation for inhibition of VEGFR-2, it was concluded that a less hydrophobic molecule with ortho-substituent(s), exerting less steric hindrance and para-substituent devoid of hydrogen-bond acceptor property augment the inhibition action. Besides, a 3-substituent transmitting a higher negative field effect is advantageous to improve the pIC50 value of a compound. The correlation equation, derived for the inhibition of PDGFRα, has revealed that a less hydrophobic molecule, having a 3-substituent which transmits a more negative resonance effect, is helpful in raising its activity. Likewise, in the middle phenyl ring, absence of a fluoro substituent augments the inhibitory activity. Based on derived QSAR equations pertaining to VEGF-2 and PDGFα receptors, the drawn guidelines for selection of substituents, may be used to synthesize potent compounds in future.
Introduction
Angiogenesis play an important role in the growth of most solid tumors and the progression of metastasis [Citation1,Citation2]. Recently, it was reported that the growth of many types of solid tumors is suppressed by the specific inhibition of tumor-induced angiogenesis and thus it may prove a novel therapeutic approach against such tumors Citation3-5. The vascular endothelial growth factor (VEGF) is a key angiogenic agent, which is secreted by malignant tumors, which induces the proliferation and the migration of vascular endothelial cells Citation6-10. A number of compounds inhibiting the biological effect of VEGF has been reported and have been evaluated in a clinical trial. These include, antibodies to VEGF [Citation11] or its receptor [Citation12], small molecule receptor tyrosine kinase inhibitors [Citation13,Citation14] such as the 3-substituted indolinones Citation15-18, the 4-anilino- quinazolines Citation19-22 and the anilinophthalazines [Citation23,Citation24]. The latter studies have shown that an antiVEGF antibody exhibits significant effects in patients with colon and renal cancers [Citation25,Citation26].
More recently, a synthetic study was performed [Citation27] with a view to find novel tyrosine kinase inhibitors for VEGF receptor 2 (VEGFR-2). Such inhibitors were identified through the screening with in-house compounds, which were synthesized earlier as platelet-derived growth factor receptor (PDGFR) tyrosine kinase inhibitors Citation28-31. The inhibitory activities of these compounds for VEGFR-2 and PDGFRα phosphorylations were estimated through cell-based assay. The concentration of a molecule required to bring out 50% inhibition of the receptor concerned was expressed as IC50. The initial structure-activity relationship (SAR) study on these compounds was, however, directed only to alteration of the substituents at different positions of the structure but no rationale was provided to reduce the trial-and-error factors. Hence, a quantitative SAR (QSAR) on these analogues has been performed since QSAR not only provides the rationale for drug design but also illuminates their plausible mechanism of action at the molecular level.
Materials and methods
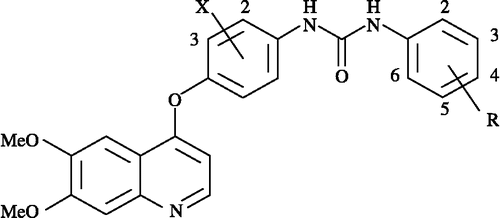
The QSAR analysis was carried out on a reported series [Citation27] comprising of the derivatives of N-Phenyl-N′-{4-(4-quinolyloxy)phenyl}urea. These compounds, having the general structure shown in , along with their biological effects for VEGFR-2 and PDGFRα are compiled in . The biological effects, evaluated by cell-based assay and measured as IC50 for the two receptors, are expressed on the negative logarithmic scale, pIC50, on a molar basis. The suitable quantifying parameters, used to derive final equations, are only listed in this Table. Amongst them, the partition parameter, P, the measure of hydrophobicity of the molecule, was calculated as ClogP using ChemDraw software [Citation32]. Whereas the physicochemical parameters, the Taft's steric, Es, resonance, R, hydrogen-bond acceptor, HA are taken from the literature Citation33-35 and are given in along with an indicator variable, reflecting upon some special structural feature present in the compounds. The subscripted numerals associated to the descriptors represent the varying positions in the titled compounds. The multiple regression analysis (MRA), employing the method of least squares, is used to derive significant correlation equations to ascertain the predictive power of the study and to explore the possible mode of action. The derived significant QSAR equations were further validated by the leave-one-out (LOO) method [Citation36]. This method is frequently employed for internal validation of a statistical model equation. The derived parameter q2, from LOO, then accounts for the predictivity or more precisely the robustness of the model. The method has been discussed briefly in one of our earlier publications [Citation37].
Table I. QSAR parameters and inhibitory activities of N-Phenyl-N′-{4-(4-quinolyloxy) phenyl}urea derivatives for VEGFR-2 and PDGFRα (see for structures).
Results and discussion
lists the compounds where the alteration in substituents occurred at the middle and terminal phenyl rings attached to the two ends of the urea moiety. To account for the effects produced by different substituents, a large number of descriptors related to hydrophobic, electronic and steric interactions were initially examined for the varying positions of both phenyl rings. In the preliminary attempt, the selected parameters for various substituents for varying positions were hydrophobicity, π, hydrogen-bond donor or acceptor, HD or HA, electronic (meta and para), σ, field, F, resonance, R, dipole moment, μ, Taft's steric, Es, molar refraction, MR, molecular weight, MW and van der Waals volume, Vw. This resulted into a large number of QSAR equations, which were then subjected to different statistical tests. The correlation equations, which returned the highest correlation coefficient, r and F-statistic and lowest standard deviation, s were finally retained for further discussion. The inhibition activity for VEGFR-2 correlating different quantifying parameters is finally shown in Equation (1) and its development steps [(i) – (iii)] are given in .
Table II. Stepwise development of Equation (1); pIC50 = a0 + a1ClogP + a2Es2 + a3F3 + a4HA4 + a5Es6
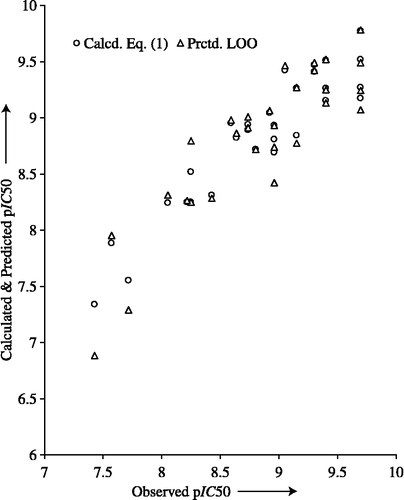
In the above and subsequent correlation equations, n is the number of data points, F is the F-ratio between the variances of calculated and observed activities, and the ± data within the parentheses are the 90% confidence intervals. Except the parameter, ClogP which was calculated for the whole molecule, the remaining quantifying parameters of Equation (1) stand for R-substituents of the terminal phenyl ring. From this equation it appears that the 2- and 6-substituents exert steric hindrance, the 3-substituents are involved in electronic interactions and the 4-substituents are engaged in hydrogen-bond acceptor interaction. Besides other interactions, the ClogP, accounting for hydrophobicity of a molecule appears to play an important role in the inhibition action. The statistical parameters of Equation (1), are sound enough to indicate significant results as the r2 value accounts for 86% of the variance in the observed activities and 99% level of significance [F4,23(0.01) = 4.264]. The higher value obtained for q2 expressed the robustness of the QSAR model. That the variables used in deriving Equation (1) had no mutual correlation is shown in . The calculated activity values, using this equation and listed in Table I, are in close agreement with the observed ones. The plot between observed versus calculated and predicted activities is shown in to identify their systematic variations and to understand the goodness of fit directed by the model equation. From Equation (1), it appears that a less hydrophobic molecule with ortho-substituent(s), exerting less steric hindrance and para-substituent devoid of hydrogen-bond acceptor property augment the inhibition action. Besides, a 3-substituent transmitting a higher negative field effect is advantageous to improve the pIC50 value pertaining to VEGFR-2.
Table III. Intercorrelation Matrixa among the predictors of Equation (1)
Further, the PDGFR tyrosine kinase inhibition activity of the title compounds was also analyzed quantitatively in terms of the QSAR parameters (Table I). Such parameters have revealed a correlation Equation (2), whose development is given in .
Table IV. Stepwise development of Equation (2); pIC50 = b0 + b1ClogP + b2R3 + b3IX
This equation highlights the importance of hydrophobicity of the molecule and the resonance effect of 3-substituents of the terminal phenyl ring. Occurrence of a fluoro-substituent reflected through the indicator variable, IX, in the middle phenyl ring is also predominant in governing the inhibitory activity of a compound. A value either 1 or 0 for IX, in that order, indicates the presence or absence of a fluoro-substituent in the middle phenyl ring. The significant role played by the variable IX, is apparent in steps (ii and iii; ) in developing the above model equation. Except the r-value which is accounting for 79% (r2 = 0.788) of the variance, the other statistical parameters of Equation (2) tune to a high level of significance. The F-value remained significant at 99% level [F3,25(0.01) = 4.676] while q2 expressed a reasonable QSAR model. Equation (2), as such, reflects upon the parametric requirement and needs further improvement. The residuals [pIC50(obsd.) – pIC50(calcd.; Equation 2)] obtained for all the data points were, therefore, further analyzed to trace out unusual behavior of certain congeners. Compound 7 was the lone analogue whose calculated activity value largely deviated from the observed one. The 4-methyl, being a non-polar substituent, enhances hydrophobicity of this molecule, which may not allow effective binding with some hydrophilic pocket present at the receptor. Compound 15 still has a more hydrophobic 4–Cl substituent, but being polar in nature, may favor interaction with a hydrophilic pocket leading to enhancement of activity. The predicted activity of this compound, therefore, becomes closer to the observed one. Elimination of compound 7 further revealed the correlation shown in Equation (3)
The statistical parameters, r, F and q2 are increased while the s and 90% confidence intervals associated to regression coefficients are lowered. All these parameters now account for highly significant results. The calculated pIC50 values using Equation (3), listed in Table I, are in close agreement with the observed ones. The required orthogonality conditions amongst predictor variables of this equation are evinced in . From Equation (3), it appears that a less hydrophobic molecule having a 3-substituent which transmits a more negative resonance effect, is helpful in raising its activity. Likewise, in the middle phenyl ring, absence of a fluoro- substituent augments the inhibitory activity. At present, the substitutional pattern in the middle phenyl ring cannot be concluded precisely as the reported data set has a limited number of substituents in this ring.
Table V. Intercorrelation Matrixa among the predictors of Equation (3)
The conclusions deduced from Equations (1) and (3) may serve as guidelines and assist in obtaining more potent analogues in a further synthesis of similar compounds. The present study, therefore, provides a basis for rationalization of substituent selection in designing more potent analogs of N-Phenyl-N′-{4-(4-quinolyloxy)phenyl}urea.
Acknowledgements
We are grateful to the Department of Chemistry, S K Government College and Department of Engineering Chemistry, Sobhasaria Engineering College for providing the necessary facilities to complete this work.
References
- Folkman J. Anti-angiogenesis: New concept for therapy of solid tumors. Ann Surg 1972; 175: 409–416
- Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels and pulmonary metastases following tumor implantation, Cancer Res 1974; 34: 997–1004
- Eckhardt SG. Angiogenesis inhibitors as cancer therapy. Hosp Pract 1999; 63–84
- Bergers G, Javaherian K, Lo K-M, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 1999; 284: 808–812
- Fan T-PD, Jaggar R, Bicknell R. Controlling the vasculature: Angiogenesis, antiangiogenesis and vascular targeting of gene therapy. Trends Pharmacol Sci 1995; 16: 57–66
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993; 362: 841–844
- Kolch W, Martiny-Baron G, Kieser A, Marme D. Regulation of the expression of VEGF/VPS and its receptors: Role in tumor angiogenesis. Breast Cancer Res Treat 1995; 36: 139–155
- Leenders WPJ. Targetting VEGF in anti-angiogenic and anti-tumor therapy: Where are we now?. Int J Exp Pathol 1998; 79: 339–346
- Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, Hui TH, Tang C, Levitzki A, Gazit A, Chen I, Keri G, Orfi L, Risau W, Flamme I, Ullirich A, Hirth KP, Shawver LK. Fik-1 as a target for tumor growth inhibition. Cancer Res 1996; 56: 3540–3545
- Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, Strawn LM. Dominant-negative inhibition of Fik-1 suppresses the growth of many tumor types in vivo. Cancer Res 1996; 56: 1615–1620
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993; 362: 841–844
- Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hopper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res 1999; 59: 5209–5218
- Dumas J. Protein kinase inhibitors: Emerging pharmacophores 1997–2000. Exp Opin Ther Pat 2001; 11: 405–429
- Connell RD, Beebe JS. Patent focus on cancer chemotherapeutics. II Angiogenesis agents: April 2000–September 2000. Exp Opin Ther Pat 2001; 11: 77–114
- Sun L, Tran N, Tang F, App H, Hirth P, McMahon G, Tang C. Synthesis and biological evaluations of 3-substituted Indolin-2-ones: A novel class of tyrosine kinase inhibitors that exhibit selectivity toward particular receptor tyrosine kinases. J Med Chem 1998; 41: 2588–2603
- Fong TAT, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Fik-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization and growth of multiple tumor types. Cancer Res 1999; 59: 99–106
- Sun L, Tran N, Liang C, Tang F, Rice A, Schreck R, Waltz K, Shawver LK, McMahon G, Tang C. Design, synthesis and evaluation of substituted 3-[(3- or 4-carboxyethyl-pyrrol-2-yl)methyidenyl]indolin-2-ones as inhibitors of VEGF, FGF and PDGF receptor tyrosine kinases. J Med Chem 1999; 42: 5120–5130
- Laird AD, Vajkoczy P, Shawver LK, Thurnher A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard SR, Blake RA, Fong TAT, Strawn LM, Sun L, Tang C, Hawtin R, Tang F, Shenoy N, Hirth KP, McMahon G, Cherrington JM. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res 2000; 60: 4152–4160
- Hennequin LF, Thomas AP, Johnstone CE, Stokes ESE, Ple P, Lohmann J-JM, Ogilvie DJ, Dukes M, Wedge SR, Curwen JO, Kendrew J, Lambert-van-der-Brempt C. Design and structure-activity relationship of a new class of potent VEGF receptor tyrosine kinase inhibitors. J Med Chem 1999; 42: 5369–5389
- Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Curwen JO, Hennequin LF, Thomas AP, Stokes ESE, Curry B, Richmond GH, Wadsworth PF. ZD4190: An orally active inhibitor of vascular endothelial growth factor signaling with broadspectrum antitumor efficacy. Cancer Res 2000; 60: 970–975
- Hennequin LF, Stokes ESE, Thomas AP, Johnstone C, Ple P, Ogilvie DJ, Dukes M, Wedge SR, Kendrew J, Curwen JO. Novel 4-anilinoquinazolines with C-7 basic side chains: Design and structure-activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J Med Chem 2002; 45: 1300–1312
- Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, Graham GA, Hughes GD, Thomas AP, Stokes ESE, Curry B, Richmond GHP, Wadsworth PF, Bigley AL, Hennequin LF. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 2002; 62: 4645–4655
- Bold G, Altmann K-H, Frei J, Lang M, Manley PW, Traxler P, Wietfeld B, Bruggen J, Buchdunger E, Cozens R, Ferrari S, Furet P, Hofmann F, Martiny-Baron G, Mestan J, Rosel J, Sills M, Stover D, Acemoglu F, Boss E, Emmenegger R, Lasser L, Masso E, Roth R, Schlachter C, Vetterli W, Wyss D, Wood JM. New anilinophthalazines as potent and orally well absorbed inhibitors of the VEGF receptor tyrosine kinases useful as antagonists of tumor driven angiogenesis. J Med Chem 2000; 43: 2310–2323
- Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei, Hofmann F, Mestan J, Mett H, O'Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch K-H, Schneider MR, Drevs J, Martiny-Baron G, Totzke F, Marme D. PTK787/ZK222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 2000; 60: 2178–2189
- Fernand NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol 2003; 39–50, 3 (Suppl 6)
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber JD, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an antivascular endothelial growth factor antibody, for metastatic renal cancer. N Eng J Med 2003; 349: 427–434
- Kubo K, Shimizu T, Ohyama S, Murooka H, Iwai A, Nakamura K, Hasegawa K, Kobayashi Y, Takahashi N, Takahashi K, Kato S, Izawa T, Isoe T. Novel potent orally active selective VEGFR-2 tyrosine kinase inhibitors: Synthesis, structure-activity relationships and antitumor activities of N-Phenyl-N′-{4-(4-quinolyloxy)phenyl}ureas. J Med Chem 2005; 48: 1359–1366
- Kubo K, Shimizu T, Ohyama S, Murooka H, Nishitoba T, Kato S, Kobayashi Y, Yagi M, Isoe T, Nakamura K, Osawa T, Izawa T. A novel series of 4-phenoxyquinolines:Potent and highly selective inhibitors of PDGF receptor autophosphorylation. Bioorg Med Chem Lett 1997; 23: 22935–22940
- Yagi M, Kato S, Kobayashi Y, Kubo K, Ohyama S, Shimizu T, Nishitoba T, Isoe T, Nakamura K, Ohashi H, Kobayashi N, Iinuma N, Osawa T, Onose R, Osada H. Selective inhibition of platelet-derived growth factor (PDGF) receptor autophosphory- lation and PDGF-mediated cellular events by quinoline derivatives. Exp Cell Res 1997; 234: 285–292
- Yagi M, Kato S, Kobayashi Y, Kobayashi N, Iinuma N, Nakamura K, Kubo K, Ohyama S, Murooka H, Shimizu T, Nishitoba T, Osawa T, Nagano N. Beneficial effects of a novel inhibition of platelet-derived growth factor receptor autophosphorylation in the rat with mesangial proliferative glomerulonephritis. Gen Pharmacol 1998; 31: 765–773
- Kubo K, Ohyama S, Shimizu T, Takami A, Murooka H, Nishitoba T, Kato S, Yagi M, Kobayashi Y, Iinuma N, Isoe T, Nakamura K, Iijima H, Osawa T, Izawa T. Synthesis and structure-activity relationship for new series of 4-phenoxyquinoline derivatives as specific inhibitors of platelet-derived growth factor receptor tyrosine kinase. Bioorg Med Chem 2003; 11: 5117–5133
- ChemDraw Ultra 6.0 and Chem 3D Ultra, Cambridge Soft Corporation, Cambridge, USA.
- Hansch C, Leo A, Unger SH, Kim KH, Nikaitani D, Lien EJ. Aromatic substituents constants for structure-activity correlations. J Med Chem 1973; 16: 1207–1216
- Unger SH, Hansch C. Quantitative models of steric effects. Prog Phys Org Chem 1976; 12: 91–118
- Hansh C, Leo A. Substituents constants for correlation analysis in chemistry and biology. John Wiley, New York 1979
- Wold S. Validation of QSAR's. Quant Struct-Act Relat 1991; 10: 191–193
- Singh P, Kumar Rajesh, Sharma BK. Quantitative structure-activity relationship study of 5-iodo- and diaryl-analogues of tubercidin: Inhibitors of adenosine kinase. J Enz Inhib Med Chem 2003; 18: 395–402