Abstract
In recently, there has been a great interest in natural antioxidants as bioactive components of food, nutraceuticals or potential drugs against several diseases. In our study, 88 extracts from various parts of plants from European Asteraceae and Cichoriaceae were assayed for radical scavenging activity by means of DPPH (1,1-diphenyl-2-picryl hydrazyl radical) test using the SIA (Sequential injection analysis) method developed for this purpose in our laboratory. DPPH radical scavenging activity of all tested plant extracts was evaluated according to the IC50 parameter. 29 extracts exhibited IC50 value lower than 0.1 mg/mL. The leaves of Leuzea carthamoides (IC50 = 0.046 mg/mL) were chosen as the most promising sample for a subsequent phytochemical study, which resulted in isolation of seven natural compounds, namely, 4′,5,7-trihydroxy-6-methoxyflavone (hispidulin) (1), 5, 7, 3′, 4′- tetrahydroxyflavanone (eriodictyol) (2), 3′,4′,5,7-pentahydroxy-6-methoxyflavonol (patuletin) (3), eriodictyol-7-β-glucopyranoside (4), 6-hydroxykaempferol-7-O-(6″-O-acetyl-β-D-glucopyranoside) (5), 4-hydroxybenzoic acid (6) and 3,4-dihydroxybenzoic acid (protocatechuic acid) (7). Antioxidant activity of the isolated compounds was evaluated by DPPH test and ferric reducing antioxidant power (FRAP) test and compared with trolox and quercetin. Both tests evaluated the flavonoid (5) as the most active antioxidant. This result was confirmed by comparison with known data concerning the structure/activity relationships of flavonoids.
Introduction
The Oxidative stress plays an important role in the pathogenesis of diseases including cancer, cardiovascular disease, neurodegenerative disorders, such as Alzheimer's disease or aging generally Citation1-4. Owing to this fact, antioxidants are receiving increased attention in medicine and pharmacy Citation4-7.
There have been many studies on the biological effects of plants and their secondary metabolites acting as potential drugs against pathological states related to oxidative unbalance Citation8-13. Many other studies are dealing with the search for new antioxidants or summary plant extracts with little toxicity, that can be used in the prevention or therapy of various diseases [Citation14]. The evaluation of natural antioxidants can be performed from randomly chosen plants, or can be e.g. inspirated from traditionally used plant remedies (Chinese traditionally medicine). Recently, several screening methods have been also developed to determine plants with a high potential antioxidant activity [Citation14,Citation15].
Natural antioxidants are polyphenolic plant secondary metabolites (polyphenols) such as simple phenolic acids, hydroxycinnamic acids, napthoquinones, coumarins, flavonoids or tannins [Citation16]. Beside their antioxidant activity, plant polyphenols exhibit a wide range of other positive biological properties, such as anti-allergenic, anti-atherogenic, anti-inflammatory, anti-microbial, anti-thrombotic, cardioprotective or vasodilatory effects [Citation16,Citation17]. Due to these effects on health the consumption of plants with a high content of these compounds is generally recommended [Citation8].
The chemical diversity and thus also the therapeutical importance of plants depends on such factors, as cultivation area, climatic conditions, vegatation phase or genetic modifications [Citation18]. It is also well-known that the composition and quantity of secondary metabolites differ in various anatomical parts of an individual plant (e.g. leaves can contain another type of secondary metabolites than the root).
However, the scientific information on the antioxidant properties of various plants, particularly those that are less widely used in culinary activities and medicine, is still insufficient. Therefore, the evaluation of the antioxidant potential of herbs is still a useful task, particularly for finding new sources of natural antioxidants and nutraceuticals.
The first goal of this work was preliminary DPPH radical scavenging screening of 88 extracts of various anatomical parts () of 70 Central European taxons belonging to the families Asteraceae and Cichoriaceae with the view to find a prospective taxon for further testing; that means isolation of active substances and evaluation of their antioxidant activity. For the purpose of the antioxidant screening study the DPPH test using the sequential injection analysis (SIA) system was used. This method, developed in our laboratory, was evaluated for fast and sensitive DPPH radical scavenging screening of large series of complex natural samples [Citation15].
Table I. Tested plants and their DPPH radical scavenging activity.
As mentioned above, the next goal of this study was determination of the antioxidant-active substances from the most active taxon of the screening study. During the isolation procedure the DPPH radical scavenging activities of prepared subextracts were measured. Then, 7 natural compounds () from the most antioxidant-active subextract were isolated. The DPPH radical scavenging activity and ferric reducing antioxidant power (FRAP) of isolated compounds were evaluated. Antioxidant activities of isolated compounds were compared with known the standards trolox and quercetin. The structure/antioxidant activity relationships of isolated compounds were compared with already published data for polyphenols.
Material and methods
Preparation of 88 summary extracts
The plant material was harvested from the various localities in Czech the Republic during 2001 and 2002. All 88 final extracts were prepared by ethanolic extraction (15–75 mL of aqueous 70% ethanol), filtration and lyophilisation of plant material (1–5 g) according to a procedure described by Polasek et al. [Citation15]. Final extracts were stored and preprared for DPPH analysis. All used reagents were purchased from Sigma-Aldrich (Prague, Czech Republic).
Preparation of tested solutions for DPPH analysis using SIA method
Stock solutions of final plant extracts, 7 isolated compounds from Leuzea carthamoides, trolox and quercetin were prepared by dissolving 5 mg of the tested sample in 5 mL of aqueous 50% (v/v) ethanol under 10 min sonication in a Bandelin Sonorex Super 10P ultrasound bath (sonication level 10) (Progen Scientific Ltd, Mexborough, Great Britain). Stock solutions of subextracts of L. carthamoides were prepared similarly by disolving ∼3–10 mg of subextract with an appropriate volume of aqueous 50% (v/v) ethanol. Before dissolution, the subextracts were evaporated to dryness. Aqueous 50% (v/v) ethanol was used for appropriate dilution of the stock solution. The prepared concentrations of tested samples were 1 mg/mL, 0.5 mg/mL, 0.25 mg/mL, 0.1 mg/mL, 0.05 mg/mL, 0.025 mg/mL, 0.01 mg/mL and 0.005 mg/mL. All used reagents were purchased from Sigma-Aldrich (Prague, Czech Republic).
DPPH assay using sequential injection analysis (SIA)
The DPPH radical (2,2′-diphenyl-1-picrylhydrazyl hydrate) scavenging activity of 88 final extracts, subextracts of L. carthamoides, isolated compounds, trolox and quercetin was determinated using a PC-controlled FIAlab 3000 analyser (FIAlab Instruments Inc., Bellevue, USA) according to the method described by Polasek et al. [Citation15]. DPPH radical scavenging activity of tested samples was expressed by the parameter IC50 (concentration providing 50% inhibition of DPPH). All used chemicals were obtained from Sigma-Aldrich (Prague, Czech Republic).
FRAP assay
The Ferric reducing antioxidant power (FRAP) assay modified to be used in 96-well microplates Brand 400 μl (Fisher Scientific, Pardubice, Czech Republic) by Microplate reader Anthos 2010 (ASYS Hitech GmbH, Eugendorf, Austria) for direct determination of the reducing capacity of 7 isolated compounds from L. carthamoides and trolox was used. This method is based on the ability of the antioxidants to reduce Fe3 + to Fe2 + . All experiments were carried out according to the method of Firuzi et al. [Citation19]. Reducing activity of tested compounds was described by FRAP values (μM) at the time interval 4 and 60 min, which were calculated according to Firuzi et al. [Citation19]. All used chemicals were obtained from Sigma-Aldrich (Prague, Czech Republic).
Extraction of L. carthamoides
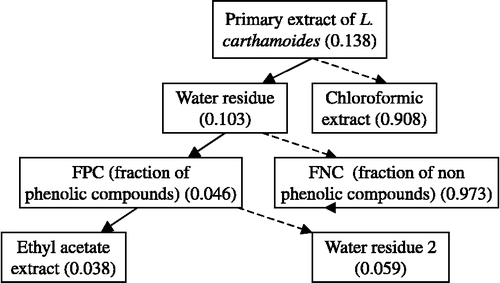
The dried powdered leaves (10.5 kg) of Leuzea carthamoides (Willd.) DC (Asteraceae), obtained from Radka Simakova (Medicinal plants cultivation, Ohnisov, Czech Republic) were percolated with 95% ethanol at an ambient temperature 22 ± 1°C and evaporated at a pressure of 1 kPa and temperature ≤ 50°C. The scheme of the extraction procedure for L. carthamoides is shown in . The primary extract was dissolved in destilled water (50°C) to an approximate volume 9 L, filtered through medium-porosity filter paper and extracted six times with chloroform (ratio water:chloroform = 4:1). Water residue was subjected to polyamide (100–200 mesh) column chromatography (CC). The fraction of non-phenolic compounds was eluted with water (FNC), and the fraction of phenolic compounds (FPC) with ethanol (95%). FPC (201 g) was dissolved in water (2.2 l) and extracted six times with ethyl acetate (ratio water:ethyl acetate = 4:1) to give water residue 2 () and ethyl acetate extract (66 g). All used reagents were purchased from Sigma-Aldrich (Prague, Czech Republic).
NMR analysis
NMR analysis of isolated substances were recorded on a VARIAN Mercury – Vx BB 300 spectrometer (Varian, Inc. Corporate Headquarters, Palo Alto, USA): 1H-NMR 300 MHz and 13C-NMR 75.46 MHz.
Results
DPPH screening study of 88 summary extracts
The results of the DPPH radical scavenging activity of 88 plant extracts from the families Asteraceae and Cichoriaceae are summarized in . It was found that the majority of lyophilised ethanolic extracts showed a significant antioxidant activity. Nevertheless, significant differences in IC50 values of tested extracts were found. The IC50 values of tested extracts were found between 0.046 mg/mL (the most active sample; leaves of L. carthamoides) and 0.542 mg/ml (the least active sample; flower of Centaurea cyanus). 29 extracts exhibited an IC50 value lower than 0.1 mg/mL. The most antioxidant active plants were: Leuzea carthamoides (0.046 mg/mL; leaves), Inula salicifolia (IC50 = 0.048 mg/mL; flowering herb), Inula hirta (0.049 mg/mL; flow. herb), Petasites hybridus (0.050 mg/mL; leaves), Artemisia pontica (0.053 mg/mL; herb), Tanacetum vulgare (0.054 mg/mL; leaves), and Achillea ptarmica (0.057 mg/mL; flow. herb).
Antioxidants of L. carthamoides
The evaluation of the DPPH radical scavenging activity (IC50) of the obtained fractions of L. carthamoides provided data about the distribution of the active antioxidant components in the plant ().
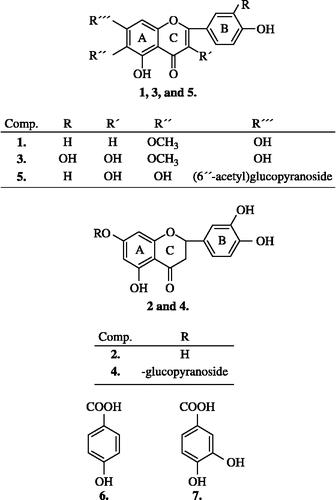
From the obtained subextracts, the chloroform extract and fraction of non-phenolic compounds were the least active against the DPPH radical. Compared to the other fractions, the ethyl acetate extract (IC50 = 0.038 mg/mL) exhibited the highest DPPH radical scavenging activity. Consequently, ethyl acetate extract was subjected to Si-gel (100–200 mesh, 1:120) CC (column chromatography) using a chloroform/ethanol mixture. Obtained fraction with similar Rf values by TLC were combined together. Fractions were recrystallized using the chloroform/ethanol mixture and purified to yield seven compounds, three flavonoid aglycons, namely, 4′,5,7-trihydroxy-6-methoxyflavone (hispidulin) (1), 5, 7, 3′, 4′-tetrahydroxyflavanone (eriodictyol) (2), 3′,4′,5,7-pentahydroxy-6-methoxyflavonol (patuletin) (3), two flavonoid glycosides, namely, eriodictyol-7-β-glucopyranoside (4), 6-hydroxykaempferol-7-O-(6″-O-acetyl-β-D-glucopyranoside) (5) and two phenolic acids, namely, 4-hydroxybenzoic acid (6) and 3,4-dihydroxybenzoic acid (protocatechuic acid) (7) (Figure 1). Structures of the compounds were elucidated by comparison of their 1H and 13C NMR spectral data with those in the literature or with authentic samples Citation20-25.
DPPH assy
DPPH radical scavenging activity was meaured according to the SIA method described by Polasek et al. [Citation15]. The results of DPPH radical scavenging activities (IC50) of seven isolated compounds, trolox and quercetin are summarized in .
Table II. DPPH radical scavenging activities (IC50) of isolated compounds, trolox and quercetin.
These results showed that four of the five isolated flavonoids (2–5) are powerful DPPH radical scavengers with IC50 values lower than 100 μM. The IC50 value of the most antioxidant active flavonoid (5), (IC50 = 29.9 μM) was close to that of trolox (IC50 = 27.8 μM) and quercetin (IC50 = 25.3 μM). Compared to the flavonoids 2–5, DPPH radical scavenging activity of hispidulin (1), was very weak (IC50 = 769.6 μM).
Our results showed that the isolated phenolic acids (6–7) were weak to moderate DPPH radical scavengers in our test. 4-hydroxybenzoic acid (6) did not affect DPPH quenching even at the highest measured concentration and therefore its IC50 value was not determinated. The IC50 value of 3,4-dihydroxybenzoic acid (7) was approximately six times higher than that of trolox and quercetin ().
FRAP assay
The reducing ability of compounds was measured by the FRAP test as described by Firuzi et al. [Citation19]. Results of measuring of isolated compounds are demonstrated as FRAP values at 4 and 60 min and are summarized in the . The highest FRAP values were obtained with 6-hydroxykaempferol-7-O-(6″-O-acetyl-β-D-glucopyranoside) (5) (33.1 μM; 65.3 μM) and 3′,4′,3,5,7-pentahydroxy-6-methoxyflavone (patuletin) (3) (31.6 μM; 63.6 μM). Flavonoids 2, 4 and 3,4-dihydroxybenzoic acid (7) are also potent ferric reducing agents with FRAP values at 4 min from 15.1 μM to 23.3 μM and FRAP values at 60 min from 27.9 μM to 52.0 μM. Compared to trolox, the flavonoids (2–5) and simple phenolic acid (7) were more efficient reducing agents. Contrariwise, the flavonoid hispidulin (1) and 4-hydroxybenzoic acid (6) were poor reducing agents in our test.
Table III. Results of FRAP values of isolated compounds and trolox at 4 and 60 min.
Discussion
In recent years, there has been a great interest in the use of natural antioxidants for the prevention or therapy of many deseases including allergy, atherosclerosis, inflammation, microbial infection, or cardiovascular diseases. This is due to the implication of oxidation stress in the pathology of various disorders [Citation11].
Plant polyphenols, one of the most widespread group of plant secondary metabolites (about 8 000 currently known structures) are often an important part of the human diet (fruit, vegetable, beverages) [Citation11]. After consumption, these compounds participate in the maintenance of human redox balance. Human intervention studies have shown the positive health effects of consumption of natural polyphenol-rich food on, for example, the cardiovascular system (tannins), carcinogenesis (flavones), bone health among postmenopausal woman (isoflavones), or improvement of energy metabolism (catechins) [Citation26].
A result of the intensive research on natural antioxidants has been for example the introduction of polyphenol-rich bark extract of Pinus maritima (Pycnogenol®), which is generally used as a cardioprotective preparation [Citation27]. Therefore, the evaluation of the antioxidant potential of plants is still a useful task for discovering more effective natural drugs or nutraceuticals.
In our study, the antioxidant activity of 88 final extracts of various parts of Central European plants belonging to the families Asteracae and Cichoriaceae was tested. For this purpose the highly reproducible DPPH radical scavenging method using SIA system was used [Citation15]. This method, developed in our laboratory, faciliated the fast antioxidant screening of such a huge number of samples. The results of DPPH radical scavenging activity showed that the majority of extracts exhibited antioxidant power. From comparison of the calculated IC50 values of all final extracts, L. carthamoides was chosen as the most promising plant for detailed isolation and antioxidant studies. Nevertheless, other highly active taxons were noted for possible future antioxidant studies.
Generally, L. carthamoides (Rhaponticum carthamoides (Asteraceae)) is a widespread and often used medicinal plant. Originally L. carthamoides was an endemic plant of southern Siberia, now it is widely grown in Central and Eastern Europe. The principal bioactive constituents of the whole plant are ecdysteroids, flavonoids, and phenolic acids. The aerial parts also contain sesquiterpene lactones of the guaianolide type, while the roots contain thiophene-based polyines [Citation28]. There have been two recent studies about antioxidant properties of L. carthamoides. The first study dealt with the antioxidant screening of 12 medical plants and concludes that the extract of L. carthamoides possesses high radical scavenging activity [Citation18]. The second study identifies 7 natural compounds of L. carthamoides by means of on-line LC-DAD-SPE-NMR system. Nevertheless, incomplete evaluation of the radical scavenging or antioxidant activity of L. carthamoides extracts or pure compounds is occured [Citation20].
During the isolation procedure, DPPH radical scavenging activity by means of the SIA method of prepared subextracts was measured and from the most antioxidant active extract seven phenolic compounds were isolated. Compounds 5,6 and 7 have been reported previously as constituents of L. carthamoides [Citation20,Citation29].
The DPPH test using SIA method for determination of radical scavenging activity of all isolated compounds and antioxidants trolox and quercetin was used. The results of the DPPH radical quenching activities of the isolated flavonoids were compared with known data concerning the structure/antioxidant activity relationships of flavonoids. Generally, important structural criteria for high activity of the flavonoids included 1) the ortho-dihydroxy groups (catechol structure) in the B-ring or in the A-ring, 2) the 3-hydroxyl group or the 3-galloyl group (catechol structure) in the C-ring, and 3) the 2,3-double bond in conjugation with the 4-oxo function (carbonyl group) in the C-ring [Citation12,Citation30]. All tested flavonoids (1–5) satisfied at least one of these criteria nevertheless the radical scavenging activity of 4′,5,7-trihydroxy-6-methoxyflavone (hispidulin) (1) was low. This result indicates that alone the conjugation of the 2,3-double bond with the 4-oxo function (hispidulin, (1)) contributes very little to the antioxidant activity of the flavonoid. On the other hand, the structure of the highly active eriodictyol (2) involves only a catechol structure in the B-ring. This result confirmed the high importance of this structural feature for the high antioxidant activity of flavonoids.
According to our study, 4-hydroxybenzoic acid (6) did not scavenge the DPPH radical. Also other studies found this phenolic acid as a weak antioxidant [Citation30,Citation31]. Contrariwise, the well known antioxidant 3,4-dihydroxybenzoic acid (7) exhibited a moderate (IC50 = 163.0 μM) DPPH radical scavenging activity in our study. This result confirmed the importance of the number and position of hydroxyl groups in the molecule for high antioxidant activity of phenolic acids and is in a good correlation with the study by Sroka and Cisowski [Citation32,Citation33].
Among the isolated flavonoids, 6-hydroxykaempferol-7-O-(6″-O-acetyl-β-D-glucopyranoside) (5) and 3′,4′,5,7-pentahydroxy-6-methoxyflavonol (patuletin) (3) showed the highest ferric reducing activity in the antioxidant FRAP test. Both compounds are flavonoids with a 3-hydroxy group, 2,3-double bond and 4-oxo function in the C-ring and the catechol structure in the A- or B-ring. These structural features are the highest contributors to the reducing activity of the flavonoids. Flavonoid structures (2,4) which did not contain all these “active groups” in their structure were less active than the flavonoids 3 and 5. Reducing test also confirmed (similar to the DPPH test) that the flavonoid 1 did not possess antioxidant activity. This conclusion corresponds with already the published structure/reducing activity relationship data of the flavonoids evaluated by Firuzi et al. [Citation19]. The study of Firuzi et al. evaluated quercetin (among 18 structurally different flavonoids) as the most potent reducing flavonoid with FRAP value 4 min = 65.0 μM and FRAP value 60 min = 95.9 μM [Citation19]. None of isolated flavonoids (the FRAP values of the most active compound (5) were 33.1 μM resp. 65.3 μM) exhibited such a high reducing potential. Compared to trolox, isolated flavonoids (2–5) exhibited higher ferric reducing activity.
The catechol structure in 3,4-dihydroxybenzoic acid (7) is responsible for a significant ferric reducing activity. Similarly as in the DPPH test, elimination of the 3-hydroxy group (7) significantly decreased reducing activity.
In conclusion, from the group of 88 extracts from European Asteraceae and Cichoriaceae, L. carthamoides was investigated as one of the most promising sources of natural antioxidants. Consequently, an isolation and antioxidant study determined 7 natural compounds. The results of DPPH and FRAP tests evaluated 6-hydroxykaempferol-7-O-(6″-O-acetyl-β-D-glucopyranoside) (5) as the most antioxidant active compound. This result was confirmed by comparison with known data for the structure/activity relationship of natural compounds. Our study evaluated L. carthamoides as a promising plant for future antioxidant study with the purpose of proposing a new nutraceutical preparation. Nevertheless, significance to health of dietary antioxidants depends also on their mechanism of absorption and biotransformation; further investigations on the bioavailability and in vivo antioxidant properties of extracts of L. carthamoides are required.
Acknowledgements
This work was supported by a grant from Charles University (Czech Republic) No. GAUK 124/2005/B-BIO/FaF and a research project from the Ministry of Education of the Czech Republic (MSM 0021620822).
References
- Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res 2003; 523–524: 9–20
- Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Neurobehavioral aspects of antioxidants in aging. Int J Dev Neurosci 2000; 18: 367–381
- Surh YJ, Chun KS, Cha HH, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res 2001; 480–481: 243–268
- Koleckar V, Jun D, Opletal L, Jahodar L, Kuca K. Assay of radical scavenging activity of antidotes against chemical warfare agents by DPPH test using sequential injection technique. J Appl Biomed 2007; 5: 81–84
- Yang L, Gong J, Wang F, Zhang Y, Wang Y, Hao X, Wu X, Bai H, Stockigt J, Zhao Y. Synthesis and antioxidant evaluation of novel silybin analogues. J Enz Inhib Med Chem 2006; 21: 399–404
- Dwivedi VK, Chandra M, Misra PC, Misra A, Misra MK. Status of some free radical scavenging enzymes in the blood of myocardial infarction patients. J Enz Inhib Med Chem 2006; 21: 43–46
- Kontogiorgis CA, Savvoglou K, Hadjipavlou-Litina DJ. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J Enz Inhib Med Chem 2006; 21: 21–29
- Katalinic V, Milos M, Kulisic T, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem 2006; 94: 550–557
- Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of thailand. Food Chem 2005; 92: 491–497
- Chang WC, Sei CK, Soon SH, Bong KC, Hye JA, Min YL, Sang HP, Soo KK. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 2002; 163: 1161–1168
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem 2006; 99: 191–203
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat Res-Fund Mol M 2005; 579: 200–213
- Kondratyuk TP, Pezzuto JM. Natural product polyphenols of relevance to human health. Pharm Biol 2004; 42: 46–63
- Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. Screening of medicinal plant extracts for antioxidant activity. Life Sci 2003; 73: 167–179
- Polasek M, Skala P, Opletal L, Jahodar L. Rapid automated assay of anti-oxidation/radical-scavenging activity of natural substances by sequential injection technique (SIA) using spectrophotometric detection. Anal Bioanal Chem 2004; 379: 754–758
- Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 1998; 56: 317–333
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem 2006; 99: 191–203
- Miliauskas G, Venskutonis PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 2004; 85: 231–237
- Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta 2005; 1721: 174–184
- Miliauskas G, van Beek TA, de Waard P, Venskutonis RP, Sudhoolter EJR. Identification of radical scavenging compounds in rhaponticum carthamoides by means of LC-DAD-SPE-NMR. J Nat Prod 2005; 68: 168–172
- Nishida J, Kawabata J. DPPH radical scavenging reaction of hydroxy- and methoxychalcones. Biosci Biotechnol Biochem 2006; 70: 193–202
- Roemmelt S, Zimmermann N, Rademacher W, Treutter D. Formation of novel flavonoids in apple (Malus_domestica) treated with the 2-oxoglutarate-dependent dioxygenase inhibitor prohexadione-Ca. Phytochemistry 2003; 64: 709–716
- Gaind KN, Singla AK, Wallace, James W. Flavonoid glycosides of kalanchoe spathulata. Phytochemistry 1981; 20: 530–531
- Agrawal PK, Thakur RS, Bansal MC. Carbon-13 NMR of flavonoids, PK Agrawal. Elsevier Science Publishers, Amsterdam (Netherlands) 1989; 95–182
- Phytochemical methodssecond ed., JB Harborne. Chapman and Hall, London 1984
- Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 2005; 81: 243–255
- Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radical Bio Med 1999; 27: 704–724
- Opletal L, Sovova M, Dittrich M, Solich P, Dvorak J, Kratky F, Cerovsky J, Hofbauer J. Phytotherapeutic aspects of diseases of the circulatory system. 6. Leuzea carthamoides (WILLD.) DC: the status of research and possible use of the taxon. Ceska Slov Farm 1997; 46: 247–255
- Vereskovskii VV. Chekalinskaya II. Phenolic compounds of Rhaponticum carthamoides (Willd) Iljin Vestsi Akademii Navuk BSSR, Seryya Biyalagichnykh Navuk. 1978; 2: 14–18
- Cai YZ, Mei Sun, Jie Xing, Luo Q, Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci 2006; 78: 2872–2888
- Zhou K, Yin JJ, Yu L. ESR determination of the reactions between selected phenolic acids and free radicals or transition metals. Food Chem 2006; 95: 446–457
- Sroka Z, Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol 2003; 41: 753–758
- Villano D, Fernandez-Pachon MS, Moya ML, Troncoso AM, Garcia-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007; 71: 230–235