Abstract
Radix Polygoni multiflori is a herb used effectively to prevent graying and treat skin depigmentation diseases in traditional Chinese medicine but its active ingredients have not been discovered yet. In this investigation, we tested six compounds isolated from Radix Polygoni multiflori, to discover the active component on melanogenesis. Three experiments were performed in the present investigation: mushroom tyrosinase activity, melanin content B16 cell proliferation assay. Among all the six components tested, THSG showed the most potent effects on tyrosinase activation and melanogenesis; it was shown to be a potent tyrosinase activator and a melanogenesis stimulator in this study. On the other hand, we found that gallic acid significantly inhibited tyrosinase and, in addition, anthraquinones were cytotoxic to melanoma cells. They were both harmful to melanogenesis. Therefore, we propose that THSG acts as the active ingredient of Radix Polygoni multiflori on melanogenesis.
Introduction
Tyrosinase (EC 1.14.18.1) is an enzyme catalyzing the rate-limiting step for melanin biosynthesis [Citation1]. This enzyme catalyses two different reactions: the hydroxylation of monophenols to o-diphenols (monophenolase activity), and the oxidation of o-diphenols to o-quinones (diphenolase activity), which, in turn, are polymerized to melanin [Citation2]. Therefore, tyrosinase is a key enzyme involved in melanin biosynthesis and of particular interest in repigmentation research.
Radix Polygoni multiflori (“Heshouwu” in chinese) is a useful herb for preventing graying and treating the depigmentation disease in traditional Chinese medicine and is the dried root of the plant Polygonum multiflorum Thunb.(Ploygonaceae) [Citation3]. In chinese medicine, Radix Polygoni Multiflori is used to improve the liver and kidney functions, as well as rejuvenate and blacken the hair. Although it has been used in repigmentation from ancient time in China, its active ingredients have not been studied previously.
Six components:2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside, chrysophanol, emodin, physcion, aloe-emodin and gallic acid, have been isolated from it in our laboratory (methods and data not shown). In this investigation, we tested these six isolated compounds, to discover the active component on melanogenesis.
Materials and methods
Reagents
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (THSG), chrysophanol, emodin, physcion, aloe-emodin and gallic acid were isolated from Radix Polygoni multiflori in our laboratory (purity>95%). Mushroom tyrosinase, L-3,4-dihydroxyphenylalanine (L-DOPA) and 8-Methoxypsoralen (8-MOP) was purchased from Sigma Chemical Co. (St. Louis, U.S.A.). RPMI-1640 powdered medium, trypsin, fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-iphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) was obtained from Gibco(Gibco-BRL, USA). All other reagents were analytical grade.
Cell culture
Cultured murine B16 melanoma cells were from the Cell bank of Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, China). The B16 cells were cultured in RPMI 1640 medium with 10% FBS, 1% penicillin–streptomycin at 37°C in a humidified CO2 incubator.
Mushroom tyrosinase activity assay
Mushroom tyrosinase activity was measured by the rate of oxidation of L-DOPA. The assay was performed according to methods described previously [Citation4] with minor modifications. The initial rate of dopachrome formation was determined by the increase in absorbance at 492 nm per min (A492/min). Reactive reagents were added to 96-well microplates (TPP, Switzerland) with a total volume 200 μL. The assay mixture contains 50 μL L-DOPA (3 mmol/L), 15 μL solvent with or without sample, and 85 μL phosphate buffer (pH 6.8), which was mixed with 50 μL of tyrosinase (100 U/mL). After incubation at 25°C for 2 min, the reaction was monitored at 492 nm using the microplate reader (Multiskan MK 3, Labsystems, Holland) immediately. The control reaction was conducted without the test sample.
The activation of tyrosinase was expressed as follows: activation (%) = (A–B)/B × 100%, where A represents the absorbance of the test sample well, and B represents the absorbance of the control well.
Melanin content assay
The melanin content assay was carried out as previously described [Citation5]. B16 cells were plated in 12-well plates at a density of 2 × 104 cells/mL. Various concentrations of test samples were prepared by dissolving in DMSO and diluting in culture medium. After adding the test samples, the plates were incubated at 37 °C in a CO2 incubator for 3 days. The final concentration of DMSO in the culture was 0.33% and this concentration was found to be non-toxic to B16 cells (data not shown).
After incubation, the medium was removed and the cells were washed with sterile phosphate buffered saline (PBS, pH 7.4) and trypsinized using trypsin/EDTA at 37°C for 3–5 min. Cells were collected and centrifuged at 500 rpm for 10 min. A pellet was formed and the media was removed. After washing twice with fresh PBS, the remaining pellet was dissolved in 1.0 M NaOH and vortexed for 30 min. The melanin content was calculated on the basis of the absorbance at 492 nm.
Cell proliferation assay
The study of cell proliferation was carried out as previously described [Citation6], using the MTT-based colorimetric assay. B16 cells were diluted with RPMI 1640 medium. Aliquots (100 μL) containing 0.6 × 104 cells were inoculated into 96 well microplates using a Gilson Pipette. A 50 μL solution of each compound was added into 6 replicates of 100 μL of cells in 96-well microplates. The final concentration of each compound in the well was 0.2–12.5 μg/mL. After adding compounds into the B16 cells (96-well plate), the plates were incubated at 37 °C in an incubator with 5% CO2 and 90% humidified air for 3 days.
At the end of the incubation, MTT solution (20 μL, 0.5 mg/mL in phosphate-buffered saline) was added to the wells. After another 4 h incubation, the medium was removed, and 100 μL DMSO was added to dissolve the formazan produced in the cells. The absorbance of each well was then read at 570 nm using a Microplate Reader (Multiskan MK 3, Labsystems, Holland). The optical density of formazan formed by control cells was assumed to be 100%.
Statistical analysis
Results are expressed as mean ± standard error. Data were analyzed using the Student's t-test. Values of P < 0.01 were considered significant.
Results
Mushroom tyrosinase activity assay
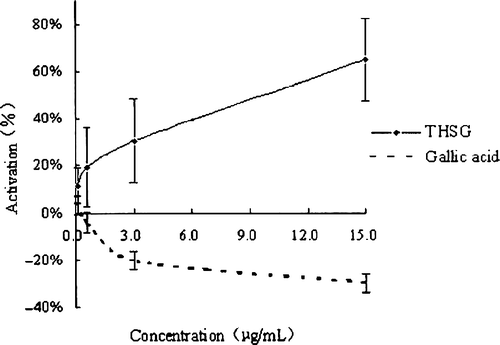
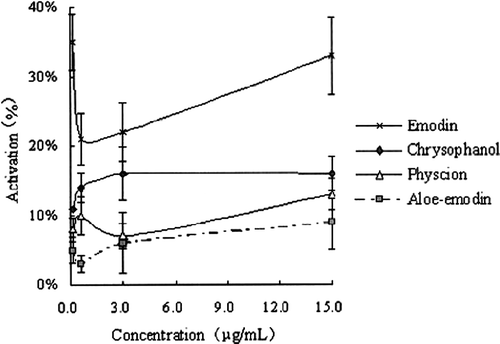
Mushroom tyrosinase has been widely used as the target enzyme for screening and characterizing potential tyrosinase regulators. The diphenolase activity of this enzyme was assayed using L-DOPA as the substrate. THSG, gallic acid and 4 anthraquinones, which were isolated from Radix Polygoni Multiflori, were tested for their effects on the oxidation of L-DOPA by mushroom tyrosinase. The results showed that THSG activated the diphenolase activity, while gallic acid inhibited it (). Dose-dependent effects on enzyme activity were exhibited by both THSG and gallic acid, respectively. The effects of the anthraquinones are shown in . The 4 anthraquinones showed mild activating effects on tyrosinase, and there was no dose-dependency at the test concentrations. Thus, it suggests that THSG and the 4 anthraquinones are the active candidates, which will be further investigated.
Melanin content in cultured B16 cells
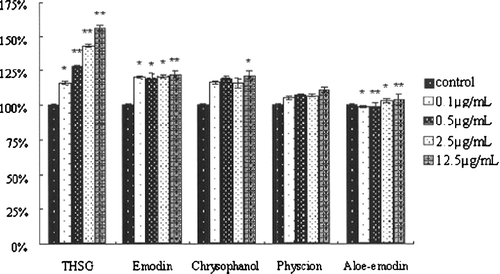
The melanin content of B16 melanoma cells is an important indicator for evaluation of melanogenesis activators since they can usually improve melanin content in melanoma cells. THSG, chrysophanol and emodin showed significant melanogenesis stimulation activities over the range of concentrations from 0.1 to 12.5 μg/ml (). Physcion and aloe-emodin stimulated melanogenesis slightly. These results suggested that THSG, chrysophanol and emodin can improve melanogenesis in B16 cells and THSG is the most effective stimulator.
Effects of THSG on tyrosinase activity in cultured B16 cells
The effects of THSG and 8-MOP on B16 cell tyrosinase are shown in . Both THSG and 8-MOP showed significant activation effects on B16 cell tyrosinase. Similarly, they activated tyrosinase over a broad range of concentrations (from 0.1–25μg/mL) in a dose-dependent manner (). Yet, although THSG showed more potent effects than 8-MOP, the difference was not statistically significant (P > 0.05). The results showed that THSG is a potent activator of tyrosinase.
Cell proliferation
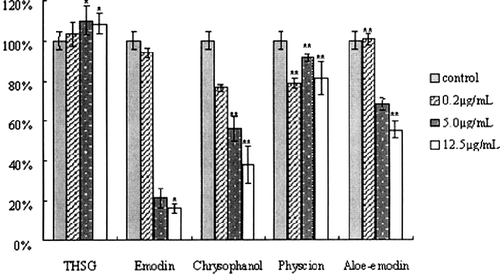
The 5 components with the tyrosinase activation effects were tested in this assay. B16 cells were exposed to the samples over a concentration range from 0.2 to 12.5 μg/mL. We found that THSG-stimulated proliferation of B16 cells occurs over a broad range of concentrations, from 0.1–12.5 μg/mL, with maximal stimulation at 5.0 μg/mL (). While the anthraquinones inhibited the proliferation of B16 cells in almost all the concentrations tested (). To conclude, this study demonstrated that THSG stimulated the proliferation of melanocytes, and, therefore, it would be beneficial to melanogenesis. On the contrary, the anthraquinones were found to be cytotoxic.
Discussions
In our investigation, several experiments were performed on Radix Polygoni multiflori components in order to discover the substance with the most activating effect on melanogenesis. Among all the 6 components tested, THSG showed the most potent effects on tyrosinase activation and melanogenesis. It proved to be a potent tyrosinase activator and a melanogenesis stimulator and, therefore, it acts as the activating ingredient of Radix Polygoni multiflori on melanogenesis. On the other hand, we found that gallic acid inhibited tyrosinase significantly. In addition, anthraquinones were cytotoxic to melanoma cells. Thus, they are both harmful to melanogenesis.
THSG is one of the stilbene derivatives which are distributed abundantly in plants and have a number of interesting biological activities [Citation7,Citation8]. Although THSG is similar to other stilbene derivatives in their chemical structures, their effects on tyrosinase activity differ greatly. Some studies showed that several stilbene derivatives can inhibit tyrosinase activity [Citation9,Citation10,Citation11,Citation12,Citation13]. Among them, oxyresveratrol (2,3′,4,5′-tetrahydroxy-trans-stilbene) [Citation12] and gnetol (2,3′,5′,6-tetrahydroxy-trans-stilbene) [Citation13] are the representative. We compared the structures of THSG, oxyresveratrol and gnetol in order to determine the possible causes for their different regulatory activities. There are 4 hydroxy groups on the phenyl rings in all the 3 stilbene derivatives, however, there is one additional glycosylation on THSG. Likhitwitayawuid's study showed that the glycosylation of the parent hydroxystilbene could abolish the inhibitory effect and lower the affinity to enzymes [Citation14]. The glycosylation of polyphenols can help to protect these molecules from enzymatic oxidation, which is the reason that THSG can coexist with tyrosinase without being oxidised. As a result, glycosylation on THSG is crucial for its activating effect on tyrosinase.
To conclude, we found that THSG, the active component of Radix Polygoni multiflori, activates melanogenesis and, therefore, can be used as a candidate for depigmentation disease. At the same time, compounds, such as gallic acid and anthraquinones, were found to be harmful to melanogenesis.
References
- Gold HS, Moellering RC. Antimicrobial-drug resistance. N Engl J Med 1996; 335: 1445–1453
- Bossche VH, Marichal P, Odds FC. Molecular mechanisms of drug resistance in fungi. Trends Microbiol 1994; 10: 393–400
- Cohen ML. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 1992; 257: 1050–1055
- Terzioğlu N, Karalı N, Gürsoy A, Kiraz M, Erturan Z. Synthesis and antimicrobial activity of new triazole and thiadiazole derivatives of 4(3H)-quinazolinones. Acta Pharmaceutica Turcica, XXXX 1998; 2: 77–82
- Varvaresou A, Kakolidou AT, Papastaikoudi TS, Tiligada E. Synthesis and biological evaluation of indole containing derivatives of thiosemicarbazide and their cyclic 1,2,4-triazole and 1,3,4-thiadiazole analogs. Arzneim.-Forsch./Drug Res 2000; 50(I)48–54
- Gülerman NN, Doğan HN, Rollas S, Johansson C, Çelik C. Synthesis and structure elucidation of some new thioether derivatives of 1,2,4-triazoline-3-thiones and their antimicrobial activities. Farmaco 2001; 56: 953–958
- Servi S, Genç M, Gür S, Koca M. The synthesis and antimicrobial activity of some new methyl N-arylthiocarbamates, dimethyl N-aryldithiocarbonimidates and 2-arylamino-2-imidazolines. Eur J Med Chem 2005; 40: 687–693
- Dogan HN, Duran A, Rollas S, Sener G, Uysal MK, Gulen D. Synthesis of new 2,5-disubstituted-1,3,4-thiadiazoles and preliminary evaluation of anticonvulsant and antimicrobial activities. Bioorg Med Chem 2002; 10: 2893–2898
- Kassim EM, Abd-El-Nour KN, El-Diwany AI, Abou-Aiad TH. Synthesis and relation between the antimicrobial activity and dipole moment of some phthalazinone derivatives. Ind J Chem 1996; 35B: 692–696
- Kassem EM, Kamel MM, El-Zahar M. New 1(2H)-phthalazinones of potential antimicrobial activity. Pharmazie 1990; 45: 215–216
- Demirayak S, Karaburun AC, Beis R. Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur J Med Chem 2004; 39: 1089–1095
- Doğruer DS, Küpeli E, Yeşilada E, Şahin MF. Synthesis of new 2-[1(2H)-phthalazinon-2-yl]acetamide and 3-[1(2H)-phthalazinon-2-yl]propanamide derivatives as antinociceptive and anti-inflammatory agents. Arch Pharm Pharm Med Chem 2004; 337: 303–310
- National Committee for Clinical Laboratory Standards. Method for broth dilution antifungal susceptibility testing yeast; approved standard, M27-A, 15, 10, NCCLS, VA Medical Center, Tuscon, 1996.
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, third ed. Approved standard. NCCLS document M100-S12, NCCLS, Wayne, PA, USA.
- Hassanien AZ. Abd El-Baset. Phosphorus, Phthalazinone in Heterocyclic Synthesis: Synthesis of some s-Triazole, s-Triazolothiadiazine, s-Triazolothiadiazine, and s-Triazolothiadiazole derivatives as pharmaceutical interest. Phosphorus sulfur and silicon and the related elements 2003; 178: 1987–1997
- El-Masry AH, Fahmy HH, Abdelwahed SHA. Synthesis and antimicrobial activity of some new benzimidazole derivatives molecules 2000; 5: 1429–1438