Abstract
Antioxidants are compounds that can delay, inhibit, or prevent the oxidation of materials that can be oxidized by scavenging free radicals and help in diminishing oxidative stress. They belong to different chemical classes. Recently there are studies related to pyridazinone derivatives for their antioxidant activities. Since there are evidences implicates reactive oxygen species and nitric oxide as mediators of inflammation and/or tissue damage in inflammatory and arthritic disorders it was though that compounds that have both antioxidant and anti-inflammatory activities would have been essential for the inflammatory diseases. Based on these findings a series of 2H-pyridazine-3-one and 6-chloropyridazine analogues that have anti-inflammatory activity was tested in vitro on superoxide formation and effects on lipid peroxidation were determined against α-tocopherol. Most of the compounds have strong inhibitory effect on superoxide anion (between 84% – 99%) at 10− 3 M concentration. In addition, these compounds showed similar activity to α-tocopherol at 10− 3 M concentrations.
Introduction
Oxidative damage of important cellular constituents such as lipids, proteins and DNA, can be involved in aging as well as may play a role in the pathogenesis of various diseases such as cancer, atherosclerosis, rheumatoid arthritis and ischemic injuryCitation1-3. Oxidative stress is an imbalance situation, where excessive quantities of reactive oxygen species (ROS) such as superoxide radical anion, hydrogen peroxide, and hydroxyl radical are present at higher levels than those required for normal cell function and overwhelm endogenous antioxidant capacity and repair.
Pyridazine derivatives possess many activities like vasorelaxant[Citation4], anti-convulsant[Citation5], anti-hypertansive[Citation6] and recently antioxidant[Citation7]. 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H) dione detects superoxide, peroxynitrite and hydrogen peroxide in cell free systems as well as in isolated mitochondria[Citation8]. 5-Substituted pyrrolo[1,2-b]pyridazines exhibit profound inhibition of lipid peroxidation in vitro[Citation7]. There are evidences implicates ROS and nitric oxide as mediators of inflammation in inflammatory and arthritic disorders[Citation9]. Free radical species play a key role in modulating inflammation/infection induced alterations in skeletal muscle function. Inflammation and oxidative stress share an important role in the etiology of a variety of chronic diseases[Citation10,Citation11]. The vital role of chronic inflammation in disease development continues especially in neurodegenerative disorders. Using aminopyridazine oxidative and inflammatory cytokine pathways can be inhibited[Citation12].
Based on these findings it was thought that compounds that have both antioxidant and anti-inflammatory activities would have been crucial for inflammatory diseases since, side effects and/or insufficient pharmacokinetic profiles have made most of the drug candidates undesirable. We investigated the capacity of a series of 2H-pyridazine-3-one and 6-chloropyridazine analogues as antioxidant. Synthesis and physical properties of these compounds were published by our group earlier with their significant anti-inflammatory activity resultsCitation13-16.
Materials and methods
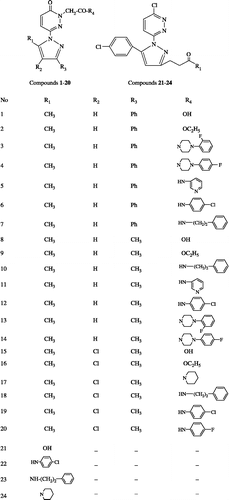
Xanthine, xanthine oxidase, cytochrome c, 2,2,diphenyl-1-picrylhydrazyl (DPPH), butylated hydroxytoluene and α-tocopherol thiobarbituric acid (TBA) were purchased from Sigma Chemical Co. (St Louis, MO, USA). The chemical reagents used in synthesis were purchased from Sigma (Germany) and Aldrich (USA). Preparation, physical properties and anti-inflammatory activity of the 2H-pyridazine-3-one and 6-chloropyridazine were published earlier by our group (compounds 1 to 713, 8 to 1414, 15 to 2015 and 21 to 2416).
Antioxidant activity studies
Superoxide radical scavenging activity
The capacity of 2H-pyridazine-3-one and 6-chloropyridazine derivatives to scavenge superoxide anion was determined spectrophotometrically on the basis of inhibition of cytochrome c reduction according to the modified method of McCord et al [Citation17]. Superoxide anion was generated in the xanthine/xanthine oxidase system. The incubation mixture (1 ml, total volume) was consisted of phosphate buffer (pH 7.8, 0.05M), xanthine oxidase (0.32 Units/mL), xanthine (50 μM), cytochrome c (60 mM) and different concentrations of pyridazine derivatives at 100 μL. The reaction was started by the addition of xanthine oxidase to this mixture. The absorbance was measured at 550 nm for 3 min for cytochrome c reduction. Each experiment was triplicated, and the results are expressed as a percent of the control. Solvent of the compounds was used as a control.
Assay of lipid peroxidation
The effect of the 2H-pyridazine-3-one and 6-chloropyridazine derivatives on rat liver homogenate induced with FeCl2-ascorbic acid was determined. Lipid peroxidation (LP) was examined by the method of Mihara et al [Citation18]. Procedures involving the animals and their care conformed to institutional guidelines, in compliance with national and international laws and guidelines for the use of animals in biomedical research. Animals were starved for 24 h. prior to sacrifice and then sacrificed by decapitation under anesthesia. The livers were immediately removed and washed in ice-cold distilled water, then immediately homogenized with a Teflon homogenizer. LP was measured spectrophotometrically by estimation of thiobarbituric acid reactants (TBARS). Amounts of TBARS were expressed in terms of nmol malondialdehyde (MDA/ g tissue). A typical optimized assay mixture contained 0.5 mL of liver homogenate, 0.1 mL of Tris-HCl buffer (pH 7.2), 0.05 mL of 0.1 mM ascorbic acid, 0.05 mL of 4 mM FeCl2 and 0.05 mL of various concentrations of pyridazine derivatives or α-tocopherol, and was incubated for 1 h at 37°C. After incubation, 3.0 mL of H3PO4 and 1 mL of 0.6% TBA were added and shaken vigorously. The mixture was boiled for 30 min. After cooling the mixture to room temperature, n-butanol was added and mixed strongly. The n-butanol phase was separated at 3000 rpm for 10 min. The absorbance of the supernatant was read at 532 nm against a blank, which contained all reagents except liver the homogenate.
Statistical analysis
The values are expressed as mean ± SD. The analysis was performed using by SPSS (version 11 for Windows, SPSS Inc, Chicago, IL). Mean differences were determined by least significant difference multiple range test. All the experiments were performed in duplicate.
Results and discussion
In the present study in vitro antioxidant capacity of 2H-pyridazine-3-one and 6-chloropyridazine derivatives was studied using two different in vitro assays, superoxide radical scavenging activity and effect on lipid peroxidation. The inhibitory effects of different concentrations of pyridazine derivatives on superoxide anion and lipid peroxidation are presented in . The results showed that some of the pyridazine derivatives at the 10− 3M concentrations showed significant superoxide anion scavenging effect, and the scavenging rates were in the range of 15–99%. Compounds 3, 4, 6, 19, 20, 22, 23, and 24 have strong inhibitory effect on superoxide anion (94%, 93%, 84%, 94%, 99%, 87%, 98%, and 96%, respectively) at 10− 3 M concentration. In addition, these compounds showed similar activity to α-tocopherol at 10− 3 M concentrations. Compounds 1 and 12 had weak scavenger effect on superoxide anion by about 15% and 25%, respectively, at 10− 3 M concentration.
Table I. Effects of the compounds on superoxide anion and lipid peroxidationa.
Results gave a prospect to evaluate the antioxidant properties of 2H-pyridazine-3-one 2H-pyridazine-3-one (1-20) and 6-chloropyridazine (21-24) derivatives. Almost all the 6-chloropyridazines (22-24) represented significant superoxide anion scavenging effect while only five of the 2H-pyridazine-3-one showed the similar activity. Among the 2H-pyridazine-3-one derivatives, compounds bearing chloro/flourophenyl amine and flourophenylpiperazine side chains on pyridazine ring (R4) revealed the highest activity. Also it was observed that compound 20 that has the maximum activity has chloro substitution (R2) in the imidazole ring. According to the findings superoxide anion scavenging activity of substituted 2H-pyridazine-3-one and 6-chloropyridazine derivatives might be related to the chemical structure of these compounds.
All the compounds had no effect on DPPH radical scavenging activity that was tested by measuring the pyridazine derivatives ability to bleach the stable radical 2,2,diphenyl-1-picrylhydrazyl (DPPH)[Citation19] (Data are not shown).
In the lipid peroxidation process, the initiation of a peroxidation sequence refers to the attack of a ROS, This attack easily generates free radicals from polyunsaturated fatty acids. OH radical is the most efficient ROS to do that attack. In this study a lipid peroxidation system which forms malondialdehyde and is readily detected using thiobarbituric acid, was used to assess hydroxyl radical formation.
Using the LP assay pyridazine derivatives showed rather weak inhibitory effect on MDA formation at 10− 3 M and 10− 4 M. Except compounds 4, 19 and 20 that have chloro/flourophenyl amine and flourophenylpiperazine side chains on pyridazine ring no significant activity was observed concerning inhibition of LP. In addition 6-chloropyridazine derivatives (21-24) have no effect on the inhibition of LP. This result shows that the tested pyridazine derivatives in general act as potent superoxide radical scavengers rather than hydroxyl radical scavengers in in vitro conditions.
The superoxide anion radical has been implicated in several pathophysiological processes, due to its transformation into more reactive species including hydroxyl radical that initiates lipid peroxidation. Superoxide has also been observed to directly initiate lipid peroxidation[Citation20]. Therefore, the scavenging of superoxide anion radical by compounds 3, 4, 6, 19, 20, 22, 23, and 24 and inhibition of LP by compounds 4, 19, 20 are likely to make them promising antioxidants.
References
- Suzen S. Recent developments of melatonin related antioxidant compounds. Com Chem High Thorough Synt 2006; 9(6)409–419
- Suzen S, Bozkaya P, Coban T, Nebioğlu D. Investigation of in vitro antioxidant behavior of some 2-phenylindole derivatives: Discussion on possible antioxidant mechanisms and comparison with melatonin. J Enz Inhib Med Chem 2006; 21(4)405–411
- Gurer-Orhan H, Orhan H, Püsküllü MO, Buyukbingol E, Suzen S. Synthesis and evaluation of in vitro antioxidant capacities of some benzimidazole derivatives. J Enz Inhib Med Chem 2006; 21(2)241–247
- Kots AY, Grafov MA, Khropov YV, Betin VL, Belushkina NN, Busygina OG, Yazykova MY, Ovchinnikov Kulikov AS, IV, Makhova NN, Medvedeva NA, Bulargina V, Severina IS. Vasorelaxant and antiplatelet activity of 4,7-dimethyl-1,2, 5-oxadiazolo[3,4-d]pyridazine 1,5,6-trioxide: Role of soluble guanylate cyclase, nitric oxide and thiols. Br J Pharmacol 2000; 129(6)1163–1177
- Moreau S, Coudert P, Rubat C, Vallee-Goyet D, Gardette D, Gramain JC, Couquelet J. Synthesis and anticonvulsant properties of triazolo- and imidazopyridazinyl carboxamides and carboxylic acids. Bioorg Med Chem 1998; 6(7)983–991
- Gil-Longo J, de los Reyes Laguna M, Verde I, Castro ME, Orallo F, Fontenla JA, Calleja JM, Ravina E, Teran C. Pyridazine derivatives. XI: Antihypertensive activity of 3-hydrazinocycloheptyl[1,2-c]pyridazine and its hydrazone derivatives. J Pharm Sci 1993; 82(3)286–290
- Ostby OB, Gundersen LL, Rise F, Antonsen O, Fosnes K, Larsen V, Bast A, Custers I, Haenen GR. Synthesis of 5-substituted pyrrolo[1,2-b]pyridazines with antioxidant properties. Arch Pharm (Weinheim) 2001; 334(1)21–24
- Imada I, Sato EF, Miyamoto M, Ichimori Y, Minamiyama Y, Konaka R, Inoue M. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal Biochem 1999; 271(1)53–58
- Cuzzocrea S. Role of nitric oxide and reactive oxygen species in arthritis. Curr Pharm Res 2006; 12(27)3551–3570
- Supinski G, Callahan LA. Free radical mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol 2007; 102: 1649–1657
- Peake JM, Suzuki K, Coombes JS. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J Nutr Biochem 2007; 18: 357–371
- Craft JM, Watterson DM, Frautschy SA, Van Eldik LJ. Aminopyridazines inhibit β-amyloid-induced glial activation and neuronal damage in vivo. Neurobiol Aging 2004; 25(10)1283–1292
- Banoğlu E, Akoğlu C, Ünlü S, Küpeli E, Yeşilada E, Şahin MF. Amide derivatives of [6-(5-methyl-3-phenylpyrazol-1-yl)-3(2H)-pyrazinone-2-yl]acetic acids as potential analgesic and anti-inflammatory compounds. Arch Pharm Pharm Med Chem 2004; 337: 7–14
- Banoğlu E, Akoğlu C, Ünlü S, Çalışkan-Ergun B, Küpeli E, Yeşilada E, Şahin MF. Synthesis of amide derivatives of [6-(3,5-dimethylpyrazol-1-yl)-3(2H)-pyrazinone-2-yl]acetic acid and their analgesic and anti-inflammatory properties. Arz Forsch Drug Res 2005; 55: 520–527
- Şüküroğlu M, Çalışkan-Ergun B, Ünlü S, Şahin MF, Küpeli E, Yeşilada E, Banoğlu E, Akoğlu C. Synthesis, analgesic and anti-inflammatory activities of [6-(3,5-dimethyl-4-pyrazol-1-yl)-3(2H)-pyrazinone-2-yl]acetamides. Arch Pharm Res 2005; 28: 509–517
- Şüküroğlu M, Çalişkan-Ergün B, Das-Evcimen N, Sarikaya M, Banoğlu E, Suzen S. Screening and evaluation of rat kidney aldose reductase inhibitory activity of some pyridazine derivatives. Med Chem Res 2007, in print
- McCord J, Fridowich I. An enzymic function for erythrocuprein. J Biol Chem 1969; 243: 6049–6055
- Mihara M, Uchiyama M, Fukuzawa K. Thiobarbituric acid value of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem Med 1980; 23: 303–311
- Blois MS. Antioxidant determination by the use of stable free radical. Nature 1958; 181: 1199–1200
- Halliwell B. Free radicals and antioxidants: A personal view. Nutrition Rev 1994; 52: 253–265