Abstract
The anti-AChE activity of phosphoramidates has been noticed for many years. Because of the wide application of phosphoramidates in recent years, there has been a continuing research for synthesis, purification and identification of effective and safe derivatives. In this study some rodenticides with the general formula Me2NP(O)(p-OC6H4-X)2, where X = H, CH3, Cl, have been synthesized in water (without organic solvent) and characterized by 31P, 31P {1H}, 13C and 1H NMR spectroscopy. Since lipophilicity has been recognized for its importance in QSAR studies, efforts have been made to determine the logP values. The ability of these rodenticides to inhibit human acetylcholinesterase (hAChE) has been predicted with PASS (Prediction of Activity Spectra for Substances) software (version 1.917) and then has been evaluated by a modified Ellman's assay and spectrophotometric measurements.
Introduction
Cholinesterases are targets for phosphoramidates which have wide applications in medicine (treatment of cancer, AIDS and Alzheimer's disease), agriculture (insecticides, herbicides, rodenticides and fungicides), industry (stabilizers, oxidants) and other interesting scientific fields. Correlation between the in vitro inhibition of AChE and the acute LD50 measured in vivo provide further evidence confirming the central role of the sensivity of AChE as the principal determinate of acute toxicity of phosphoramidates[Citation1]. The physiological role of acetylcholinesterase (AChE) is to hydrolyze the neurotransmitter acetylcholine (ACh) released in the process of cholinergic transmission of nerve impulses[Citation2]. Phosphoramidates rodenticides that usually act by inhibiting AChE and ChE activity, resulting in an accumulation of acetylcholine in neural and non-neural tissues. Accumulation of ACh in neuromuscular junction and synaptic cleft is belived to be the major cause of death in the target species [Citation3]. AChE active center, according to classical theory, consists of an anionic site that binds with the inhibitor and an catalytic site that participates in the enzymes phosphorylation. Substrates such as acetylcholine react at the catalytic site. Phosphoramidates usually phosphylate the catalytic site of AChE, and that reaction inhibits the enzyme[Citation4]. Substrates and reversible ligands protect cholinesterases from phosphylation by OP compounds. Even when reversible ligands bind only to the anionic site of the enzyme, the catalytic site can be protected from phosphylation[Citation5]. Binding sites of reversible ligands and their affinities for cholinesterases can be evaluated by different approaches. For details concerning the evaluation of binding sites and the mechanism of action of cholinesterase inhibitors, the reader is referred to extensive publications[Citation4]. Screening of cholinesterase inhibition has been done by determining the IC50 value[Citation6,Citation7].
The basic philosophy in Structure-Activity relationships is that the structural changes that affect the biological activities of a set of congeners are of three major types: electronic, steric, and hydrophobic. Other factors, such as hydrogen bonding, polarizability, and dipole moment, appear to play less important roles[Citation8]. Thus, the biological activity of chemical compounds can be related to their physicochemical properties by several functions:
LogP: hydrophobic parameter, σ: Hammet electronic constant, Es:Taft steric constant.
Since lipophilicity (the affinity of a molecule or a moiety for a lipophilic environment) has been recognized for its importance in QSAR (Quantitative Structure-Activity Relationship) studies, efforts have been made to determine the logP (logarithm of partition coefficient in biphasic systems, such as n-octanol/water) value. This parameter is closely related to the transport properties of rodenticides and their interaction with receptors. This parameter can be either determined experimentally or calculated[Citation9].
Rodenticides such as warfarin, bromodiolone and difenacoum, are defined as any substance that is used to kill rats, mice, and other rodent pests. It has been found that certain esters of amidophosphoric acids or of amidothiophosphoric acids especially those shown with the following general formula (1) can be used with great advantage as rodenticides in the surface spraying process[Citation10].
Where X represents a member selected from the group consisting of oxygen and sulfur, aryl preferably represents phenyl radicals, which furthermore may be substituted with halogen or nitro groups, especially with chlorine groups; R1, R2 represents hydrogen, a lower alkyl or alkenyl radical or halogen-substituted lower alkyl radical or aryl group.
In this research three kinds of phosphoramidates with the general formula Me2NP(O)(p–OC6H4–X)2, with X = H,CH3,Cl that have application in agriculture as rodenticides, have been selected. The basis of this selection was the Fujita hydrophobic constants (π parameter). After synthesis and characterization, LogP values for the target compounds have been experimentally determined by the “shake-flask” method. PASS software (version 1.917) was utilized for prediction of anti-AChE and other biological activity. Inhibitory potency were experimentally determined by Ellman's method.
Methods
NMR spectra were recorded on a Bruker DOX-250 instrument. CDCl3 was used as solvent. 1H and 13C chemical shifts were determined relative to internal TMS and 31P chemical shifts relative to 85% H3PO4 as external standard. Absorbances were measured with PERKIN-ELMER Lambda5 UV spectrophotometer.
Synthesis
The target rodenticides were prepared by treatment of POCl3 with the N,N-dimethylamine salt and then phenolic derivatives.
N,n-dimethyl Phosphoramidic Acid Di,phenyl Ester, Me2np(o)(p-oc6h5)2
Phenol (15.06 g, 160 mmol) was dissolved in a 10% aqueous solution containing 6.40 g of sodium hydroxide (160 mmol). This solution was cooled at 10°C; then 6.48 g (40 mmol) dimethyl amidophosphoric acid dichloride (from reaction between P(O)Cl3 and (CH3)2NH.HCl in reflux system) was added slowly into this solution. By cooling the reaction temperature was kept at about 4°C. After the addition had been completed the mixture was stirred for a further 4 h. Then the two layers were separated and the lower oily layer washed twice with 500 mL of water. The remaining water was removed by distillation in vacuum[Citation10]. Flash gradient chromatography method was used for the purification of the product (silicagel, hexane-ethyl acetate 4:1).
Red liquide, υmax/cm− 1(KBr): 1250(P = O), 1108(P–O–C), 740(P–N), 1H NMR (CDCl3), δ(ppm): 2.70 (6 H, d, 3JP–H = 2.5 Hz, 2 CH3), 6.76–7.22 (10 H, m, ArH); 13C NMR (CDCl3), δ(ppm): 152.00 (d), 129.70 (s), 124.90 (s), 120.00 (s), 36.70 (s); 31P{1H} NMR δ(ppm): 1.60 (s); 31P NMR, δ(ppm): 1.29–1.89 (hept., 3JP–H = 10.1 Hz), yield:80%.
Lipophilicity study
Hydrophobicity is the affinity of a molecule or a moiety for a lipophilic environment. It is commonly measured by its distribution in a biphasic system, either liquid-liquid (e.g., partition coefficient in 1-octanol/water) or solid/solid (retention on reversed-phase high performance liquid chromatography (RP-HPLC) or thin-layer chromatography (TLC) system). Partition coefficient in octanol/water (logP) has long been used to parameterize hydrophobic character in QSAR studies. In our research logP values for the target compounds were experimentally determined by the shake-flask method. Calculation of logP values was performed as follows[Citation11]:
where: P – partition coefficient, y – total mass of compound (mg), x – mass of compound in the buffer phase after partitioning (mg), Vbuffer – volume of buffer (ml), Voct – volume of n-octanol (ml).
With different concentrations of compound in buffer, a calibration graph was plotted. Then 0.047 g of compound was dissolved in 5 mL of n-octanol and 10, 20, 30 mL of buffer was added. The phases were shaken together on a mechanical shaker for 30 min, centrifuged for 20 min to afford complete phase separation, and the n-octanol phase was removed. Absorbance of the buffer phase was measured using a UV/VIS spectrophotometer at 272 nm. The concentration was then calculated from the calibration graph and logP value was determined by formula (2) ().
Table I. Log P value for (Me2N)P(O)(O–C6H5)2 by the shake-flask method.
In vitro evaluation of Acetylcholinesterase inhibition:
ATCh(acetylthiocholine) is a suitable substrate for AChE. The enzyme used in this work was of human erythrocyte AChE from Sigma (Cat. No. C0663). The colorimetric Ellman method is the most commonly used assay for the determination of AChE activity through acetylthiocholine hydrolysis to acetic acid and thiocholine. The assay makes use of the thiocholine-mediated cleavage of the chromogenic disulfide DTNB[Citation10] and activity is measured by following the increase in absorbance at 412 nm.
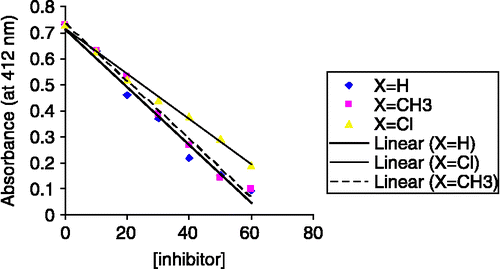
Absorption of organophosphorus compounds in vivo is commonly assessed by measuring the decrease in acetylcholinesterase activity. Inhibition curves were obtained with acetylthiocholine in the different concentrations of rodenticide compounds[Citation13].
The enzyme samples, 3μL (8 u) were incubed at room temperature in different concentrations of rodenticide in the phosphate buffer (70 mM). Then the enzyme activity was determined using ATCh as the substrate[Citation14], (final ATCh conc. during enzyme assay = 0.5 mM). At concentrations ranging from 10 to 60 mM the phosphoramidic acid showed a reversible inhibition with an IC50 of 31.3 mM, ().
The two following compounds were prepared and their logP and IC50 values were determined similarly.
N,n-dimethyl Phosphoramidic Acid Bis-(4-methyl-phenyl) Ester, Me2np(o)(p-oc6h4-ch3)2
Yellow liquid, υmax/cm− 1(KBr): 1235(P = O), 1090(P–O–C), 650(P–N), 1H NMR (CDCl3), δ(ppm): 2.85 (3 H, s, p–CH3), 3.32 (6H, d, 3JP–H = 7.5 HZ, 2 CH3), 7.66 (8 H, s, ArH); 13C NMR (CDCl3), δ(ppm): 152.00 (d), 134.20 (s), 130.00 (s), 119.70 (s), 36.75 (s), 20.60 (s); 31P{1H} NMR δ(ppm): 2.15 (s); 31P NMR, δ(ppm): 1.95–2.35 (hept., 3JP–H = 10.1 HZ). yield:74%.
N,n-dimethyl Phosphoramidic Acid Bis-(4-chloro-phenyl) Ester, Me2np(o)(p-oc6h4-cl)2
Yellow liquid, υmax/cm− 1(KBr): 1310(P = O), 1090(P–O–C), 810(P–N), 1H NMR(CDCl3), δ(ppm): 2.78 (6 H, d, 3JP–H = 35.0 HZ, 2 CH3), 6.65–7.30 (8 H, m, ArH); 13C NMR (CDCl3), δ(ppm): 149.20 (d), 129.80 (s), 121.00 (s), 116.70 (s), 36.60 (s); 31P{1H} NMR δ(ppm): δ = 1.68 (s); 31P NMR, δ(ppm): 1.38–1.99(hept., 3JP–H = 10.1 HZ), yield:89%.
Computational evaluation of lipophilicity and biological activity:
Because experimental measurements are time consuming and sometimes difficult, computational methods are very valuable tools for calculation of logP values. A number of different computer programs for prediction of lipophilicity have been recently developed[Citation9]. In our work, miLogP 1.2., computer program[Citation15] for predicting logP have been compared with experimental data. PASS software[Citation16] for computational evaluation of biological activity has been used, too.
miLogP 1.2
– The miLogP 1.2. program calculates log P values as a sum of group contributions and correction factors. The group contributions were obtained by fitting calculated logP values with experimental logP values for a training set of several thousands of drug-like molecules[Citation9].
PASS (Prediction of activity spectra for substances)
This is a software product for predicting the biological activity spectrum for chemical substances on the basis of their structural formulas[Citation17]. Biological activity spectra for our target compounds have been predicted as probable with pa > 0.7 (pa: probabilities to be active), (version 1.917-July 2005). Biological activity spectrum for N,N-Dimethyl phosphoramidic acid di,phenyl ester are shown in .
Table II. PASS output for N,N-Dimethyl phosphoramidic acid di,phenyl ester.
Results and discussion
Physicochemical properties (δ of 31P{1H} NMR, measured and calculated logP) and anti-AChE activity experimental and predictive values for target rodenticides are summarized in . There are many various methods for synthesis of phosphoramidates, and the utilization of non-safe organic solvents such as benzene and pyridine is incidental to all of them. long time and high temperature are disadvantages for these. Target compounds have been synthesized previously by different methods, but here we have synthesized them, in water (without organic solvents), in high yield and at room temperature.
Table III. Physicochemical properties and anti-AChE activity experimental and predictive values for target rodenticides.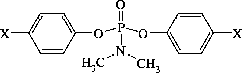
Reproducibility and low standard deviation for hydrophobicity results confirm that the shake-flask method in its simplicity, is a suitable method for logP measurements. Qualitative comparison between measured and calculated logP results show that Cl- and CH3- substituents increase the affinity of a molecule for lipophilic environment in concordance with Fujita constants (π). One should not lose sight of the fact that, even at the present stage of development, computer calculation of logP has often prompted remeasurement of a solute with the result that the more carefully measured value agrees well with the calculated.
Conformity between computational and in vitro evaluation of anti-AChE activity denote reliable prediction by PASS software. Medium hAChE inhibition activity for the rodenticides under study show that these compounds can be used widely in the agricultural industry.
The most important result in our research is a decrease in inhibitory activity in lieu of an increase in lipophilicity that can assist to the preparation of new, safe rodenticides. Although the electronic parameter has an important role in QSAR studies no correlation was observed between the electronic parameter (δ) and IC50, so showing that the hydrophobic parameter has a more important role.
Acknowledgements
We are sincerely grateful to the Department of Biology for providing us with a biochemistry lab and thankful to the New Ideas Research Institute for the gift of AChE.
References
- Kendall B, Wallace J. Species specificity in the chemical mechanisms of organophosphorus anticholinesterase activity. Chem Res Toxical 1991; 4: 41–49
- Dauterman WC, OBrien RD. Cholinesterase variation as a factor in organophosphate selectivity in insects. J Agric Chem 1964; 12: 318–319
- Singh AK. Quantitative structure-activity relationships for phosphoramidothioate toxicity in housefly. Com Biochem Physio 1999; 123: 241–255
- PrimoŽiČ I, OdŽak R, Tomić S, Simeon-Rudolf V, Reiner E. Pyridinium, imidazolium, and quinucludinium oximes: synthesis, interaction with native and phosphylated cholinesterases, and antidotes against organophosphorus compounds. J Med Chem Def 2004; 2: 1–30
- Reiner E. Inhibition of acetylcholinesterase by 4,4′-bipyridin and its effect upon phosphorylation of the enzyme. Croat Chem Acta 1986; 59: 925–931
- Gholivand Kh, Shariatinia Z, Ghadimi S, Mojahed F, Madani Alizadegan A, Khajeh Kh, Naderi-manesh H. Acetylcholinesterase inhibition by diaza- and dioxophosphole compounds: Synthesis and determination of IC50 values. J Enz Inhib Med Chem 2004; 19(5)403–407
- Gholivand Kh, Shariatinia Z, Khajeh k, Naderimanesh H. Synthesis and spectroscopic characterization of some phosphoramidates as reversible inhibitors of human acetylcholinesterase and determination of their potency. J Enz Inhib Med Chem 2006; 21: 31–35
- Hansch C, Leo A, Exploring QSAR. Fundamentals and applications in chemistry and biology. ACS professional references book, Washington, DC 1995; 1–161
- Medic M, Mornar S. Lipophilicity study of salicylamide. Acta Pharm 2004; 54: 91–101
- U.S. 2,146,356.
- Medic-Saric M, Mornar A, Badovinac-Crnjevic T, Jasprica I. Experimental and calculation procedures for molecular lipophilicity: A comparative study for 3,3′-(2-methoxybenzylidene)bis(4-hydroxycoumarin). Croat Chem Acta 2004; 77: 367–370
- Hoenicka M. Ellman Assay. Anal Biochem 1968; 25: 192–205
- Skrinjaric-Spoljar M, Sinko G, Reiner E, Simeon-Rudolf V. Acetylcholinesterase from rat cells and cholinesterase of Pseudomonas aeruginosa: Different types of inhibition by atropine. Molecular and Cellular Biochemistry 1981; 34:95–99
- Sing AK, White T, Spassova D, Jiang Y. Physicochemical, molecular-orbital and electronic properties of acephate and methamidophos. Biochem Physiol 1998; 119: 107–117
- http://www.molinspiration.com
- PASS., software. version 1.917-July2005.
- Lagunin A, Stepanchikova A, Filimonov D, Poroikov V. PASS. Bioinformatics applications note 2000; 16: 747–748