Abstract
Some useful therapeutic agents inhibit certain carbonic anhydrase (CA) isozymes to varying degrees. We have conducted enzyme kinetics studies in a 4-nitrophenyl acetate (4-NPA) hydrolysis assay with the marketed antiepileptic drugs topiramate (1) and zonisamide (2) to determine if their full inhibition of human CA-II and CA-I requires extended preincubation conditions. We found that neither 1 nor 2 requires appreciable preincubation with either enzyme to manifest full inhibitory activity. We also examined the sulfamide cognate of topiramate (3) to characterize its CA inhibitory activity, and confirmed that it is a very weak inhibitor, unlike 1 or 2. In a CO2 hydration assay, 3 behaved as a very weak, partial inhibitor of CA-II and CA-I. We conclude that topiramate (1), zonisamide (2), and sulfamide 3 do not require extended exposure to human CA-I or CA-II to manifest full inhibitory activity (4-NPA assay).
Introduction
Carbonic anhydrases (CAs) are important enzymes that have attracted considerable interest [Citation1,Citation2]. Given the roles of CAs in many physiological and pathophysiological processes, CA inhibitors can serve as useful therapeutic agents for treating numerous diseases, including glaucoma, cancer, and obesity Citation2-4. Topiramate (TPM; 1) and zonisamide (ZNS; 2) are widely marketed antiepileptic drugs that evince some CA inhibitory actions. Although these drugs have relatively little structural similarity, they do possess analogous sulfamate and sulfonamide moieties, respectively. Additionally, these drugs share several pharmacological properties in that they inhibit some types of neuronal voltage-gated Na+ and Ca2 + channels [Citation5], and certain isoforms of CA[Citation2]. When their CA inhibitory activity was first identified in the 1980's, these compounds were viewed as comparatively weak inhibitors (e.g., relative to acetazolamide), with Ki values for ZNS in the range of 4–12 μMCitation6-8 and Ki values for TPM in the range of 1–120 μMCitation9-11 (for CA activity in erythrocytes, brain, or kidney; in various species). As knowledge about the various CA isozymes developed, and as CA isozymes became available in purified forms, it has become possible to establish more accurate Ki values for each CA isozymeCitation1-4.
Recently, we have conducted studies involving four distinct assay procedures to determine Ki or Kd values for the interaction of TPM with human CA-II and CA-ICitation12-15. The Ki or Kd values from the four procedures consistently ranged from 0.3–0.6 μM for CA-II and 90–140 μM for CA-I. In contrast, another research group has reported much more potent inhibition of human CA-II and CA-I by TPM, with Ki values of 0.005–0.010 μM and 0.25 μM, respectively[Citation16,Citation17]. This apparent discrepancy in Ki values of approximately 60-fold (CA-II) and 400-fold (CA-I) provided an impetus for us to seek independent verification through a direct thermodynamic method (ThermoFluor®), which afforded a Kd value of 0.3 μM for TPM[Citation14]. Since the marked differences in Ki (or Kd) values between the two research groups have yet to be adequately resolved, we became intrigued by the possibility that slow-tight binding kinetics might play a role. Indeed, a recent publication reports slow-tight binding behavior for ZNS with CA-II[Citation18]. According to De Simone et al.[Citation18], establishing a true Ki for ZNS as an inhibitor of CA-II requires one-hour preincubation of ZNS with CA-II prior to initiation of the catalytic reaction. More specifically, preincubation of ZNS for 15 minutes with CA-II yielded a Ki of 10.3 μM; however, preincubation for 60 minutes yielded a much more potent Ki of ∼35 nM[Citation18]. We have performed experiments to replicate these findings with ZNS, as well as to determine if the Ki values for TPM and its sulfamide cognate (3; JNJ-17065984) would require such an extended preincubation period. In contradistinction, we found that preincubation of up to one hour had essentially no effect on the Ki value for ZNS with either CA-II or CA-I. The same behavior was observed for TPM and sulfamide 3. Additionally, the present study confirms our prior observations that sulfamide 3 is an exceedingly weak inhibitor of CA-II and CA-ICitation13-15.
Materials and methods
Test compounds
Topiramate (1) and its sulfamide cognate 3 were synthesized as described earlier[Citation13]. Zonisamide (2) and 4-nitrophenylacetate (4-NPA) were purchased from Sigma Chemical Co. (St. Louis, MO). Test compounds were dissolved in DMSO at 100 mM and diluted in purified water just prior to the kinetics measurements.
Enzyme reactions
In most of the experiments we used 4-nitrophenylacetate (4-NPA) as the substrate for enzyme catalysis (). 4-NPA (colorless) is hydrolyzed to 4-nitrophenol (yellow) and acetate, and the reaction is monitored by the emergence of color[Citation19,Citation20]. A detailed description of the assays used in this study was reported previously[Citation12,Citation15]. The incubation medium was buffered with HEPES (10 mM) and the pH was adjusted to 7.3 with Tris. For each sample, the reaction volume included 70 μL of the HEPES–Tris solution, 10 μL of an aqueous solution of test compound (10 μL of H2O for catalyzed control sample), 10 μL of a freshly prepared aqueous solution of purified human erythrocyte CA-II (∼0.02 to 0.1 μg) or CA-I (∼0.1 to 0.5 μg) (10 μL of H2O for uncatalyzed reaction samples), and 10 μL of a solution in which 4-NPA was dissolved in DMSO, then diluted 10-fold with absolute ethanol. The concentration of 4-NPA in the reaction medium was 3 mM. The reaction was performed at 21–23°C in a 96-well plate. The reaction was initiated by adding 4-NPA stock solution either within a few minutes, or at 60 min, after addition of the enzyme. In each experiment, test compounds were evaluated in quadruplicate. Immediately after initiating the reaction, the plate was placed in a SPECTRAmax 384 spectrophotometer (Molecular Devices, Sunnyvale, CA) and the formation of reaction product was monitored at 1-min intervals for 15–20 min at 400 nm. The concentration of 4-NPA was at least 25-fold lower than the Km of 4-NPA as a CA-II substrate[Citation19,Citation20]. According to the Cheng–Prusoff equation[Citation21], under these conditions the IC50 and Ki values differ by less than 3%, and they are regarded as virtually synonymous in this study.
Figure 1 Graphic representation of the percent inhibition of CA-II by ZNS as a function of the concentration of ZNS and the incubation (Inc) time after initiation of the enzymatic reaction (4-NPA hydrolysis assay). The increase in the optical density was monitored at 1-minute intervals. The data derive from one experiment performed in quadruplicate.
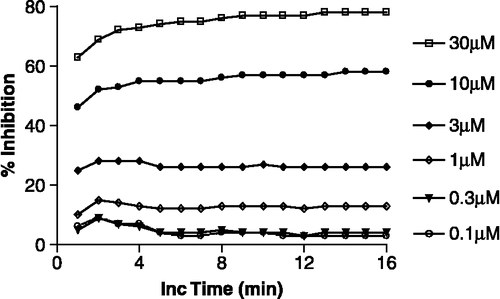
Figure 2 Concentration–inhibition graphs illustrating the effect of preincubation on the inhibition of CA-II and CA-I by ZNS (4-NPA assay). The data are the mean ( ± SEM) of 4 or 5 experiments, each of which was performed in quadruplicate. The curve-fit analysis of the data assumed normal saturation and one-site competition. Filled circles are paired with the dashed line.
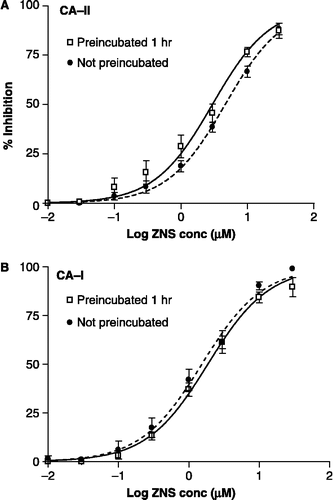
Figure 3 Concentration–inhibition graphs showing the effect of preincubation on the inhibition of CA-II and CA-I by TPM (4-NPA assay). The data are the mean ( ± SEM) of 4 or 5 experiments (each done in quadruplicate). The curve-fit analysis of the data was performed with a sigmoidal saturation equation that allows for a variable slope. Filled circles are paired with the dashed line.
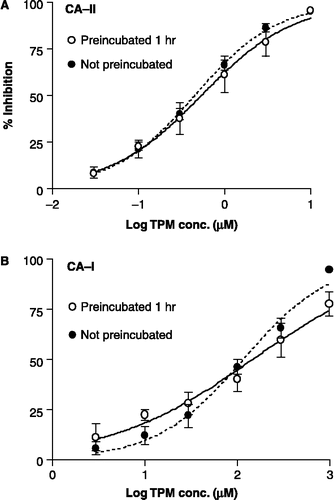
Figure 4 Concentration–inhibition graphs illustrating the effect of preincubation on the inhibition of CA-II and CA-I by 3 (4-NPA assay). The data are the mean ( ± SEM) of 3 or 6 experiments (each done in quadruplicate). The curve-fit analysis of the data was performed using a sigmoid saturation equation that allows for a variable slope. Filled circles are paired with the dashed line.
Data analysis and calculation of Ki values
The relative difference in the spectrophotometric optical density between the uninhibited CA-catalyzed reaction and the uncatalyzed reaction (nonspecific activity) was calculated and assigned a value of 100. The data for each test compound were referenced to the two sets of control data, from which the percent inhibition of CA activity was calculated. Replicates for each experiment were averaged. Each compound was tested in two or three separate experiments at six concentrations. From the concentration–inhibition data, IC50 (Ki) values were calculated by using PRISM 4 (Graphpad, San Diego, CA). Two curve-fit equations were used to analyze the data. One equation assumed single site normal competition saturation kinetics [%I = (100 – 0)/(1 + 10(LogX-LogEC50)]; where 100 and 0 represent the maximum and minimum possible % inhibition (I), respectively, and X is the inhibitor concentration) and the other allowed the saturation kinetics to vary and generate a variable Hill slope with maximum inhibition of ≤ 100% [%I = (100 – 0)/(1 + 10((LogEC50-LogX)*B)); where 100, 0, I, and X are the same as above, and B is the Hill slope].
Results
At the outset, we conducted an experiment with ZNS involving CA-II-catalyzed 4-NPA hydrolysis to ascertain the rate at which the CA-II inhibitory effect approached equilibrium when there was no prior exposure of the enzyme to ZNS (). Our results indicate that the binding equilibrium is achieved within 15 minutes even in the absence of preincubation.
In subsequent experiments, we determined the effect of 60-minute preincubation of ZNS with CA-II or CA-I on the calculated Ki (). For one set of samples, the hydrolysis of 4-NPA was initiated within a few minutes after ZNS and the enzyme (CA-II or CA-I) were combined in the incubation medium, after which the samples were “read” every minute over a 20-minute period. The raw data from this 20-minute experiment were used to calculate the Ki values. Another set of samples was treated identically except that there was a 60-minute delay prior to initiating the hydrolysis of 4-NPA. The concentration–inhibition data for each of the four sets of samples modeled well to the equation describing normal saturation for one-site competition kinetics (). The Ki (95% confidence interval, 95% CI) values from this analysis for inhibition of CA-II were 2.98 (2.30–3.85) μM for the preincubated samples and 4.64 (4.06–5.30) μM for the samples that were not preincubated (). The corresponding Ki (95% CI) values for the inhibition of CA-I were 1.86 (1.62–2.15) μM and 1.53 (1.22–1.92) μM for the preincubated and non-preincubated samples, respectively ().
A similar set of experiments with CA-II or CA-I was conducted for TPM to determine the effect of preincubation on the Ki value (). The concentration–inhibition data were subjected to a curve-fit analysis assuming one-site competition with normal saturation kinetics, as well as a curve-fit analysis that used a sigmoidal dose-response with a variable slope. From this analysis, the Ki (95% CI) values for the inhibition of CA-II were 0.54 (0.38–0.76) μM for the preincubated samples and 0.45 (0.38–0.53) μM for the non-preincubated samples (). (The Ki values derived from the two curve-fitting procedures differed by less than 1%.) Since the inhibition of CA-I by the preincubated set of samples exhibited a low Hill slope (0.55), we elected to use a curve-fit analysis with a variable slope (). The corresponding Ki (95% CI) values for the inhibition of CA-I were 147 (93–234) and 117 (92–150) μM for the preincubated and non-preincubated experiments, respectively ().
We also examined the sulfamide cognate of TPM (3; JNJ-17065984) in this manner, since the Ki values for 3 reported by us and Supuran's group have differed widely (as mentioned above). For 3, we have obtained consistent Ki values of 340, 456, and 650 μM in three independent experiments, as well as a Kd value of 25 μMCitation13-15. On the other hand, Supuran and coworkers reported a Ki value of 2 μM[Citation17]. Could this discrepancy in the Ki values be attributable to differences in preincubation time? To address this issue, we not only performed a set of experiments similar to those performed for ZNS and TPM, but also an experiment involving CA-catalyzed hydration of CO2, which is described in detail elsewhere[Citation13,Citation15]. The concentration–inhibition data for the 4-NPA hydrolysis assay with 3 were subjected to the two curve-fit analyses, as described above for TPM. The inhibition of both CA-II and CA-I exhibited relatively low Hill slopes (0.74). For this reason, the curve-fit analysis using the variable slope was chosen again. The Ki (95% CI) values for the inhibition of CA-II by 3 were 382 (315–464) μM for the preincubated samples and 489 (367–652) μM for the non-preincubated samples (). The corresponding Ki (95% CI) values for the inhibition of CA-I by 3 were 569 (347–933) and 707 (506–989) μM for the preincubated and non-preincubated samples, respectively ().
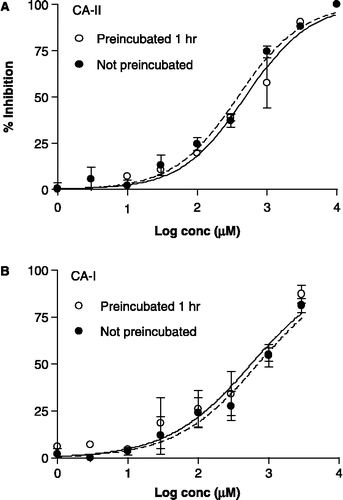
In a previous study of 3 with the CO2 hydration procedure, we found it to be a weak, partial inhibitor of CA-II[Citation15]. To substantiate that observation, we now performed experiments with expanded concentration ranges: 0.1 μM to 10 mM for CA-II () and 10 μM to 10 mM for CA-I (). With both enzymes, the inhibitory activity at the 10 mM concentration of 3 was far below the maximum possible inhibition of 100%. A PRISM curve-fit analysis indicated that the maximum inhibition of CA-II and CA-I by 3 was just ∼50% and ∼33%, respectively. The IC50 values obtained by curve-fit analysis were converted to Ki values by using the Cheng–Prusoff equation[Citation21]. The Ki (95% CI) value for inhibition of CA-II was 243 (3.3–17800) μM, with a low Hill slope of 0.48 ± 0.24 (SEM) and maximum % inhibition of 50 ± 25. The exceptionally wide 95% CI for the Ki is attributable to the very shallow concentration–inhibition curve (), which turned out to cause rather large standard errors. The Ki value for inhibition of CA-I was 48.7 (21.2–112) μM, with a Hill slope of 2.0 ± 1.4 (SEM) and a maximum % inhibition of 33 ± 5 μM. Evidently, the CO2 hydration assay has limitations for assessing very weak inhibitors such as 3.
Figure 5 Concentration–inhibition graphs depicting the inhibitory effect of 3 on the activity of human CA-II and CA-I as determined with a CO2 hydration assay (described in detail previously[Citation15]), instead of the 4-NPA assay. The data are the mean ( ± SEM) of 2–6 experiments (each performed in triplicate). The curve-fit analysis of the data was performed with a sigmoidal saturation equation that allowed for a variable slope and variable maximum % inhibition. The Ki was 2.4-fold and 4.6-fold lower than the IC50 for CA-II and CA-I, respectively.
![Figure 5 Concentration–inhibition graphs depicting the inhibitory effect of 3 on the activity of human CA-II and CA-I as determined with a CO2 hydration assay (described in detail previously[Citation15]), instead of the 4-NPA assay. The data are the mean ( ± SEM) of 2–6 experiments (each performed in triplicate). The curve-fit analysis of the data was performed with a sigmoidal saturation equation that allowed for a variable slope and variable maximum % inhibition. The Ki was 2.4-fold and 4.6-fold lower than the IC50 for CA-II and CA-I, respectively.](/cms/asset/cbdcbee2-e0bb-43e4-ad93-024e1007bffe/ienz_a_250567_f0005_b.gif)
Discussion
The results of our investigation indicate that the interaction of ZNS with CA-II or CA-I reached equilibrium within a 15-minute period. Also, extending the exposure time prior to initiating the catalytic reaction with CA-II or CA-I had little or no effect on the Ki values generated with the 4-NPA hydrolysis assay. In the same vein, extending the exposure of TPM or sulfamide 3 with either isozyme to one hour prior to adding 4-NPA had little or no effect on the Ki values (relative to those obtained at 15 minutes). On the basis of these results, extended preincubation of the inhibitor with the enzyme cannot be responsible for the disparate Ki values reported by usCitation12-15 and Supuran's group[Citation16,Citation17]. Moreover, the Ki values for the inhibition of CA-II by 3 of 380 μM (preincubated) and 490 μM (non-preincubated) are consistent with our prior observations of exceedingly weak inhibition of CA-IICitation13-15. In fact, we were the first to note that sulfamides appear to be much weaker inhibitors of CA-II than their sulfamate congeners[Citation13]. Later, we substantiated this point with an independent assay based on direct binding to CA-II (ThermoFluor® method)[Citation22], which yielded thermodynamically-based Kd values[Citation14]. For 3, the ThermoFluor technique afforded a Kd value of 25 μM, which is 50-fold weaker than the Kd value for TPM[Citation14]. The weaker potency of a sulfamide relative to its sulfamate cognate may be ascribed to the former being much less acidic, with a larger pKa value by nearly 2 units[Citation14]. This ∼100-fold lower acidity for the sulfamide would mean that it has a much lower population of the anionic form required for binding to Zn(II) in the CA active site. An alternative steric explanation for 3 being a weaker inhibitor of CA-II than TPM (1) was proposed by Winum et al. from an examination of the X-ray co-crystals of 1√CA-II and 3√CA-II[Citation17].
The Kd (ThermoFluor) and Ki (CO2 hydration assay) values for an inhibitor compound, have generally shown reasonable consistency ( ≤ 3-fold difference)[Citation14]. However, there was divergence from this picture in the case of sulfamide 3, for which the Ki was ∼25-fold higher than the Kd (Ki = 650 μM vs. Kd = 25 μM). In a subsequent study, we compared sulfamates to sulfamides as inhibitors of CA-II and CA-I, and obtained Ki values by utilizing both the CO2 hydration and 4-NPA hydrolysis assays[Citation15]. Thus, we encountered very shallow concentration–inhibition curves in the CO2 hydration assay with several sulfamides, reflecting characteristics of partial and/or mixed inhibition. By contrast, the same sulfamides demonstrated full inhibition in the 4-NPA hydrolysis assay. To investigate this issue further for sulfamide 3, we expanded the concentration conditions to encompass a 100,000-fold range in the current study (). It was hoped that such a broad range would establish the maximum inhibition of CA-II more accurately and thereby furnish a more accurate Ki value. However, despite this expanded concentration range, the Ki value of 243 μM from the PRISM curve-fit analysis had an extremely wide 95% confidence interval of 3.3–17800. Consequently, in the CO2 hydration assay sulfamide 3 is a very weak, partial inhibitor of CA-II, such that it is difficult to establish a reasonably accurate Ki value for 3.
In the various assays that have been employed to test compounds for inhibition of CA isozymes, the biochemical principles and experimental conditions can vary greatly. For example, assays based on the hydration of CO2 are typically performed at lower temperatures (0–5°C) to minimize uncatalyzed hydration of CO2, whereas the ThermoFluor assay is performed at higher temperatures (50–60°C) to monitor the molecular folding state of the enzyme. The temperature differences in these two assays are likely to have some influence on the determined inhibitor–enzyme affinity values. The operative question is: What would be considered reasonable differences in Ki or Kd values generated by different assays performed in various laboratories? In this respect, the following issues come into play: (1) the intrinsic properties of the assay procedure, including the design of the experiment (e.g., number of concentrations and replicates for each inhibitor; the concentration(s) of the substrate); (2) the method used to analyze the data; and (3) normal experimental error that is inherent for each assay procedure. Because of the numerous variables involved in generating the raw data and the various ways of calculating Ki or Kd values, differences of as much as 3-fold would be standard and acceptable. However, when the differences exceed 5-fold, and especially 10-fold, it becomes important to identify and understand the cause(s). Our current study was performed with this aspect in mind. First, we sought to identify the source of the 26-fold difference between the Kd and Ki values for sulfamide 3 obtained in our own laboratories[Citation14], as described above. Second, we sought to resolve the issue related to the 60-fold to ∼400-fold difference between our reported Ki and Kd values for TPM (0.3–0.6 μM for human CA-II; 90–150 μM for human CA-I)Citation12-15 and the Ki values reported by Supuran and coworkers (0.005–0.010 μM for human CA-II; 0.25 μM for human CA-I)[Citation16,Citation17]. In the present study, our CA-II Ki value for 3 of 380–490 μM still differs by ca. 200-fold from the CA-II Ki value of 2 μM reported by Winum et al[Citation17].
Winum et al.[Citation17] insinuated that the CA inhibition data produced in our published studies may be flawed due to the presence of Zn++ chelators in the incubation medium, without proffering any evidence, experimental or otherwise, that there could be such an effect. Perhaps, their suggestion may be based on the notion that dithiothreitol (DTT) is included in some CO2 hydration-based assays[Citation23], and that DTT at very high concentrations can bind to CA-bound Zn, thereby attenuating the CA activity[Citation24]. We have performed experiments without DTT in the reaction medium, or with DTT at the concentration recommended by Cammer et al.[Citation23], and found no differential effects on the activity of CA-II or CA-I (control experiments; not shown). Additionally, with or without DTT, there were no differences in the potencies of CA-II or CA-I inhibition for TPM or the reference inhibitor acetazolamide (control experiments; not shown). Along this line, it is important to note that EDTA, a well-known Zn++ chelator, actually enhances the affinity of various sulfonamide inhibitor ligands for CA-II and CA-I; it does not diminish their affinity[Citation22]. Consequently, a Zn++ chelator is highly unlikely to be responsible for the higher Ki and Kd values that we have reported for TPMCitation12-15.
Time and again, through several independent lines of investigation, we have demonstrated the key points of our claims. From the work described herein, we firmly corroborate the Ki values for zonisamide (2), topiramate (1), and the sulfamide cognate of topiramate (3; JNJ-17065984). Moreover, we conclude that zonisamide, topiramate, and sulfamide 3 do not require extended preincubation to express full activity as inhibitors of CA-II and CA-I. Finally, we wish to underscore that sulfamide 3 is an exceedingly weak inhibitor of both CA isozymes.
References
- Wistrand PJ. The carbonic anhydrases: New horizons, WR Chegwidden, ND Carter, YH Edwards. Birkhäuser Verlag, Basel 2000; 597–609
- Scozzafava A, Mastrolorenzo A, Supuran CT. Expert Opin Ther Pat 2004; 14: 667–702
- Maren TH. The carbonic anhydrases: New horizons, WR Chegwidden, ND Carter, YH Edwards. Birkhäuser Verlag, Basel 2000; 425–436
- Parkkila S, Parkkila A-K, Kivela J. Carbonic anhydrase: Its inhibitors and activators, CT Supuran, A Scozzafava, J Conway. CRC Press, Boca Raton, FL 2004; 283–301
- Rogawski MA, Löscher W. Nat Rev Neurosci 2004; 5: 553–564
- Matsumoto K, Miyazaki H, Fujii T, Hashimoto M. Chem Pharm Bull 1989; 37: 1913–1915
- Masuda Y, Karasawa T. Arzneim-Forsch 1993; 43: 416–418
- Masuda Y, Noguchi H, Karasawa T. Arzneim.-Forsch 1994; 44: 267–269
- Maryanoff BE, Nortey SO, Gardocki JF, Shank RP, Dodgson SP. J Med Chem 1987; 30: 880–887
- Shank RP, Gardocki JF, Vaught JL, Davis CB, Schupsky JJ, Raffa RB, Dodgson SJ, Nortey SO, Maryanoff BE. Epilepsia 1994; 35: 450–460
- Dodgson SJ, Shank RP, Maryanoff BE. Epilepsia 2000; 41: S35–S39, (Suppl. 1)
- Shank RP, Doose DR, Streeter AJ, Bialer M. Epilepsy Res 2005; 63: 103–112
- Maryanoff BE, McComsey DF, Costanzo MJ, Hochman C, Smith-Swintosky V, Shank RP. J Med Chem 2005; 48: 1941–1947
- Klinger AL, McComsey DF, Smith-Swintosky V, Shank RP, Maryanoff BE. J Med Chem 2006; 49: 3496–3500
- Shank RP, McComsey DF, Smith-Swintosky VL, Maryanoff BE. Chem Biol Drug Design 2006; 68: 113–119
- Casini A, Antel J, Abbate F, Scozzafava A, David S, Waldeck H, Schäfer S, Supuran CT. Bioorg Med Chem Lett 2003; 13: 841–845
- Winum J-Y, Temperini C, Cheikh KE, Innocenti A, Vullo D, Ciattini S, Montero J-L, Scozzafava A, Supuran CT. J Med Chem 2006; 49: 7024–7031
- De Simone G, Di Fiore A, Menchise V, Pedone C, Antel J, Casini A, Scozzafava A, Wurlb M, Supuran CT. Bioorg Med Chem Lett 2005; 15: 2315–2320
- Thorslund A, Lindskog S. Eur J Biochem 1967; 3: 117–123
- Pocker Y, Stone JT. Biochemistry 1967; 6: 668–678
- Cheng Y, Prusoff WH. Biochem Pharmacol 1973; 22: 3099–3108
- Matulis D, Kranz JK, Salemme FR, Todd MJ. Biochemistry 2005; 44: 5258–5266
- Cammer W, Fredman T, Rose AL, Norton WT. J Neurochem 1976; 27: 165–171
- Husic HD, Hsieh S, Berrier AL. Biochim Biophys Acta 1991; 1078: 35–42
