Abstract
Some novel ‘tailor-made’ compounds, 6,6-dimethyl-7,9-diaryl-1,2,4,8-tetraazaspiro[4.5]decan-3-thiones 23–27 have been studied for their in vitro antibacterial activity against Staphylococcus aureus, β-Heamolytic streptococcus, Vibreo cholerae, Salmonella typhii, Shigella felxneri, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa and anti-fungal activity against Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem. Compounds 24 and 25 exerted potent antibacterial activity against S. aureus, β-H. streptococcus, E. coli and P. aeruginosa whereas all compounds 23–27 exerted strong in vitro antifungal activity against A. flavus, Mucor and Rhizopus.
Introduction
Synthesis of bioactive compounds in the field of organic chemistry received significant attention resulting in substantial advances both in the synthetic and medicinal aspects. Bioactive heterocyclic ring systems having 2,6-diaryl-piperidine-4-one nucleus with different substituents at 3- and 5-positions of the ring have aroused great interest due to their wide variety of biological properties such as antiviral, antitumour [Citation1,Citation2], central nervous system [Citation3], local anesthetic [Citation4], anticancer [Citation5], antimicrobial activity [Citation6] and their derivative piperidine are also biologically important and act as neurokinin receptor antagonists [Citation7], analgesic and anti-hypertensive agents [Citation8].
The 1,2,4-triazole nucleus Citation9, Citation10, Citation11, Citation12 has been incorporated in a wide variety of therapeutically interesting drugs including H1/H2 histamine receptor blockers, cholinesterase active agents, CNS stimulants, anti anxiety agents, sedatives, analgesics, and anti convulsants.
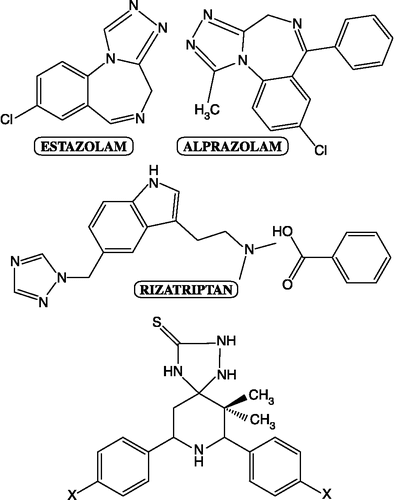
Due to an increase in the number of immuno-compromised hosts, [Citation13], over the past decades, the incidence of systemic microbial infections has been increasing dramatically. The increasing incidence of bacterial resistance to a large number of antibacterial agents such as glycopeptides (vancomycin, inhibition cell walls synthesis), sulfonamide drugs (inhibitors of tetrahydrofolate synthesis), β-lactam antibiotics (penicillins and cephalosporins), nitroimidazoles and quinolones (DNA inhibitors), tetracyclins, chloramphenicol and macrolides (erythromycin, inhibiting protein synthesis) is becoming a major concern [Citation14]. For the past several years, vancomycin has been considered the last line of defense agent against Gram-positive infections and no alternative drugs for treating diseases that have become resistant to vancomycin [Citation15]. Patients undergoing organ transplants, anticancer chemotherapy or long treatment with antimicrobial agents and patients with AIDS are immuno suppressed and very susceptible to life threatening systemic fungal infections like Candidiasis, Cryptococcosis and Aspergillosis. Antifungal azoles, fluconazole and itraconazole which are strong inhibitors of lanosterol 14α-demethylase (cytochrome P45014DM) and orally active have been widely used in antifungal chemotherapy. Reports are available on the developments of resistance to currently available antifungal azoles in Candida spp., as well as clinical failures in the treatment of fungal infections Citation16, Citation17, Citation18, Citation19. Furthermore, most of the present antifungal drugs are not effective against invasive Aspergillosis and the only drug of choice in such patients is the injectable amphotericin B. Some examples of 1,2,4-triazole based antibacterial and antifungal drugs are estazolam [Citation20], alprazolam [Citation21] and rizatriptan [Citation22]. It is known from that some clinically useful drugs contain a 1,2,4-triazole moiety.
These observations places new emphasis on the need to search for alternative new and more effective antimicrobial agents with a broad spectrum. Recently, we exploited the synthesis of 2,6-diarylpiperidin-4-one derivatives Citation23, Citation24, Citation25 with a view to incorporate various other bioactive heterocyclic nucleus such as 1,2,3-selenadiazoles, 1,2,3-thiadiazoles, diazepans intact for evaluation of associated antibacterial and antifungal activities. In the interest of above, we planned to synthesize a system, which comprises both piperidine and triazolidin-3-thione components together to give a compact structure like title piperidinyl spiro-1,2,4-triazolidin-3-thiones.
Experimental
Chemistry
Performing TLC assessed the reactions and the purity of the products. All the reported melting points were taken in open capillaries and were uncorrected. IR spectra were recorded in KBr (pellet forms) on a Nicolet-Avatar–330 FT-IR spectrophotometer and note worthy absorption values (cm− 1) alone are listed. 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz respectively on Bruker AMX 400 NMR spectrometer using DMSO-d as solvent. The ESI + ve MS spectra were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on Carlo Erba 1106 CHN analyzer.
By adopting the literature procedure [Citation26], 3,3-dimethyl-2,6-diarylpiperidin-4-ones were prepared 13–17.
General method of preparation of 3,3-dimethyl-2,6-diarylpiperidin-4-one thiosemicarbazones 18–22
A mixture of 3,3-dimethyl-2,6-diarylpiperidin-4-one (0.01 mol) and thiosemicarbazide (0.01 mol) in ethanol (40 mL) was refluxed on a steam bath for 2 h and was concentrated to one-third of its original volume. After cooling, the mixture was poured over crushed ice. The solid product thus obtained was filtered off and recrystallized twice from ethanol to give 3,3-dimethyl-2,6-diarylpiperidin-4-one thiosemicarbazones as crystalline solid.
Typical procedure for the synthesis of 6,6-dimethyl-7,9-diphenyl-1,2,4,8-tetraazaspiro[4.5]decan-3-thione 23
A solution of 3,3-dimethyl-2,6-diphenylpiperidin-4-one thiosemicarbazone 18 (0.005 mol) in dichloromethane (50 mL) was treated with m-chloroperbenzoic acid (0.01 mol) and stirred for 1 h at (0–5)oC. The reaction mixture was decomposed with an ice-cold solution of sodium hydrogen carbonate solution. The organic layer was washed with brine solution, and then with excess of water and dried over anhydrous sodium sulfate. After evaporation of the solvent under reduced pressure, a gummy mass was obtained, which was solidified on treatment of petroleum ether (bp40–60). Final purification of 7,9-diphenyl-6,6-dimethyl-1,2,4,8-tetraazaspiro[4.5]decan-3-thione was done by column chromatography using silica gel (100–200 mesh), with ethyl acetate -Petroleum ether (bp40–60) in the ratio (3:7) as eluent. IR (KBr) (cm− 1): 3424, 3366, 3146, 3030, 2971, 2928, 1289, 764, 701; 1H NMR (δ ppm): 0.94 (s, 3H, CH3 at C-6), 1.15 (s, 3H, CH3 at C-6), 2.63 (s, 1H, H8), 2.13-2.19 (dd, 1H, H10a), 3.22–3.29 (dd, 1H, H10e), 3.66 (s, 1H, H2) 3.77–3.83 (dd, 1H, H9a, J9a,10e = 2.92, J9a,10a = 17.12), 7.13 (s, 1H, H1), 7.26–7.58 (m, 10H, Harom), 8.32 (s, 1H, H4); 13C NMR (δ ppm): 20.8, CH3 at C-6, 21.3 CH3 at C-6, 32.6, C-10, 42.4, C-6, 59.9, C-9, 69.7, C-7, 79.1, C-5, 126.7–128.9 − Carom,140.4, 144.1 ipso-C, 179.0 C-3.
The compounds 24–27 were synthesized correspondingly.
6,6-dimethyl-7,9-bis(4-methyphenyl)-1,2,4,8-tetraazaspiro[4.5]decan-3-thione 24
IR (KBr) (cm− 1): 3429, 3315, 3102, 3022, 2972, 2924, 1246, 817, 745; 1H NMR (δ ppm): 1.02 (s, 3H, CH3 at C-6), 1.08 (s, 3H, CH3 at C-6), 2.07 (s, 6H, CH3 at arom. ring) 2.49 (s, 1H, H8), 2.18–2.27 (dd, 1H, H10a), 2.94–3.10 (dd, 1H, H10e), 3.61 (s, 1H, H2) 3.67–3.71 (dd, 1H, H9a, J9a,10e = 2.12, J9a,10a = 15.52), 7.08 (s, 1H, H1), 7.10–1.42 (m, 8H, Harom), 8.50 (s, 1H, H4); 13C NMR (δ ppm): 20.6, CH3 at C-6, 21.1 CH3 at C-6, 22.7, CH3 at arom. ring 32.3, C-10, 42.2, C-6, 59.8, C-9, 69.5, C-7, 79.0, C-5, 126.5–128.7 − Carom, 136.1, 137.5, 141.1, 159.7 ipso-C, 180.0 C-3.
6,6-dimethyl-7,9-bis(4-methoxyphenyl)-1,2,4,8-tetraazaspiro[4.5]decan-3-thione 25
IR (KBr) (cm− 1): 3427, 3314, 3246, 3158, 2969, 2929, 1246, 832, 750; 1H NMR (δ ppm): 0.92 (s, 3H, CH3 at C-6), 1.16 (s, 3H, CH3 at C-6), 2.49 (s, 1H, H8), 2.13–2.31 (dd, 1H, H10a), 3.47–3.51 (dd, 1H, H10e), 3.59 (s, 1H, H2) 3.69–3.74 (dd, 1H, H9a, J9a,10e = 2.16, J9a,10a = 16.04), 3.87 (s, 6H, OCH3 at arom. ring), 7.13 (s, 1H, H1), 7.33–7.97 (m, 8H, Harom), 8.30 (s, 1H, H4); 13C NMR (δ ppm): 20.8, CH3 at C-6, 22.7 CH3 at C-6, 32.7, C-10, 42.6, C-6, 55.0, 54.9, OCH3 at arom. ring, 59.7, C-9, 69.1, C-7, 79.0, C-5, 126.6–129.8 –Carom, 132.5, 136.2, 158.4, 159.9 ipso-C, 179.0 C-3.
6,6-dimethyl-7,9-bis(4-fluorophenyl)-1,2,4,8-tetraazaspiro[4.5]decan-3-thione 26
IR (KBr) (cm− 1): 3427, 3369, 3254, 3149, 2975, 2931, 1291, 823, 748; 1H NMR (δ ppm): 1.12, (s, 3H, CH3 at C-6), 1.17 (s, 3H, CH3 at C-6), 2.60 (s, 1H, H8), 2.31–2.39 (dd, 1H, H10a), 3.53–3.58 (dd, 1H, H10e), 3.68–3.76 (dd, 1H, H9a, J9a,10e = 2.15, J9a,10a = 16.38), 7.18 (s, 1H, H1), 7.37–7.81 (m, 8H, Harom), 8.37 (s, 1H, H4); 13C NMR (δ ppm): 21.5, CH3 at C-6, 22.3 CH3 at C-6, 32.8, C-10, 42.8, C-6, 60.1, C-9, 69.9, C-7, 80.6, C-5, 127.8–129.3 − Carom, 137.9, 141.6, 158.3, 158.74 ipso-C, 180.2 C-3.
6,6-dimethyl-7,9-bis(4-chlorophenyl)-1,2,4,8-tetraazaspiro[4.5]decan-3-thione 27
IR (KBr) (cm− 1): 3425, 3368, 3251, 3147, 2972, 2929, 1289, 824, 745; 1H NMR (δ ppm): 1.16, (s, 3H, CH3 at C-6), 1.19 (s, 3H, CH3 at C-6), 2.61 (s, 1H, H8), 2.30–2.38 (dd, 1H, H10a), 3.51–3.57 (dd, 1H, H10e), 3.69–3.74 (dd, 1H, H9a, J9a,10e = 2.16, J9a,10a = 16.40), 7.20 (s, 1H, H1), 7.43–7.83 (m, 8H, Harom), 8.39 (s, 1H, H4); 13C NMR (δ ppm): 21.4, CH3 at C-6, 22.6 CH3 at C-6, 32.6, C-10, 42.4, C-6, 60.8, C-9, 69.8, C-7, 80.1, C-5, 128.0–129.8 –Carom, 137.7, 141.4, 158.0, 158.4 ipso-C, 180.0 C-3.
Microbiology
Materials
All the clinically isolated bacterial strains namely Staphylococcus aureus, β-Heamolytic streptococcus, Vibreo cholerae, Salmonella typhii, Shigella felxneri, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa and fungal strains namely Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem are obtained from Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
The in vitro activities of the compounds were tested in Sabourauds dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by two-fold serial dilution method [Citation27]. The respective test compounds 23–27 were dissolved in dimethylsulfoxide to obtain 1 mg mL− 1 stock solution. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7 days old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. The colony forming units (cfu) of the seeded broth were determined by plating technique and adjusted in the range of 104–105 cfu/mL. The final inoculums size was 105cfu/mL for antibacterial assay and 1.1–1.5 × 102 cfu/mL for antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at a pH 5.6 for fungi (SDB). Exactly 0.4 mL of the solution of test compound was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution and so on till six such dilutions were obtained. A set of assay tubes containing only seeded broth was kept as control. The tubes were incubated in BOD incubators at 37 ± 1°C for bacteria and 72–96 h for fungi. The minimum inhibitory concentrations (MICs) were recorded by visual observations after 24 h (for bacteria) and 72–96 h (for fungi) of incubation. Ciprofloxacin was used as standard for bacteria studies and Fluconazole was used as standard for fungal studies.
Results and discussion
Chemistry
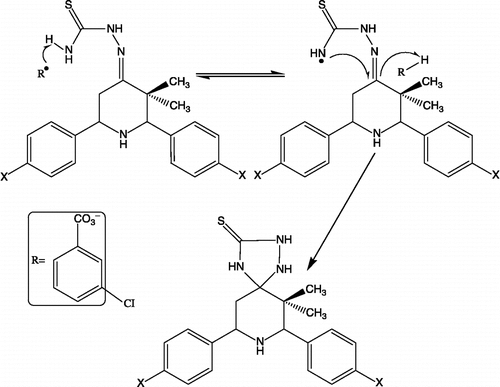
1,2,4-triazolidin-3-thione and their derivatives can be conveniently synthesized from aldehyde/ketone thiosemicarbazones and also from substituted thiosemicarbazide by cyclization using suitable reagents [Citation28]. Earlier MnO2 and H2O2 had been used for the synthesis of such compounds by the oxidative cyclization of thiosemicarbazones but the use of m-Chloroperbenzoic acid has provided better results. The schematic representation and the analytical data of compounds 23–27 are given in and , respectively. Cyclocondensation reaction of respective ketone, aldehyde and ammonium acetate in the ratio of 1:2:1, respectively afforded the formation of 2,6-diaryl-3,3-dimethyl-piperidin-4-ones 13–17. 2,6-diaryl-3,3-dimethyl-piperidin-4-ones are converted into their thiosemicarbazones 18–22 and are eventually oxidative cyclized with m-chloroperbenzoic acid at 0–5°C to afford 7,9-diaryl-6,6-dimethyl-1,2,4,8-tetraazaspiro[4.5]decan-3-thiones 23–27. The importance of the title compounds is due to their diverse potential, broad-spectrum biological activity. The structure of the newly synthesized compounds 23–27 is confirmed by melting point, elemental analysis, MS, FT-IR, one-dimensional NMR (1H & 13C) spectroscopic data. A free radical mechanism () has been proposed for the conversion of thiosemicarbazones to the piperidinyl spiro-1,2,4-triazolidin-3-thiones.
Scheme 2. Synthetic route for the formation of 7,9-diaryl-6,6-dimethyl-1,2,4,8-tetraazaspiro[4,5]-decan-3-thlones.
![Scheme 2. Synthetic route for the formation of 7,9-diaryl-6,6-dimethyl-1,2,4,8-tetraazaspiro[4,5]-decan-3-thlones.](/cms/asset/9361fad8-65ad-401b-b181-a7f48b282dba/ienz_a_318976_f0002_b.gif)
Table I. Physical and analytical data for 7,9-diaryl-6,6-dimethyl-1,2,4,8-tetraazaspiro[4.5]decan-3-thiones (23–27).
Antibacterial activity
New spiro-1,2,4-triazolidin-3-thione derivatives, 7,9-diaryl-6,6-dimethyl-1,2,4,8-tetraazaspiro[4.5]decan-3-thiones 23–27 were tested for their antibacterial activity in vitro against S. aureus, β-H.streptococcus, V. cholerae, S. typhii, S. felxneri, E. coli, K. pneumonia and P. aeruginosa. Ciprofloxacin was used as standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . Compounds 24 and 25 exerted potent antibacterial activity against S. aureus, β-H. streptococcus, E. coli and P. aeruginosa.
Table II. In vitro antibacterial activity (MIC) values for compounds 23–27.
Antifungal activity
The in vitro antifungal activity of piperidinyl spiro-1,2,4-triazolidin-3-thiones 23–27 was studied against the fungal strains viz., A.flavus, Mucor, Rhizopus and M.gypsuem. Fluconazole was used as a standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . All the synthesized compounds 23–27 exerted strong in vitro antifungal activity against Aspergillus flavus, Mucor and Rhizopus.
Table III. In vitro antifungal activity (MIC) values for compounds 23–27.
Conclusion
The microbiological screening studies carried out to evaluate the antibacterial and antifungal potencies of the newly synthesized piperidinyl spiro-1,2,4-triazolidin-3-thiones 23–27 are clearly known from Tables and . A close inspection of the in vitro antibacterial and antifungal activity profile in differently electron donating (CH3 and OCH3) functional group substituted phenyl rings of novel piperidinyl spiro-1,2,4-triazolidin-3-thiones 24 and 25 exerted strong anti-bacterial activity against the tested bacterial strains viz. S. aureus, β-H.streptococcus, E. coli and P. aeruginosa. Results of the anti-fungal activity study show that the nature of substituents on the phenyl ring viz., methyl, methoxy, fluoro and chloro functions at the para positions of the aryl moieties are determinant for the nature and extent of the anti-fungal activity of all the synthesized compounds 23–27 over fungal strains namely A.flavus, Mucor and Rhizopus. The method of action of these compounds is unknown. These observations may promote a further development of our research in this field. Further development of this group of piperidinyl spiro-1,2,4-triazolidin-3-thiones may lead to compounds with better pharmacological profile than standard antibacterial and antifungal drugs.
Acknowledgements
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording spectra. Two of our authors namely J.Thanusu and V.Kanagarajan are highly thankful for Annamalai University authorities for providing financial support in the form of Research Fellowship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- HI El-Subbagh, SM Abu-Zaid, MA Mahran, FA Badria, and AM Alofaid. (2000). Synthesis and biological evaluation of certain α, β-Unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem 43:2915–2921.
- AA Watson, GWJ Fleet, N Asano, RJ Molyneux, and RJ Nugh. (2001). Polyhydroxylated alkaloids. Natural occurrence and therapeutic applications. Phytochemistry 56:265–295.
- CR Ganellin, and RG Spickett. (1965). Compounds affecting the central nervous system. 1,4-piperidones and related compounds. J Med Chem 8:619–625.
- RE Hagenbach, and H Gysin. (1952). Piperidones as potential antitumour agents. Experimentia 8:184–187.
- B Ileana, and V Dobre. (1985). Nicluescu-Duvaz I Spin labeled nitrosoureas. Potential anticancer agents. J Prakt Chem 327:667–674.
- IG Mokio, AT Soldatenkov, VO Federov, EA Ageev, ND Sergeeva, S Lin, EE Stashenku, NS Prostakov, and EL Andreeva. (1989). Antimicrobial activity of aliphatic-aromatic ketones, β-ketols and α-glycols. Khim Farm Zh 23:421–427.
- JR Dimmock, and P Kumar. (1997). Anticancer and Cytotoxic Properties of Mannich Bases. Curr Med Chem 4:1–22.
- H Kubota, A Kakefuda, Y Okamoto, M Fujii, O Yamamoto, Y Yamgiwa, M Orita, K Ikeda, M Takenchi, and T Shibanuma. (1998). Fsomura Y Synthesis of ( ± )-N-[2-(3,4-dichlorophenyl)-4-(spiro-substituted piperidin-1′-yl)butyl]-N-methylbenzamides and evaluation of NK[1]-NK[2] dual antagonistic activities. Chem Pharm Bull 46:1538–1544.
- ND Heindel, and JR Reid. (1980). 4-Amino-3-mercapto-4 H-1,2,4-triazoles and Propargyl Aldehydes: A New Route to 3-R-8-Aryl-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazepines. J Heterocycl Chem 17:1087–1088.
- RA Glennon, MR Seggel, WH Sonie, K Herrick-Davis, RA Lyon, and M Titeler. (1988). Iodine-125 labeled 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane: An iodinated radioligand that specifically labels the agonist high-affinity state of 5-HT2 serotonin receptors. J Med Chem 31:5–7.
- S Stankovsky, F Jedlovska, and K Spirkova. (1993). Synthesis of some triazolyl acetanilides. Collect Czech Chem Commun 58:2211–2214.
- MZ Krimer, VP Tashch, YB Kalyan, FZ Bakaev, YG Putskin, and Y Molchanov. (1995). Synthesis and antimicrobial activity of halogenated derivatives of 2-benzylidene-1-(1,2,4-triazol-1-ylmethyl)-cyclohexanols. Khim Farm Zh 29:764–769.
- J Davies. (1996). Bacteria on the rampage. Nature 383:219–220.
- DTW Chu, JJ Plattner, and L Katz. (1996). New directions in antibacterial research. J Med Chem 39:3853–3874.
- RV SperaJr, and BF Farber. (1994). Multidrug-resistant Enterococcus faecium. An untreatable nosocomial pathogen. Drugs 48:678–688.
- FC Odds. (1993). Resistance of yeasts to azole-derivative antifungals. J Antimicrob Chemother 31:463–471.
- CA Hitchock. (1993). Resistance of Candida albicans to azole antifungal agents. Biochem Soc Trans 21:1039–1047.
- EM Johnson, DW Warnock, J Luker, and SR Porter. (1995). Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother 35:103–114.
- JH Rex, MG Rinaldi, and MA Pfaller. (1995). Resistance of Candida species to flucanazole. Antimicrob Agents Chemother 39:1–8.
- The Merck Index & Co, Inc. USA: Whitehouse Station NJ (2001). p 3737.
- The Merck Index & Co, Inc. Whitehouse Station NJ USA 2001. p 320
- The Merck Index & Co, Inc. Whitehouse Station NJ USA 2001. p 8324
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Design, synthesis, characterization, antibacterial and antifungal activities of novel class of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-selenadiazoles J Enz Inhib Med Chem, 2008 (in press)
- M Gopalakrishnan, J Thanusu, and V Kanagarajan. (2008). Synthesis and biological evaluation of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-thiadiazoles. Med Chem Res (in press)
- M Gopalakrishnan, P Sureshkumar, J Thanusu, V Kanagarajan, R Govindaraju, and G Jayasri. (2007). A convenient ‘one-pot’ synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enz Inhib Med Chem 22:709–715.
- CR Noller, and V Baliah. (1948). The preparation of some piperidine derivatives by the Mannich reaction. J Am Chem Soc 70:3853–3854.
- MH Dhar, MM Dhar, BN Dhawan, BN Mehrotra, and C Ray. (1968). Screening of Indian plants biological activity. Part I. Indian J Exp Biol 6:232–247.
- C Temple. In: Wiley, editor. Triazoles-1,2,4 The Chemistry of Heterocyclic Compounds Montgomery. New York: (1981).