Abstract
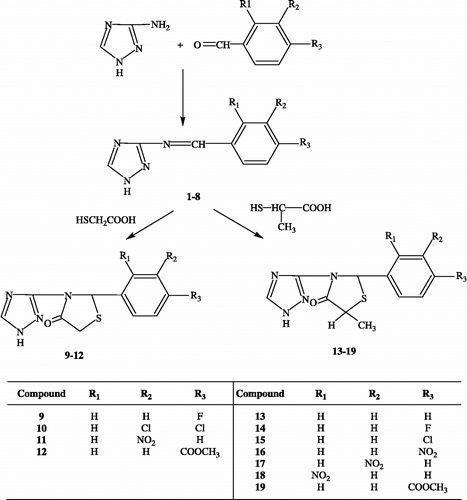
A new series of 3-(1,2,4-triazol-3-yl)-4-thiazolidinone derivatives has been synthesized by the reaction of Schiff bases of 3-amino-1,2,4-triazoles with mercaptoacetic acid and 2-mercaptopropionic acid. Their antibacterial and antifungal activities were evaluated against S. aureus, S. epidermidis, C. albicans and C. glabrata
Introduction
The clinical relevance of fungal diseases has increased over the past 30 years due to an increasing population of immunocompromised patients who have cancer, AIDS or have received transplants.
Almost all antifungal agents currently in use in human mycoses target the ergosterol biosynthetic pathway, an important component of fungal membranes. A member of the ergosterol biosynthesis pathway is the cytochrome P-450 dependent 14α-sterol demethylase (CYP51) enzyme, which catalyzes the oxidative removal of the 14α-methyl group of lanosterol to give Δ14,15 desaturated intermediates[Citation1,Citation2]. Triazole derivatives such as fluconazole and voriconazole have been shown to exhibit antifungal activity by inhibiting the fungal CYP51 enzyme [Citation3] hence inhibiting the synthesis of ergosterol. The widespread use of antifungal agents has led to the development of drug resistance [Citation4]. In addition the clinical value of current antifungal agents has been limited by high risk of toxicity and pharmacokinetic deficiencies of the compounds.
Therefore there is still an existing need for broad spectrum antifungal compounds.
Recent reports describe the synthesis and evaluation of new azoles with antifungal activity Citation5, Citation6, Citation7. 4-Thiazolidinones exhibit varius biological activities such as antibacterial Citation8, Citation9, Citation10, antiviral [Citation11], antituberculosis, anticancer [Citation12], and antifungal [Citation13,Citation14].
In order to take advantage of the antifungal and antibacterial properties of both triazoles and 4-thiazolidinones, we synthesized new triazole derivatives combined with 4-thiazolidinone moiety. Structural elucidation of these compounds was performed by UV, IR, 1H-NMR, 13C-NMR, mass spectroscopy and elemental analysis.
Material and methods
All chemicals were obtained from Merck (Schuchardt, Germany). Melting points were determined on a Buchi 530 capilllary melting point apparatus (Flawil, Switzerland) in open capillaries and uncorrected. The purity of the compounds were controlled by TLC on silica gel HF 254 + 366 (E. Merck, Darmstadt, Germany). UV spectra were determined using Shimadzu 1601 UV spectrophotometer (Kyoto, Japan). IR spectra were recorded on a Perkin Elmer 1600 FT Infrared spectrophotometer in potassium bromide pellets (Norwalk, Connecticut USA). 1H and 13C-NMR spectra were recorded on Bruker AC-L 200 and 300 MHz spectrophotometers using tetramethylsilane as internal standart (Rheinstatten, Germany). All chemical shifts were reported as δ (ppm), and spin-spin couplings as J (Hz) values. EI/MS were recorded on a VG Zab Spec (70 eV) (Manchester, England). Elemental analyses were performed on a Carlo Erba 1106 elemental analyzer at the Scientific and Technical Research Council of Turkey.
General procedure for the preparation of compounds
2-Aryl-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (9-19)
0.01 mole of compounds 1-8 was refluxed with 0.6 mole of mercaptoacetic acid for compounds 9-12 and with 2-mercaptoacetic acid for compounds 13-19 in dry benzene (30 mL) (care-carcinogenic) using a Dean-Stark trap for 3 days. Excess benzene was evaporated in vacuo. The residue was triturated with saturated NaHCO3 until CO2 evolution ceased and allowed to stand overnight.
The solid thus obtained was filtered, washed with H2O and crystallized from an appropriate solvent ().
2-(4-Fluorophenyl)-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (9)
Yield 28%; m.p. 197°C; IR (KBr, ν, cm− 1) 3212 (NH), 1694 (C = O); 1H-NMR (DMSO-d6, δ, ppm) 3.87, 4.07 (2H, 2d, J = 16.1 Hz, thiazole C5-H); 6.38 (1H, s, thiazole C2-H); 7.12 (2H, t, J = 8.7 Hz, Ar-H); 7.39-7.46 (2H, m, Ar-H); 8.27 (1H, s, triazole C5-H); 13.87 1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm): 31.96 (thiazole C5); 61.72 (thiazole C2); 115.05 (Ar-C); 115.40 (Ar-C); 128.58 (Ar-C); 128.73 (Ar-C); 136.40 (Ar-C1); 164.20 (Ar-C4); 144.40 (triazole C5); 159.34 (triazole C3); 170.56 (C = O); Anal. Calc.for C11H9FN4OS (M+): (264) C, 49.99; H, 3.43; N, 21.20. Found: C, 50.54; H, 2.95; N, 20.84%.
2-(3,4-Dichlorophenyl)-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (10)
Yield 80%; m.p. 192-193°C; IR (KBr, ν, cm− 1) 3212 (NH), 1686 (C = O); 1H-NMR (DMSO-d6, δ, ppm) 3.86, 4.13 (2H, 2d, J = 16.1 Hz, thiazole C5-H); 6.39 (1H, s, thiazole C2-H); 7.37 (1H, d, J = 8.4 Hz, Ar-H); 7.57 (1H, d, J = 8.3 Hz, Ar-H); 7.66 (1H, s, Ar-H); 8.22 (1H, s, triazole C5-H); 13.90 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 31.78 (thiazole C5); 60.93 (thiazole C2); 126.58 (Ar-C); 128.48 (Ar-C); 130.79 (Ar-C); 131.11 (Ar-C); 141.86 (Ar-C1); 170.52 (C = O). Anal. Calc.for C11H8Cl2N4OS (M+): (314) C,41.92; H, 2.56; N, 17.78. Found: C, 42.45; H, 2.09; N, 17.21%.
2-(3-Nitrophenyl)-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (11)
Yield 59%; m.p. 210-212°C; IR (KBr, ν, cm− 1) 3142 (NH), 1712 (C = O); 1H-NMR (DMSO-d6, δ, ppm) 3.89, 4.15 (2H, 2d, J = 16.1 Hz, thiazole C5-H);6.58 (1H, s, thiazole C2-H); 7.62 (1H, t, J = 7.9 Hz, Ar-H); 7.87 (1H, d, J = 7.7 Hz, Ar-H); 8.11 (1H, d, J = 7.9 Hz, Ar-H); 8.23 (1H, s, Ar-H); 8.25 (1H, s, triazole C5-H), 13.94 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 31.82 (thiazole C5); 61.24 (thiazole C2); 121.14 (Ar-C); 123.18 (Ar-C); 130.25 (Ar-C); 132.92 (Ar-C); 143.04 (Ar-C1); 147.77 (Ar-C3); 145.00 (triazole C5); 170.60 (C = O). Anal. Calc.for C11H9N5O3S (M+): (291) C,45.36; H, 3.11; N, 24.04. Found: C,45.80; H, 2.61; N, 24.28%.
2-[(4-Methoxycarbonyl)phenyl]-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (12)
Yield 14%; m.p. 214-215°C; IR (KBr, ν, cm− 1) 3223 (NH), 1722,1694 (C = O); 1H-NMR (DMSO-d6,δ, ppm) 3.82 (3H, s, ester CH3); 3.87, 4.08 (2H, 2d, J = 16.1 Hz, thiazole C5-H); 6.46 (1H, s, thiazole C2-H); 7.51 (2H, d, J = 8.3 Hz Ar-H); 7.89 (2H, d, J = 8.2 Hz, Ar-H); 8.17 (1H, s, triazole C5-H), 13.88 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 31.88 (thiazole C5); 52.09 (ester CH3); 61.70 (thiazole C2); 126.51 (Ar-C); 129.43 (Ar-C); 145.81 (Ar-C1); 165.70, 170.57 (C = O). Anal. Calc.for C13H12N4O3S (M+): (304) C,51.31; H, 3.97; N, 18.41. Found: C,51.77; H, 3.66; N, 18.03%.
5-Methyl-2-phenyl-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (13)
Yield 19%; m.p. 166-169°C; IR (KBr, ν, cm− 1) 3247 (NH), 1694 (C = O); 1H-NMR (DMSO-d6, δ, ppm) 1.51, 1.57 (3H, 2d, J = 6.9 Hz, CH3); 4.21-4.39 (1H,m, thiazole C5-H); 6.32, 6.36 (H, 2s, thiazole C2-H); 7.23-7.33 (5H, m, Ar-H); 8.16 (1H, s, triazole C5-H), 13.88 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 17.73, 19.48 (CH3); 39.54, 41.61 (thiazole C5); 59.99, 60.74 (thiazole C2); 126.03 (Ar-C); 126.65, 128.10 (Ar-C); 128.5 (Ar-C); 140.53 (Ar-C1); 172.69 (C = O). Anal. Calc.for C12H12N4OS (M+): (260) C,55.37; H, 4.64; N, 21.52. Found: C,55.49; H, 4.54; N, 20.95%.
2-(4-Fluorophenyl)-5-methyl-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (14)
Yield 27%; m.p. 168-174°C; IR (KBr, ν, cm− 1) 3219 (NH), 1705 (C = O); 1H-NMR (DMSO-d6, δ, ppm): 1.51, 1.57 (3H, 2d, J = 6.9 Hz, CH3); 4.18-4.36 (1H, m, thiazole C5-H); 6.32, 6.37 (1H, 2s, thiazole C2-H); 7.11 (2H, t, J = 8.7 Hz, Ar-H); 7.38-7.45 (2H, m, Ar-H); 8.29 (1H, s, triazole C5-H), 13.89 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 18.08, 19.40 (CH3); 40.86, 41.62 (thiazole C5); 59.76, 60.57 (thiazole C2); 115.12 (Ar-C); 115.55 (Ar-C); 128.57 (Ar-C); 129.45, 129.59 (Ar-C); 135.17, 136.96 (Ar-C1); 143.88 (triazole C5); 159.31, 159.45 (triazole C3); 164.16, 164.32 (Ar-C4); 173.24 (C = O). Anal. Calc.for C12H11FN4OS (M+): (278) C, 51.79; H, 3.98; N, 20.13. Found: C,52.31; H, 4.12; N, 19.94%.
2-(4-Chlorophenyl)-5-methyl-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (15)
Yield 44%; m.p. 185-186°C; IR (KBr, ν, cm− 1) 3201 (NH), 1705 (C = O); 1H-NMR (DMSO-d6, δ, ppm) 1.50, 1.55 (3H, 2d, J = 6.9 Hz, CH3); 4.18-4.36 (1H, m, thiazole C5-H); 6.32, 6.36 (1H, 2s, thiazole C2-H); 7.37 (4H, s, Ar-H); 8.16 (1H, s, triazole C5-H), 13.87 (1H, s, triazole N-H): 13C-NMR (DMSO-d6, δ, ppm) 17.71, 19.48 (CH3); 39.91, 41.61 (thiazole C5); 59.34, 60.24 (thiazole C2); 128.04, 128.49, 128.84 (Ar-C); 132.65, 132.95 (Ar-C); 138.51, 139.67 (Ar-C1); 144.14 (triazole C5); 173.00, 173.27 (C = O). Anal. Calc.for C12H11ClN4OS (M+): (294) C, 48.90; H, 3.76; N, 19.01. Found: C, 49.45; H, 3.28; N, 18.64%.
5-Methyl-2-(4-nitrophenyl)-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (16)
Yield 56%; m.p. 205-209°C; IR (KBr, ν, cm− 1) 3213 (NH), 1707 (C = O); 1H-NMR (DMSO-d6, δ, ppm) 1.50, 1.56 (3H, 2d, J = 6.8 Hz, CH3); 4.21-4.35 (1H, m, thiazole C5-H); 6.47, 6.57 (1H, 2s, thiazole C2-H); 7.65 (2H, d, J = 7.2 Hz, Ar-H); 8.17 (2H, d, J = 8.5 Hz, Ar-H); 8.41 (1H, s, triazole C5-H), 13.99 (1H, s, triazole N-H): 13C-NMR (DMSO-d6, δ, ppm) 17.44, 19.60 (CH3); 39.48, 41.59 (thiazole C5); 59.05, 59.90 (thiazole C2); 123.62 (Ar-C); 127.17 (Ar-C); 127.93 (Ar-C); 147.17 (Ar-C1); 148.69 (Ar-C4); 143.65 (triazole C5); 155.00 (triazole C3); 172.91 (C = O). Anal. Calc.for C12H11N5O3S (M+): (305) C, 47.21; H, 3.63; N, 22.94. Found: C, 47.67; H, 3.16; N, 22.43%.
5-Methyl-2-(3-nitrophenyl)-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (17)
Yield 76%; m.p. 195-201°C; IR (KBr, ν, cm− 1) 3215 (NH), 1707 (C = O); 1H-NMR (DMSO-d6, δ, ppm): 1.49, 1.55 (3H, 2d, J = 6.9 Hz, CH3); 4.18-4.32 (1H, m, thiazole C5-H); 6.68, 6.71 (1H, 2s, thiazole C2-H); 7.51-7.59 (2H, m, Ar-H); 7.70-7.78 (1H, m, Ar-H); 8.02-8.14 (2H, m, Ar-H and triazole C5-H), 13.93 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 17.67, 21.74 (CH3); 42.34 (thiazole C5); 56.59, 57.67 (thiazole C2); 125.71, 125.93 (Ar-C); 126.14, 127.44 (Ar-C); 130.04, 130.10 (Ar-C); 135.33, 135.46 (Ar-C); 136.91, 137.25 (Ar-C1); 146.99, 147.72 (Ar-C3); 144.23 (triazole C5); 155.81 (triazole C3); 173.58 (C = O). Anal. Calc.for C12H11N5O3S (M+): (305) C, 47.21; H, 3.63; N, 22.94. Found: C, 47.77; H, 3.73; N, 22.50%.
5-Methyl-2-(2-nitrophenyl)-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (18)
Yield 72%; m.p. 234-236°C; IR (KBr, ν, cm− 1) 3308 (NH), 1711 (C = O); 1H-NMR (DMSO-d6, δ, ppm): 1.50, 1.55 (3H, 2d, J = 7 Hz, CH3); 4.21-4.35 (1H, m, thiazole C5-H); 6.66, 6.69 (1H, 2s, thiazole C2-H); 7.53-7.61 (2H, m, Ar-H); 7.74 (1H, t, J = 7.1 Hz, Ar-H); 8.02-8.14 (2H, m, Ar-H and triazole C5-H), 13.89 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 16.71, 20.77 (CH3); 39.50, 41.46 (thiazole C5); 55.73, 56.77 (thiazole C2); 125.03, 125.45 (Ar-C); 126.07, 126.64 (Ar-C); 129.37 (Ar-C); 134.80 (Ar-C); 136.28, 136.60 (Ar-C1); 146.37, 147.09 (Ar-C2); 143.65 (triazole C5); 148.18 (triazole C3);172.62 (C = O). Anal. Calc.for C12H11N5O3S (M+): (305) C, 47.21; H, 3.63; N, 22.94. Found: C, 47.54; H, 3.35; N, 22.64%.
2-[(4-Methoxycarbonyl)phenyl]-5-methyl-3-(1H-1,2,4-triazol-3-yl)-1,3-thiazolidin-4-one (19)
Yield 64%; m.p. 171-174°C; IR (KBr, ν, cm− 1) 3237 (NH), 1719, 1698 (C = O); 1H-NMR (DMSO-d6,δ, ppm) 1.51, 1.57 (3H, 2d, J = 6.9 Hz, thiazole CH3); 2.49 (3H, s, ester CH3), 4.21-4.40 (1H, m, thiazole C5-H); 6.41, 6.44 (1H, 2s, thiazole C2-H); 7.48-7.53 (2H, m, Ar-H); 7.86-7.99 (2H, m, Ar-H); 8.22 (1H, s, triazole C5-H), 13.90 (1H, s, triazole N-H). 13C-NMR (DMSO-d6, δ, ppm) 17.71, 19.59 (CH3); 39.95, 41.70 (thiazole C5); 52.07 (ester CH3), 59.67, 60.57 (thiazole C2); 126.41 (Ar-C); 127.26 (Ar-C); 129.45 (Ar-C); 146.02 (Ar-C) 143.73 (triazole C5); 155.00 (triazole C3); 165.59 (ester C = O); 172.60 (C = O). Anal. Calc.for C14H14N4O3S (M+): (318) C,52.82; H, 4.43; N, 17.60. Found: C, 53.16; H, 4.46; N, 17.16%.
Antimicrobial activity
Antibacterial activity
The disc diffusion method was used for determining the antimicrobial activity [Citation15] against staphylococcus aureus and staphylococcus epidermidis. The minimum inhibitory concentrations (MIC) (μg/mL) of active compounds were determined by the microdilution method [Citation16] using Mueller-Hinton broth serial two-fold dilutions ranged from 2500 to 2.4 μg/mL for compounds. The inoculum was prepared in broth which had been kept overnight at 37°C and which had been diluted with Mueller-Hinton broth to give a final concentration of 105 cfu/mL in the test tray. The trays were covered and placed in plastic bags to prevent drying. After incubation at 37°C for 18-20 h, the MIC was defined as the lowest concentration of compound giving complete inhibition of visible growth.
Antifungal activity
The study was designed to compare MICs obtained by the NCCLS reference M27-A2 broth microdilution method [Citation17]. Twice MIC readings were performed for compounds tested. For antimycotic assays the compounds were dissolved in DMSO. Further dilutions of the compounds and standard drugs in test medium were prepared at the required quantities of 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.562, 0.781, 0.40 μg/mL concentrations with Sabouroud dextrose broth.
In order to ensure that the solvent per se had no effect on yeast growth, a control test was also performed containing inoculated broth supplemented with only DMSO at the same dilutions used in our experiments and found inactive in culture medium.
All the compounds were tested for their in vitro growth inhibitory activity against the human pathogens yeast Candida albicans (isolated obtained from Faculty of Medicine Osmangazi University, Eskisehir, Turkey), Candida glabrata ATCC 36583. Ketoconazole was used as control drug.
The yeasts were maintained in Sabouroud dextrose broth (Difco) after overnight incubation 35 ± 1°C. The inocula of test microorganisms adjusted to match the turbidity of a Mac Farland 0.5 standard tube as determined with a spectrophotometer and the final inoculum size was 0.5-2.5 × 105 cfu/mL for antifungal assays.
Testing was carried out in Sabouroud dextrose broth (Difco) at ph 7 and the two-fold serial dilutions technique was applied. The last well on the microplates was containing only inoculated broth was kept as controls and the last well with no growth of microorganism was recorded to represent the MIC expressed in μg/mL. Every experiment in the antimicrobial assays was replicated twice in order to define the MIC values.
Results and discussion
In this study eleven new compounds incorporating the scaffold of 3-(1,2,4-triazol-3-yl)-4-thiazolidinone have been synthesized and their antibacterial and antifungal activities were evaluated.
At the first stage, Schiff's bases of 3-amino-1,2,4-triazole and aromatic aldehydes were prepared. The Schiff's bases were reacted with mercaptoacetic acid and 2-mercaptopropionic acid to give 2-aryl-3-(1,2,4-triazol-3-yl)-4-thiazolidinone and 2-aryl-5-methyl-3-(1,2,4-triazol-3-yl)-4-thiazolidinone derivatives, respectively. The structures of the compounds were confirmed by spectral methods (UV, FTIR, 1H-NMR, 13C-NMR, EI-MS) and elemental analysis.
In the IR spectra, bands in the 3308-3133 cm− 1 and 1722-1686 cm− 1 regions attributed to N-H and C = O strechings of the compounds 9-19 respectively. In the 1H-NMR spectra of compounds 9-19, lack of the CH = N signal at δ 7.78-8.83 ppm, provided confirmatory evidence for ring closure from Schiff Bases (1-8). C5-H signals of 9-12 were observed at δ 3.87-3.89 ppm and δ 4.04-4.15 ppm as double doublets due to chiral center at C2. Similarly compounds 13-19 exhibited C5-CH3 as two doublets in the δ 1.50-1.57 ppm and C2-H as two singlets in the δ 6.32-6.71 ppm region. In the 13C-NMR spectra, C = O signals appeared at δ 170.47-173.58 ppm are diagnostic for thiazolidinone formation [Citation18,Citation19]. C2 resonances were appeared in δ 60.93-61.72 ppm region as single peaks for compounds 9-12. Diastereoisomers of compounds 13-19 exhibited C2 signals at δ 55.73-60.74 ppm region as two peaks due to C2 and C5 chiral centers. EI-MS of all compounds displayed the molecular ion which confirmed their molecular weights. Molecular ions are base peaks for most of the compounds, except 10 (m/z 241), 11 (m/z 218), 17 and 18 (m/z 111).
Some of the compounds were tested for antimicrobial activity against S. aureus ATCC 6538 and S. epidermidis ATCC 12228. Compounds 10, 15 and 16 demonstrated varying degrees of antimicrobial activity against S. epidermidis ATCC 12228 ().
Table I. Antibacterial activity of tested compounds (MIC μg/mL).
The compounds were evaluated for their antifungal properties. In comparison with the control antifungal agent Ketoconazole, compounds 10 and 11 showed moderate activities against C. albicans as well as moderate activities when compared with the antifungal agent ketoconazole against C. Glabrata ().
Table II. Antifungal activity of tested compounds (MIC μg/mL).
Acknowledgements
The authors wish to thank Prof. Dr. Gülten Ötük at Istanbul University, Faculty of Pharmacy, Department of Microbiology for the antibacterial work.
This project is supported by Istanbul University Research Fund Project Numbers: T-930/061100 and UDP-78/26082002.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Q Sun, J Xu, Y Cao, W Zhang, Q Wu, D Zhang, J Zhang, H Zhao, and Y Jiang. (2007). Synthesis of novel triazole derivatives as inhibitors of cytochrome P450 14α-demethylase(CYP51). Eur J Med Chem 42:1226–1233.
- L Alcazar-Fuoli, E Mellado, G Garcia-Effron, JF Lopez, JO Grimalt, JM Cuenca-Estrella, and JL Rodriguez-Tudela. (2008). Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids 73:339–347.
- H Sanati, P Belanger, R Fratti, and M Ghannoum. (1997). A new triazole,voriconazole(UK-109,496) blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob Agents Chemother 41:2492–2496.
- D Andes. (2004). Antifungal pharmacokinetics and pharmacodynamics:understanding the implications for antifungal drug resistance. Drug Res Updates 7:185–194.
- N Karaburun, K Benkli, Y Tunali, U Uçucu, and S Demirayak. (2006). Synthesis and antifungal activities of some aryl[3-(imidazol-1-yl/triazol-1-ylmethyl) benzofuran-2-yl]ketoximes. Eur J Med Chem 41:651–656.
- S Ozkirimli, TI Apak, M Kiraz, and Y Yegenoglu. (2005). Synthesis of new triazolyl-N,N-dialkyldithiocarbamates as antifungal agents. Arch Pharmacal Res 28:1213–1218.
- F Chevreuil, A Landreau, D Seraphin, G Larcher, J Bouchara, and PJ Richomme. (2006). Synthesis and antifungal activity of new thienyl and aryl conazoles. J Enz Inhib Med Chem 21:293–303.
- F Ur, N Cesur, S Birteksöz, and G Otük. (2004). Synthesis of some new 6-methylimidazo[2,1-b] thiazole-5-carbohydrazide derivatives and their antimicrobial activities. Arzneim-Forsch/Drug Res 54:125–129.
- P Vicini, A Gernikaki, K Anastasia, M Incerti, and F Zani. (2006). Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorg Med Chem 14:3859–3864.
- A Deeb, BE Bayoumy, and M El-Mobayed. (1986). Studies on β-lactams and thiazolidinones. Part I. Synthesis and biological activity of some new triazolyl-azetidine-2-ones and 4-thiazolidine-4-ones. Egypt J Pharm Sci 27:37–41.
- A Rao, J Balzarini, A Carbone, A Chimirri, E Clercq, AM Monforte, P Monforte, C Pannecouque, and M Zappala. (2004). Synthesis of new 2,3-diaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Farmaco 59:33–39.
- O Güzel, N Terzioglu, G Capan, and A Salman. (2006). Synthesis and biological evaluation of new 5-methyl-N-(3-oxo-1-thia-4-azaspiro[4.5]-dec-4-yl)-3-phenyl-1H-indole-2-carboxamide derivatives. Arkivoc xii:98–110.
- G Capan, N Ulusoy, N Ergenc, and M Kiraz. (1999). New 6-phenylimidazo[2,1-b] thiazole derivatives: Synthesis and antifungal activity. Montsh Chem 130:1399–1407.
- CV Kavitha, S Basappa, SN Swamy, K Mantelingu, S Doreswamy, MA Sridhar, JS Prasad, and KS Rangappa. (2006). Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials. Bioorg Med Chem 14:2290–2299.
- AL Barry, and C Thornsberry. Susceptibility tests: Diffusion test procedures In: EH Lennette, A Balows, WJ Hausler, and HJ Shadomy, editors. Manual of clinical microbiology. 4th ed. Washington DC: ASM; (1985). p 978–980.
- RN Jones, AL Barry, TL Gavan, and JA Washington. Susceptibility tests: Microdilution and macrodilution broth procedures. In: EH Lennette, A Balows, WJ Hausler, and HJ Shadomy, editors. Manual of clinical microbiology. 4th ed. Washington DC: ASM; (1985). p 972–974.
- NCCLS. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standart 2nd ed. NCCLS document M27-A2. Pennsylvania USA; (2002).
- N Karalı, E İlhan, A Gürsoy, and M Kiraz. (1998). New cyclohexylidene-hydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of 3-phenyl-4(3H)-quinazolinones. Il Farmaco 53:346–349.
- N Ergenç, G Çapan, NS Günay, S Özkırımlı, M Güngör, S Özbey, and E Kendi. (1999). Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch Pharm Pharm Med Chem 332:343–347.