Abstract
A series of nine polypyridyl-ruthenium (II) complexes (N-ligands = 2,2′-bipyridines; 2,2′-6′,2′-terpyridines, di-alkyloxy-2,2′-6,2-bipyridine-3,3′-di-carboxylates), were tested against Mycobacterium tuberculosis (MBT). The complex (11) showed remarkable activity against MBT as compared to other complexes, (1–10). The aquo ligand of complex (11), as opposed to other chloro and acetonitrile derivatives, appears to play a key role in the antitubercular potency of this new class of metal-based compounds.
Introduction
Despite the ready availability of effective treatments, tuberculosis still remains a major threat worldwide. The emergence of drug resistant strains of Mycobacterium tuberculosis, particularly multiple drug resistant strains (MDR) Citation1, Citation2, Citation3, Citation4 has complicated the treatment protocol and raised the concern that tuberculosis may once again become an incurable disease in future. For this reason it is critical to discover new drug therapies with more aggressive mechanism to work against resistant strains of Mycobacterium tuberculosis. Numerous reviews recently reported in the literature are a proof of the renewed interest towards this pathology Citation5, Citation6, Citation7, Citation8, Citation9, Citation10, Citation11, Citation12.
A number of such selected metal based compounds contain metal-oxygen (M-O) hemilabile bonds e.g., (M-O = M-OH2)[Citation13,Citation14,Citation22] or (M-SOMe2)[Citation16] which have shown interesting and potential anti-tumor or antibacterial activity ().
Figure 1. Examples of some bioactive transition metal complexes containing precursors of Metal-OH2 or Metal = O moieties.
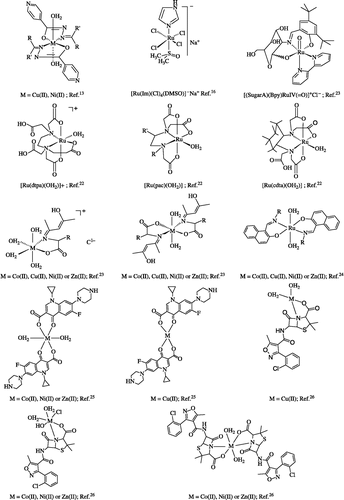
Isoniazid derived copper(II) and nickel(II) complexes with antimycobacterial in vitro activity have been reported [Citation13]. The metal complexes of 2-(1′/2′-hydroxynaphthyl)-benzoxazoles also showed significant activity (MIC < 3.12 μg/mL)[Citation14]. In an other report a nickel(II) binuclear complex has displayed a significant activity with MIC 10-fold lower than that of Rifampicin and reaches almost equal to Isoniazid, so far the sole established anti-tuberculosis drug which possesses the same MIC value against M. tuberculosis.
Instead, charged mononuclear complexes of 2,6-diacetylpyridine and bis-benzoylhydrazone in different mixtures showed MICs >12.5 mg/ mL [Citation15]. Na[trans-RuCl4(Me2SO)(Im)] (Im = imidazole), a ruthenium(III) complex shows encouraging anti-tumour and anti-metastatic properties [Citation16].
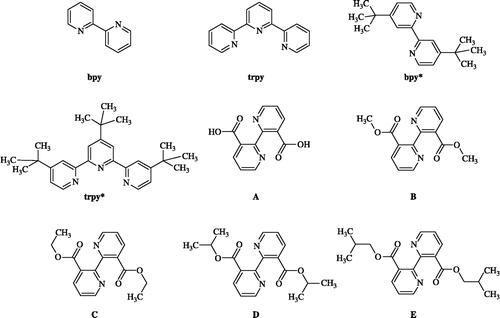
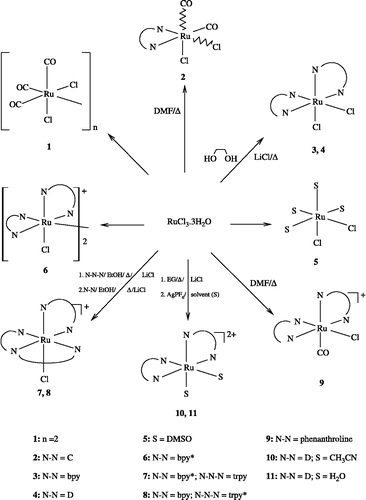
Since 1969, Dwyer et al [Citation17] reported the bacteriostatic action of metal complexes of 2,2′-bipyridyl against selected Gram-positive, Gram-negative and acid-fast bacteria. With the same idea, we have prepared and wish to report some Ruthenium complexes bearing substituted polypyridyl ligands (2,2′-bipyridines or 2,2′-6′,2″-terpyridines) bearing Ru(II)-X moieties where X = Cl, CO, CH3CN or H2O. We have also made a genuine effort to investigate and report in this paper antitubercular screening results of these complexes (). These ruthenium complexes, derived from the coordination of bipyridyl and/or terpyridyl ligands, can be obtained through a simpler and economical synthetic method. The starting material, 2,2′-bipyridine-3,3′-dicarboxylic acid, was obtained in good yield from commercially available 1,10-phenanthroline by its oxidation using the KMnO4 as described previously [Citation18a].
Materials and methods
All materials and solvents were of reagent grade as received from commercial sources. Diethyl-2,2′-bipyridine-3,3′-dicarboxylate (C) was similarly synthesized and coordinated to ruthenium(II) as described in our previous work and depicted in literature.[Citation22] 1H NMR spectra were recorded on AC 250 MHz NMR Bruker Spectrometer at ambient temperature and chemical shifts were reference to the internal tetramethylsilane. Infrared spectra were recorded in KBr pellets using a Perkin-Elmer 1310 spectrophotometer. Mass spectra were determined by platform II Micromass (ESI+, CH3CN/H2O: 50/50). Elemental analyses were performed by CNRS Service Central d'Analyse Vernaison (France).
Antitubercular Activity
Primary screening was conducted at 6.25 μg mL− 1 against M. tuberculosis H37Rv (ATCC 27294) in BACTEC 12B medium using a broth microdilution assay, the Microplate Alamar Blue Assay (MABA) [Citation19]. Compounds exhibiting fluorescence were tested in the BACTEC 460 radiometric system. Compounds demonstrating at least 90% inhibition in the primary screen were retested at lower concentrations against M. tuberculosis H37Rv to determine the MIC using MABA. MIC is defined as the lowest concentration effecting a reduction in fluorescence of 99% relative to controls[Citation19].
General procedure for the preparation of dialkyl-2,2′-bipyridine-3,3′-dicarboxylates B–E
Diethyl-2,2′-bipyridine-3,3′-dicarboxylate (C)
The ligand C was prepared by a similar procedure as described in our previous work [Citation18]. The 2,2′-bipyridine-3,3′-dicarboxylic acid was prepared from 1,10-phenanthroline by a literature procedure. Compound acid (600 mg, 2.5 mmol) and thionyl chloride (12 mL) were refluxed for 5 h. The excess amount of thionyl chloride was distilled off and the residue dried in vacuum for 5 h. Toluene (20 mL) and ethanol (2 mL) were added to it and refluxed for 3 h. Chloroform (40 mL) was added and the mixture was treated with a cold solution of sodium bicarbonate (2.5%). The organic layer was dried on sodium sulphate and the solvent removed in vacuum, giving 733 mg of crude product. The mixture was purified by chromatography on silica gel column using ether as eluent to afford C as a white solid (670 mg, 83%).
Selected data for ligand (C): Mp = 81–82°C. IR (KBr, cm− 1): 1695 (C = O, s), 1535 (C = C, w), 1415 (C = N, m), 1260 (C-O, w). 1H-NMR (250.14 MHz, CDCl3): 8.73 (dd; 2H, H6/6′, J = 4.8 and 1.7 Hz), 8.37 (dd, 2H, H4/4′, J = 7.9 and 1.7 Hz), 7.42 (dd, 2H, H5/5′, J = 7.9 and 4.8 Hz), 4.95 (m, 2H, CH, J = 6.3 Hz), 0.97 (d, 12H, 4CH3, J = 6.3 Hz). Anal. Cal. for C18H20N2O4 (328.37): C 65.85, H 6.10, N 8.53; found: C 65.78, H 6.22, N 8.38%. MS: [M]+ [m/e = 329.10] (Cal. for C18H20N2O4: 328.371).
Cis-dichloro-[{n,n′-bis-(di-iso-propyl-2,2′-bipyridine-3,3′-dicarboxylate)}]ru(ii).mono-hydrate: [Ru(d)2cl2].h2o
Following the same literature procedure, the complex cis-(Cl/Cl)-[RuCl2(D)2].H2O was synthesized: 0.262 g (1 mmol) of RuCl3.3H2O and 0.600 g (2 mmol) of ligand (D) were combined in 15 mL of ethyleneglycol. The mixture was heated gently for 25 min and allowed to cool to room temperature. The violet solid was then precipitated with water, filtered off and washed three times respectively with water and diethyl ether and dried under vacuum. An X-ray quality crystal of cis-(Cl/Cl)-[RuCl2(D)2].H2O was obtained from the recrystallization in acetone-ethanol.
Selected data for cis-(Cl/Cl)-[RuCl2(D)2].H2O (2): Yield 82%. 1H NMR (200 MHz, CDCl3) δ 10.36 (d, 2H, J = 5.4 Hz, H6), 8.25 (d, 2H, J = 7 Hz, H4), 7.78 (d, 2H, J = 6.8 Hz, H4′), 7.7 (m, 2H, H6′), 7.63 (dd, 2H, J = 7.4 and 5.4 Hz, H5), 6.9 (dd, 2H, J = 6.8 and 7.2 Hz, H5′), 4.24 (m, 8H, CH2-O), 1.23 (m, 12H, CH3). IR (KBr) ν 1731 (vs), 1576 (s) cm− 1. Anal. Calc. (found) for C32H32N4Cl2O8Ru.H2O: C 48.61 (48.27), H 4.33 (4.17), N 7.09 (6.93%); Cyclic voltammetry (CH3CN, 0.1 M TBAH, Pt/ ECS): E1/2 = 0.54 V (ΔEp = 80 mV).
The electrochemical and preliminary study of cyclic voltammetry was carried out and showed reversible and electrochemical stability properties of complex cis-(Cl)-[RuCl2(D)2].H2O.
Results and discussion
Chemistry
depicts the general reaction used to prepare ruthenium (II) complexes 1–11 which have been published previously[Citation8]. and Ref In our first published work, complexes containing (N-N)Ru, (N-N)2Ru, (N-N)(N-N-N)Ru moieties were prepared from the reaction of (RuCl3.3H2O) and (N-N and/or N-N-N) ligands in the presence of a mixture of ethanol as solvent and Et3N as a reducing agent, or a solvent alone which could play the reducing and solvolysis roles as that of dimethylformamid (DMF) or ethylenglycol (EG). [18a–c] The coordination of substituted bipyridines (bpy*, A–E) and substituted terpyridine (trpy*) instead of unsubstituted polypyridines (bpy and trpy), under the same conditions, lead to higher, but still modest, water soluble complexes.
Antimycobacterial activity
The antimycobacterial activity of the compounds was determined to identify the compounds having inhibitory activity against M. tuberculosis. Interesting results were obtained from these assays and data is reported in . The in vitro antimycobacterial activities of these polypyridine ligands and their ruthenium complexes 1–11 were inferior to that of isoniazid against M. tuberculosis H37Rv. Further, the free N,N-ligands A–E had either little or no activity (0–39%inhibition). However, none of the compounds showed activity against M. tuberculosis H37Rv suggesting that the compounds possess no specific anti-tubercular activity. This could probably be due to their low absorption (MIC >6.25 μg/mL) against M. tuberculosis. However, data in shows that only the ruthenium-aquo complex 11 of ligand D showed a high antitubercular activity compared to the rest of the free ligands (A–E), neutral complexes 1–5, monocationic or dicationic complexes 6–10 ().
Table I. Biological activity of free N-ligands and their ruthenium (II) complexes against Mycobacterium tuberculosis in vitro. [Citation19].
In this case, the degree of lipophilicity of the carboxylate substituent does not correlate positively with the antitubercular activity. A direct influence of the redox properties from the ruthenium may appears to be more important for such an activity ().
In addition to structure-activity relationships, an essential investigation is required to establish the relationship between redox potential and structure-activity which would help to understand the mechanism of metal-aquo complexes like complex 11 to inhibit various diseases such as tuberculosis, cancer and HIV. It would also be possible to address the significant area by testing their effect(s) on four cell lines, one of which has normal topoisomerase I, protease and three others have mutant (ethambutol, camptothecin and cisplatin-resistant) enzymes. If there were a difference in GI50 value this would indicate that an enzyme is a critical target for the metal based drug. A further consideration relates to the poor solubility of the Ru-OH2 derivatives in water; for the cell line assays to function it is important to prepare derivatives that are more soluble. Substituting the ester groups at 3,3′ position, with some other groups such as sugars or amides is possible and might generate the desired effects.
Interestingly, solvatation of ruthenium-Cl moiety of complex 4 with water molecules in the presence of AgTf has been demonstrated to be a powerful and optimal method for Ru-OH2 complexes in the preparation of a new efficient metal-based anti-tubercular class of compounds. This work provides for the first time a simple method for the preparation of a wide range of such compounds which are bioactive and could be clinically used as anti-tubercular agent.
The important anti-tubercular activity of this class of compounds suggests a promising novel approach to the design of prospective and significantly potential compounds for treating other bacterial infections.
As a guide for future work, the data reported herein indicates that the Ru-OH2 compounds have a definite potential efficacy that merits development through modification of both the lipophicity of bipyridyl ligands and the nature of the metal ion.
Conclusion
In this paper we report an efficient metal based anti-tubercular agent having Ru-OH2 coordination which highlights the significant feature of the presence of the metal-aquo moiety in the coordination of bioactive molecule to the metal. Antitumor and anti-HIV screening studies on complex 11 are in progress at the National Cancer Institute (NCI) which will help us in elucidating the redox potential /activity relationships.
Acknowledgements
This work was supported by grants from the Ministry of Education of Morocco (PGR-UMP-BH-2005 and CUD-UMP-BH-2007). We are indebted to Professor P.H. Dixneuf and Dr. H. Le Bozec of Rennes1 for sending us samples of some complexes and we thank the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) of United States for biological tests.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- B Heym, and ST Cole. (1997). Multidrug resistance in Mycobacterium tuberculosis. Int J Antimicrob Agents 8:61–70.
- LA Basso, and JS Blanchard. (1998). Resistance to antitubercular drugs. Adv Exp Med Biol 456:115–144.
- A Telenti, and M Iseman. (2000). Drug-resistant tuberculosis: What do we do now?. Drugs 59:171–179.
- C Loiez-Durocher, A Vachee, and N Lemaitre. (2000). Drug resistance in Mycobacterium tubercolosis: Diagnostic methods. Ann Biol Clin 58:291–297.
- K Duncan. (1997). Prospects for new anti-tuberculosis drugs. J Pharm Pharmacol 49:21–23.
- K Duncan. (1997). Tuberculosis: Annual update. Expert Opin Ther Pat 7:129–137.
- C Grassi. (1997). New drugs for tuberculosis. Expert Opin Invest Drugs 6:1211–1226.
- CE Barry. (1997). New horizons in the treatment of tuberculosis. Biochem Pharmacol 54:1165–1172.
- K. Tuberculosis Duncan. (1998). Expert Opin Ther Pat 8:137–142.
- NJC Snell. (1998). The treatment of tuberculosis: Current status and future prospects. Expert Opin Invest Drugs 7:545–552.
- DC Crick, and PJ Brennan. (2000). Antituberculosis drug research. Curr Opin Anti-Infect Invest Drugs 2:154–163.
- A Andreani, M Granaiola, A Leoni, A Locatelli, R Morigi, and M Rambaldi. (2001). Synthesis and antitubercular activity of imidazo[2,1-b]thiazoles. Eur J Med Chem 36:743–746.
- B Bottari, R Maccari, F Monforte, R Ottanaa, E Rotondo, and MG Vigorita. (2000). Antimycobacterial in vitro activity of cobalt (II) isonicotinoylhydrazone complexes. Part 10. Bioorg Med Chem Lett 10:657.
- A Kumar, and D Kumar. (2007). Synthesis and antimicrobial activity of metal complexes from 2-(1′/2′-hydroxynaphthyl)benzoxazoles. ARKIVOC (XIV):117–125.
- B Bottari, R Maccari, F Monforte, R Ottana, MG Vigorita, G Bruno, F Nicolo, A Rotondo, and E Rotondo. (2001). Nickel (II) 2,6-diacetylpyridine bis(isonicotinoylhydrazonate) and bis(benzoylhydrazonate) complexes: Structure and antimycobacterial evaluation. Part XI. Bioorg Med Chem 9:2203–2211.
- L Messori, P Orioli, D Vullo, E Alessio, and E Iengo. (2000). A spectroscopic study of the reaction of NAMI, a novel ruthenium (III) anti-neoplastic complex, with bovine serum albumin. Eur J Biochem 267:1206–1213.
- FP Dwyer, IK Reid, A Shulman, GM Laycock, and S Dixon. (1969). Biological actions of 1,10-phenanthroline and 2,2′-bipyridine hydrochlorides, quaternary salts and metal chelates and related compounds. 1. Bacteriostatic action on selected gram-positive, Gram-negative and acid-fast bacteria. Austral J Exp Bio Med Sci 47:203–218.
- N Sam, S Elkadiri, H Le Bozec, L Toupet, M Daoudi, N Bitit, T Ben Hadda, and PH Dixneuf. (1996). Novel trichloroindolizine derivatives via intramolecular acylation of a bis(chloroacyl)bipyridine. J Chem Soc Perkin Trans 15 (9):1571–1573. (b) Ben Hadda T, Sam N, Le Bozec H, Dixneuf PH. Carbonyl and chloro complexes of ruthenium (II) containing 3,3′-diester-2,2′-bipyridyl ligands, Inorg Chem Comm, 1999, 2, 460–462. (c) Ben Hadda T, Zidane I, Moya SA, LeBozec H. Soluble containing ruthenium carbonyl complexes new sterically hindered bipyridine ligands Polyhedron
- L Collins, and SG Franzblau. (1997). Microplate Alamar blue assay versus BACTEC 460 system for high- throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009.
- JK Hurst. (2005). Water oxidation catalyzed by dimeric μ-oxo bridged ruthenium diimine complexes. Coord Chem Rev 249:313–328. (b) Chatterjee D, Mitra A, Shepherd RE. Oxo-transfer catalysis from t-BuOOH with C–H bond insertion using tridentate Schiff-base-chelate complexes of ruthenium(III). Inorg Chim Acta, 357 (2004), 980–990. (c) Chanda N, Mondal, B, Puranik, VG, Lahiri GK. Ruthenium monoterpyridine complexes incorporating α,α′-diimine based ancillary functions. Synthesis, crystal structure, spectroelectrochemical properties and catalytic aspect. Polyhedron 2002;21:2033–2043. (d) Chatterjee D, Mitra A, Mukherjee S. Oxidation of benzene with tert-butylhydroperoxide catalyzed by a novel [RuIII(amp)(bipy)(H2O)]+ complex: First report of homogeneously catalyzed oxo-transfer reaction in benzene oxidation. J Mol Cata A Chem 2001;165: 295–298. (e) Chatterjee D, Mitra A. Oxidation of organic substrates catalyzed by a novel mixed-ligand [RuIII(app)(pic)(H2O)]+ complex Inorg Chem Comm, 2000;3:640–644
- F Frézard, C Demicheli, C Ferreira, and MAP Costa. (2001). Glutathione-induced conversion of pentavalent antimony to trivalent antimony in meglumine antimoniate. Antimicrob Agents Chemother 913–916.
- D Chatterjee, and A Mitra. (2006). Ruthenium polyaminocarboxylate complexes. Platinum Metals Rev 50 (1):2–12.
- ZH Chohan, M Arif, MA Akhtar, and CT Supuran. (2006). Metal-based antibacterial and antifungal agents: Synthesis, characterization, and in vitro biological evaluation of Co(II), Cu(II), Ni(II), and Zn(II) complexes with amino acid-derived compounds. Bioinorg Chem Appl 1–13.
- ZH Chohan, and HA Shad. (2008). Structural elucidation and biological significance of 2-hydroxy-1-naphthaldehyde derived sulfonamides and their first row d-transition metal chelates. J Enz Inhib Med Chem (in press)
- ZH Chohan, CT Supuranm, and A Scozzafava. (2005). Metal binding and antibacterial activity of ciprofloxacin complexes. J Enz Inhib Med Chem 20 (3):303–307.
- ZH Chohan, and CT Supuranm. (2006). Metalloantibiotics: Synthesis, characterization and in-vitro antibacterial studies on cobalt (II), copper (II), nickel (II) and zinc (II) complexes with cloxacillin. J Enz Inhib Med Chem 21 (4):441–448.