Abstract
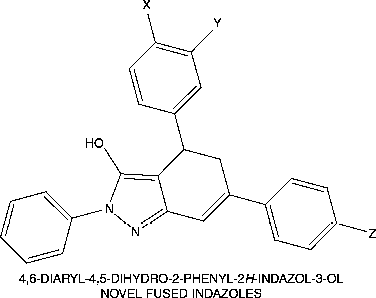
New 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ols 25-32 were designed, synthesized and in vitro microbially evaluated using clinically isolated bacterial strains viz Staphylococcus aureus, β-Heamolytic streptococcus, Vibreo cholerae, Salmonella typhii, Shigella felxneri and fungal strains viz Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem. Results of this study showed that the nature of the substituents on the phenyl rings viz., methyl, methoxy, chloro, nitro as well as the bromo functions at the meta and para positions of the aryl moieties determined the nature and extent of the activity of the fused indazolonol compounds 25-32.
Introduction
In recent years there has been a great deal of interest in exploiting more than one proximal functional groups for designing novel structures capable of performing a variety of functions. The present study describes the use of 6-carbethoxy-3,5-diarylcyclohex-2-enone [Citation1], an intermediate with three versatile functional groups i.e., ketone, olefin and ester for the synthesis of fused indazole derivatives.
A variety of structurally diverse indazole nucleus have aroused great interest in the past and recent years due to their wide variety of biological properties such as antimicrobial activity [Citation2], inhibitors of protein kinase B/Akt [Citation3], antiprotozoal agents [Citation4], antichagasic activity [Citation4], leishmanocidal activity [Citation4], trypanocidal activity [Citation4], inhibitors of S-adenosyl homocysteine/methylthio adenosine (SAH/MTA) nucleosides [Citation5], potent activator of the nitric oxide receptor [Citation6] and inhibition of platelet aggregation [Citation6].
In continuation of our earlier work on the synthesis of various bio active heterocyclic nuclei including biolabile piperidone, 1,2,3-selenadiazoles, 1,2,3-thiadiazoles, 1,2,4,5-tetrazinanes Citation7, Citation8, Citation9, Citation10, Citation11, we wish to report the development of fused indazoles on 6-carbethoxy-3,5-diarylcyclohex-2-enone derivatives thus paving the way for a novel class of 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ol.
Experimental
Microbiology
Materials
All the bacterial strains namely Staphylococcus aureus, β-Heamolytic streptococcus, Vibreo cholerae, Salmonella typhii and Shigella felxneri and fungal strains namely Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem were clinically isolated strains and were obtained from Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity (Minimum Inhibitory Concentration (MIC) method)
Minimum inhibitory concentration (MIC) in μg/mL values was carried out by two-fold serial dilution method [Citation12]. The test compounds 23-27 were dissolved in water to obtain 1 mg mL− 1 stock solution. Seeded broth (broth containing microbial spores) was prepared in nutrient broth (NB) from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7 days old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in sabourauds dextrose broth (SDB). The colony forming units (cfu) of the seeded broth were determined by plating technique and adjusted in the range of 104–105 cfu/mL. The final inoculums size was 105cfu/mL for antibacterial assay and 1.1–1.5 × 102 cfu/mL for antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at a pH 5.6 for fungi (SDB). Exactly 0.4 mL of the solution of test compound was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution and so on till six such dilutions were obtained. A set of assay tubes containing only seeded broth was kept as control. The tubes were incubated in biological oxygen demand (BOD) incubators at 37 ± 1°C for bacteria and 28 ± 1°C for fungi. The minimum inhibitory concentrations (MICs) were recorded by visual observations after 24 h (for bacteria) and 72–96 h (for fungi) of incubation. Ciprofloxacin was used as standard for bacteria studies and Fluconazole was used as standards for fungal studies.
Chemistry
Performing TLC assessed the reactions and the purity of the products. All the reported melting points were taken in open capillaries and were uncorrected. IR spectra were recorded in KBr (pellet forms) on a Nicolet-Avatar–330 FT-IR spectrophotometer and noteworthy absorption values (cm− 1) alone are listed. 1H, D2O exchanged 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz respectively on Bruker AMX 400 NMR spectrometer using DMSO-d as solvent. Two-dimensional HSQC spectra were recorded at 500 MHz and on Bruker DRX 500 NMR spectrometer using DMSO-d as solvent. The ESI + ve MS spectra were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on Carlo Erba 1106 CHN analyzer.
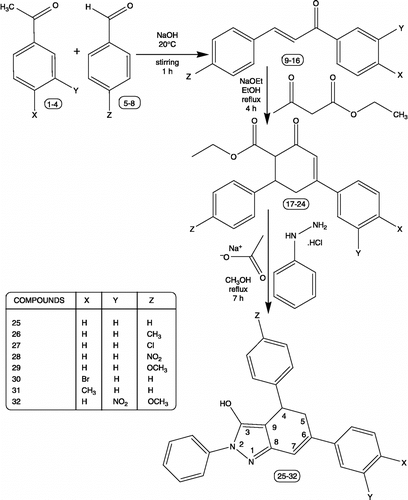
By adopting the literature procedure, 1,3-diaryl-prop-2-en-1-ones 9-16 Citation13, Citation14, Citation15 and 6-carbethoxy-3,5-diarylcyclohex-2-enone 17-24 [Citation1] were prepared.
Typical procedure for the synthesis of 4,6-diphenyl-4,5-dihydro-2-phenyl-2H-indazol-3-ol 25
A solution of 6-carbethoxy-3,5-diphenylcyclohex-2-enone, 17 (0.1 mol) in methanol (40 mL) was treated with phenyl hydrazine hydrochloride (0.15 mol) and anhydrous sodium acetate (0.15 mol) and refluxed for 7 h. The reaction mixture was cooled and then poured over crushed ice. The crude product 25 was recrystallized twice using methanol as solvent. IR (KBr) (cm− 1): 3425, 3062, 2921, 2862, 1605, 1514, 1445, 1367, 757, 705, 695; 1H NMR (δ ppm): 2.98–3.13, 3.46–3.50 (m,2H,H5), 4.34 (dd,1H,H4, J = 12.0,9.2 Hz), 6.86 (d,1H,H7, J = 7.9 Hz), 7.20–7.58 (m,15H,H-Arom.), 11.73 (s,1H,OH); 13C NMR (δ ppm): 34.4 C-4, 37.2 C-5, 99.2 C-9, 114.5 C-7; 136.6 C-8; 125.6–128.6 C-Arom.; 140.4, 142.2, 145.4 ipso-C's; 159.2 C-3.
In the D2O exchanged 1H NMR spectrum, a broad peak at 11.73 ppm due to –OH proton at C-3 was disappeared.
In the HSQC spectrum, one bond correlation (34.4/4.34) between C-4 and H4a occurs. The 13C resonance at 37.2 ppm has correlations with methylene protons H5a (37.2/2.98–3.13; 36.2/3.46–3.50) and hence C-5 resonates at 37.2 ppm. The 13C resonance at 114.5 ppm correlates with doublet at 6.86 ppm. The doublet at 6.86 ppm is conveniently assigned to H7. The cross peak (114.5/6.86 ppm) confirms that the 13C resonance at 114.5 ppm is due to C-7. The 13C resonances at 99.2, 136.6, 159.2 ppm have no correlations with protons and hence it is due to quaternary carbons. Among the quaternary carbon resonances, the 13C resonance at 140.4, 142.2, 145.4 ppm are assigned to ipso carbons. The 13C resonance at 136.6 and 159.2 ppm are due to the C-8 and C-3 carbons. The signal at 99.2 ppm is due to C-9 carbon and the C-6 carbon is merged with aromatic carbons.
Compounds 26-32 were synthesized similarly.
4,5-Dihydro-2,6-diphenyl-4-p-tolyl-2H-indazol-3-ol
26 IR (KBr) (cm− 1): 3419, 3062, 3065, 2917, 2857, 1592, 1514, 755, 708, 694; 1H NMR (δ ppm): 2.34 (s,3H,CH3 at phenyl ring); 2.95–3.10, 3.33–3.69 (m,2H,H5), 4.01 (dd,1H,H4, J = 12.4,9.6 Hz), 6.87 (d,1H,H7, J = 8.5 Hz), 7.02–7.57 (m,14H,H-Arom.), 11.35 (s,1H,OH); 13C NMR (δ ppm): 21.9 CH3 at phenyl ring; 33.9 C-4, 37.3 C-5, 99.1 C-9, 114.5 C-7; 136.3 C-8; 125.3–128.6 C-Arom.; 140.2, 141.3, 142.3 ipso-C's; 159.4 C-3.
4-(4-chlorophenyl)-4,5-dihydro-2,6-diphenyl-2H-indazol-3ol
27 IR (KBr) (cm− 1): 3404, 3053, 2926, 1602, 1534, 1492, 756, 711, 695; 1H NMR (δ ppm): 2.95–3.10, 3.26–3.40 (m,2H,H5), 4.35 (dd,1H,H4, J = 13.1,8.7 Hz), 6.87 (d,1H,H7, J = 8.0 Hz), 7.26–7.67 (m,14H,H-Arom.), 11.6 (s,1H,OH); 13C NMR (δ ppm): 34.5 C-4, 37.4 C-5, 99.3 C-9, 114.7 C-7; 136.2 C-8; 125.1–128.8 C-Arom.; 140.1, 141.2, 142.4, 144.2 ipso-C's; 159.3 C-3.
4,5-dihydro-4-(4-nitrophenyl)-2,6-diphenyl-2H-indazol-3-ol
28 IR (KBr) (cm− 1): 3421, 3075, 2922, 2846, 1597, 1515, 1433, 1346, 752, 708, 693; 1H NMR (δ ppm): 2.96–3.29, 3.40–3.55 (m,2H,H5), 4.44 (dd,1H,H4, J = 12.6,8.4 Hz), 6.90 (d,1H,H7, J = 8.2 Hz), 7.35–7.56 (m,14H,H-Arom.), 11.70 (s,1H,OH); 13C NMR (δ ppm): 34.8 C-4, 37.2 C-5, 99.2 C-9, 114.3 C-7; 136.2 C-8; 123.4-128.8 C-Arom.; 140.1, 142.4, 146.2 ipso-C's; 159.2 C-3.
4,5-dihydro-4-(4-methoxyphenyl)-2,6-diphenyl-2H-indazol-3-ol
29 IR (KBr) (cm− 1): 3416, 3064, 3051, 2932, 2835, 1606, 1511, 1441, 1372, 759, 712, 693; 1H NMR (δ ppm): 2.94–3.20, 3.22–3.46 (m,2H,H5), 3.85 (s,3H,OCH3 at phenyl ring), 4.27 (dd,1H,H4, J = 11.4,9.1 Hz), 6.85 (d,1H,H7, J = 8.6 Hz), 7.12–7.55 (m,14H,H-Arom.), 11.40 (s,1H,OH); 13C NMR (δ ppm): 34.8 C-4, 37.5 C-5, 55.3 –OCH3 at phenyl ring; 99.4 C-9, 114.3 C-7; 136.2 C-8; 125.0–128.5 C-Arom.; 137.2, 140.3, 141.0, 142.4 ipso-C's; 158.3 C-3.
6-(4-bromophenyl)-4,5-dihydro-2,4-diphenyl-2H-indazol-3-ol
30 IR (KBr) (cm− 1): 3401, 3092, 2998, 2926, 2831, 1606, 1525, 1437, 1348, 805, 713, 735; 1H NMR (δ ppm): 2.92–2.95, 2.96–3.17 (m,2H,H5), 4.02 (dd,1H,H4, J = 11.8,9.5 Hz), 6.87 (d,1H,H7, J = 8.8 Hz), 7.20–7.90 (m,14H,H-Arom.), 11.71 (s,1H,OH); 13C NMR (δ ppm): 33.8 C-4, 37.4 C-5, 99.3 C-9, 114.3 C-7; 137.0 C-8; 116.1–137.0 C-Arom.; 142.1, 142.3, 149.0, 157.2 ipso-C's; 159.0 C-3.
4,5-Dihydro-2,4-diphenyl-6-p-tolyl-2H-indazol-3-ol
31 IR (KBr) (cm− 1): 3421, 3060, 3053, 2927, 2833, 1601, 1512, 1440, 1372, 763, 715, 690; 1H NMR (δ ppm): 2.92–3.18, 3.20–3.44 (m,2H,H5), 2.28 (s,3H,CH3 at phenyl ring); 4.01 (dd,1H,H4, J = 11.1,8.8 Hz), 6.84 (d,1H,H7, J = 8.4 Hz), 7.06–7.41 (m,14H,H-Arom.), 11.70 (s,1H,OH); 13C NMR (δ ppm): 33.9 C-4, 38.7 C-5, 22.1 –CH3 at phenyl ring; 99.4 C-9, 112.7 C-7; 134.1 C-8; 123.7–127.7 C-Arom.; 137.1, 140.6, 141.4, 157.3 ipso-C's; 159.2 C-3.
4,5-Dihydro-4-(4-methoxyphenyl)-6-(3-nitrophenyl)-2-phenyl-2H-indazol-3-ol
32 IR (KBr) (cm− 1): 3427, 3020, 2924, 2851, 1607, 1523, 1487, 1446, 819, 714, 703; 1H NMR (δ ppm): 2.94–3.28, 3.30–3.44 (m,2H,H5), 3.82 (s,3H,OCH3 at phenyl ring), 3.95 (dd,1H,H4, J = 12.7,9.2 Hz), 6.85 (d,1H,H7, J = 8.2 Hz), 7.00–8.44 (m,13H,H-Arom.), 11.82 (s,1H,OH); 13C NMR (δ ppm): 34.4 C-4, 37.8 C-5, 99.5 C-9, 114.2 C-7; 135.2 C-8; 126.0–129.7 C-Arom.; 129.0, 131.4, 131.9, 139.2, 142.2 ipso-C's; 159.0 C-3.
Results and discussion
Chemistry
The schematic representation and analytical data for the synthesized compounds 25-32 are furnished in Scheme– and respectively. The synthetic strategy for the construction of 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ols 25-32, a new fused indazole derivative involves three steps, which is described as follows: Condensation of appropriate acetophenone 1-4 and appropriate benzaldehyde 5-8 in the presence of sodium hydroxide yields the respective 1,3-diaryl-prop-2-en-1-ones 9-16. The respective α,β-unsaturated ketones 9-16 on treatment with ethyl acetoacetate in the presence of sodium ethoxide gives 6-carbethoxy-3,5-diarylcyclohex-2-enones 17-24 by Knoevenagel reaction. The formed ketones 17-24 on treatment with phenyl hydrazine hydrochloride and anhydrous sodium acetate in refluxing methanol gives 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ols 25-32. The structures of the compounds are elucidated by melting points, elemental analysis, MS, FT-IR, one-dimensional NMR (1H & 13C), D2O exchanged 1H-NMR, two-dimensional HSQC spectroscopic data.
Table I. Physical and analytical data of compounds 25–32.
Antibacterial activity
All the newly synthesized novel target molecule 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ols, 25-32 were tested for their antibacterial activity in vitro against S. aureus, β-H. streptococcus, V. cholerae, S. typhii, S. felxneri. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . Ciprofloxacin was used as standard drug. Two compounds, which are having electron donating functional groups namely, (CH3, OCH3) 26 and 29 are potent against S.aureus and β-H. streptococcus. Compounds 27 and 28, which have electron withdrawing -Cl and -NO2 functional groups are active against V.cholerae and S.typhii than the standard drug Ciprofloxacin. Compound 32, which contains both electron donating methoxy group and withdrawing nitro group is more potent against S.typhii and S.felxneri. In addition, compounds 30 and 31 with substituents in the 6-aryl ring are active against S.felxneri and for 31, V.cholerae.
Table II. In vitro antibacterial activities (MIC) values for compounds 25-32.
Antifungal activity
The in vitro antifungal activity of the synthesized novel heterocyclic compounds, 25-32 was studied against the fungal strains viz., A. flavus, Mucor, Rhizopus and M. gypsuem. Fluconazole was used as a standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . In general, all the synthesized compounds exerted a wide range of modest in vitro antifungal activity against all the tested organisms, although the unsubstituted compound showed a poor spectrum. Of all the compounds tested, compounds 26, 29 and 31 are more effective against the tested A. flavus and Rhizopus. All these three compounds have electron donating methyl or methoxy functional groups. Compounds 27, 28 and 30, which contain electron withdrawing chloro, bromo or nitro groups in one of the rings, are potent against Mucor and M. gypsuem. Moreover, compound 32, a unique one having both electron donating methoxy and electron withdrawing nitro functional groups in different rings is more effective against all the tested fungal strains than the standard drug, Fluconazole.
Table III. In vitro antifungal activities (MIC) values for compounds 25–32.
Conclusion
In conclusion, the different functionalities in 6-carbethoxy-3,5-diaryl-cyclohex-2-enone 17-24 can be used advantageously in the preparation of new 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ols 25-32. Examination of the in vitro antibacterial and antifungal activity profile in differently substituted novel title compounds, 4,6-diaryl-4,5-dihydro-2-phenyl-2H-indazol-3-ols 25-32 against the tested bacterial strains viz. S. aureus, β-H. streptococcus, V. cholerae, S. typhii and S. felxneri, and the fungal strains viz., A. flavus, Mucor, Rhizopus and M. gypsuem respectively gives a structure – activity relationship, albeit with a very limited number of compounds, which may be summarised as follows: the nature of substituent on the phenyl ring viz., methyl. methoxy, chloro, nitro as well as the bromo functions at the meta and para positions of the aryl moieties determine nature and extent of the activity of the synthesized fused indazolonol compounds. These observations may promote a further development of our research in this field. Further development of this group of fused indazolonol compounds may lead to compounds with better pharmacological profile than standard drugs.
M. Gopalakrishnan, J. Thanusu & V. Kanagarajan
Design, synthesis, characterization and in vitro antimicrobial evaluation of 4,6-diaryl-4, 5-dihydro-2-phenyl-2H-indazol-3-ols
480–486
Acknowledgements
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording spectra. Two of our authors namely J.Thanusu and V. Kanagarajan are highly thankful for Annamalai University authorities for providing financial support in the form of Research Fellowship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- M Balasubramanian, and A D'Souza. (1968). Studies on the reduction of some substituted cyclohexanones. Tetrahedron 24:5399–5408.
- Cecchi L, Melani F, Filacchioni G, Tredici M. Synthesis and biological activity of some 3-(pyrazol-1′-yl)indazole derivatives Farmaco, 1984;39:945–952.
- JH Ko, SW Yeon, JS Ryu, KT Yong, SE Ha, YH Jung, PR Eun, and RC Kyu. (2006). Synthesis and biological evaluation of 5-arylamino-6-chloro-1H-indazole-4,7-diones as inhibitors of protein kinase B/Akt. Bioorg Med Chem Lett 16:6001–6005.
- A Gerpe, G Aguirre, L Boiani, H Cerecetto, M Gonzalez, C Olea-Azar, C Rigol, JD Maya, A Morello, OE Piro, VJ Aran, A Azqueta, AL de Cerain, A Monge, MA Rojas, and G Yaluff. (2006). Indazole N-oxide derivatives as antiprotozoal agents: Synthesis, biological evaluation and mechanism of action studies. Bioorg Med Chem 14:3467–3480.
- X Li, S Chu, VA Feher, M Khalili, Z Nie, S Margosiak, V Nikulin, J Levin, KG Sprankle, ME Tedder, R Almassy, K Appelt, and KM Yager. (2003). Structure-Based design, synthesis, and antimicrobial activity of indazole-derived SAH/MTA nucleosidase inhibitors. J Med Chem 46:5663–5673.
- DL Selwood, DG Brummell, J Budworth, GE Burtin, RO Campbell, S Chana, IG Charles, PA Fernandez, RC Glen, MC Goggin, AJ Hobbs, MR Kling, Q Liu, DJ Madge, S Meillerais, KL Powell, K Reynolds, GD Spacey, JN Stables, MA Tatlock, KA Wheelers, G Wishart, and C Woo. (2001). Synthesis and biological evaluation of novel pyrazoles and indazoles as activators of the nitric oxide receptor, soluble guanylate cyclase. J Med Chem 44:78–93.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, V Kanagarajan, R Govindaraju, and GA Jayasri. (2007). A convenient ‘one-pot’ synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enz Inhib Med Chem 22:709–715.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, C Prabhu, and V Kanagarajan. (2007). One-pot conversion of piperidine-4-ones to [1,4]-diazepan-5-ones under microwave irradiation using silica gel supported NaHSO4 catalyst. J Chem Res 2:80–81.
- M Gopalakrishnan, P Sureshkumar, V Kanagarajan, and J Thanusu. (2007). Design, ‘one-pot’ synthesis, characterization, antibacterial and antifungal activities of novel 6-aryl-1,2,4,5-tetrazinan-3-thiones in dry media. J Sulf Chem 28:383–392.
- T Balasankar, M Gopalakrishnan, and S Nagarajan. (2007). Synthesis and anti-bacterial activity of some 5-(4-biphenyl)-7-aryl[3,4-d]-1,2,3-benzoselenadiazoles. J Enz Inhib Med Chem 22:171–175.
- T Balasankar, M Gopalakrishnan, and S Nagarajan. (2005). Synthesis and antibacterial activity of some 5-(4-biphenylyl)-7-aryl[3,4-d][1,2,3]-benzothiadiazoles. Eur J Med Chem 40:728–731.
- MH Dhar, MM Dhar, BN Dhawan, BN Mehrotra, and C Ray. (1968). Screening of Indian plants biological activity. Part I. Indian J Exp Biol 6:232–247.
- W Guthrie, and XP Wang. (1991). The aldol condensation of acetophenone with acetone. Can J Chem 69:339.
- W Guthrie. (1991). Rate-equilibrium correlations for the aldol condensation: an analysis in terms of Marcus theory. J Am Chem Soc 113:7249.
- AT Nielson, and WJ Houlihan. (1968). The aldol condensation. Org React 16:1–438.