Abstract
Testosterone and ten of its metabolites were examined as inhibitors of butyrylcholinesterase. A significant enzyme inhibition activity (IC50 = 1.55 μM) was observed for androst-4-en-3,7-dione. The kinetic parameters of butyrylcholinesterase inhibition were determined and molecular docking was carried out in order to develop a better understanding of the inhibitor-enzyme interactions. The results showed that the inhibition was non-competitive, stabilized mainly by hydrogen bonds and hydrophobic interactions between the inhibitor and butyrylcholinesterase.
Introduction
Testosterone (1), the main male hormone, is known to be responsible for both anabolic and androgenic activities. Recent investigations have proven that testosterone plays an important role in the functions of the central nervous system. Many studies were carried out concerning the effect of endogenous androgen levels and cognition, spatial, verbal, and working memory Citation1, Citation2, Citation3, Citation4, Citation5. Morley et al. reported a significant correlation of testosterone levels with visual and verbal memory [Citation4], whereas Barrett-Connor et al. found that a higher bio-testosterone is associated with better long-term verbal memory and cognition screening test [Citation5]. Moreover, it has been reported recently that lower testosterone levels are associated with poor performance of memory and increased risk for developing Alzheimer's disease [Citation6,Citation7]. A number of other studies have shown that testosterone provides clinical benefit in Alzheimer's disease patients Citation8, Citation9, Citation10, Citation11, Citation12.
According to the cholinergic hypothesis, memory impairments in patients and senile dementia are due to a selective and irreversible deficiency in the cholinergic functions in the brain [Citation13]. In order to protect acetylcholine levels, potent acetylcholinesterase (AChE) inhibitors are employed to alleviate the symptoms. Like acetylcholinesterase, butyrylcholinesterase (BChE) inactivates the neurotransmitter acetylcholine (ACh). Greig et al. showed that selective butyrylcholinesterase inhibition elevates brain acetylcholine, and lowers Alzheimer beta-amyloid peptide [Citation14]. In addition, it was mentioned that BChE activity progressively increases in patients with Alzheimer's disease (AD), while AChE activity remains unchanged or declines [Citation15]. This suggests that development of specific BChE inhibitors will improve understanding of AD and may lead to a wider variety of potent treatment options.
Here, we have investigated the in vitro inhibitory effect of testosterone (1) and some of its derivatives against AChE and BChE by using Ellman's assay [Citation16]. This was followed by investigating the inhibition kinetics and prediction of the molecular recognition pattern of testosterone-butyrylcholinesterase complex through molecular docking.
Methods and materials
Testosterone (1) was obtained from Fluka, while its derivatives were prepared by microbial transformation of testosterone (1) using the fungi Rhizopus stolonifer and Fusarium lini [Citation17]. The structures of these compounds are given in .
Table I. Summary of the in vitro BChE inhibitory activities of compounds (1–11).
Cholinesterase inhibition assay
AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et al. [Citation16]. Electric-eel AChE (Torpedo californica type VI-S, Sigma, EC 3.1.1.7), horse-serum BChE (E.C 3.1.1.8), acetylthiocholine iodide (ATCh), butyrylthiocholine chloride (BTCh), 5, 5′-dithiobis [2-nitrobenzoic acid] (DTNB) and galanthamine were purchased from Sigma (St. Louis, MO, USA). Buffers and other chemicals were of analytical grade. Acetylthiocholine iodide and butyrylthiocholine chloride were used as substrates to assay AChE and BChE, respectively. All the inhibition studies were performed in 96-well microtiter-plates by using SpectraMax 340 (Molecular Devices, USA). The rate of the reaction was calculated from the following Ellman's equation [Citation16]:
Determination of kinetic parameters
The concentrations of test compounds that inhibited the hydrolysis of substrates (acetylthiocholine iodide and butyrylthiocholine chloride) by 50% (IC50) were determined by monitoring the effect of various concentrations of these compounds on the inhibition values. The IC50 values were then calculated using the EZ-Fit Enzyme Kinetics program (Perrella Scientific Inc., Amherst, USA). The Michaelis-Menten constant (Km) and the equilibrium constant of enzyme-inhibitor complex dissociation (Ki) were determined by the interpretation of Lineweaver-Burk plots (LWB) by measuring the initial velocities obtained over a substrate concentration range between 0.05–0.40 mM for AChE and 0.05–0.20 mM for BChE. IC50 values of compounds 1–11 against BChE are summarized in .
Results and discussion
Many testosterone derivatives were prepared by whole cell fungi transformation () [Citation17]. This method is known to produce metabolites similar to those obtained from mammalian cells [Citation18]. The preliminary screening of these compounds against AChE and BChE showed that none exhibited a significant inhibition of AChE (IC50 values >300 μM), while some exhibited a moderate inhibition of BChE (IC50 values are summarized in ). This selectivity for BChE can be explained partly by the relatively large size of these compounds, which can diffuse easily inside the larger catalytic gorge (∼200 Å3) of BChE. It has been reported that six out of the fourteen aromatic amino acid residues, lining the active site gorge of AChE are replaced by smaller aliphatic amino acid residues in BChE. This replacement may cause selective sensitivity of different inhibitors against the two enzymes [Citation19]. Hydrophobic interactions, which are more prominent in BChE, might also play an important role in the selectivity of the tested compounds toward BChE.
The results in illustrate that androst-4-en-3,17-dione (AD) (2) exhibits the highest activity (IC50 = 1.55 μM), while 11α-hydroxytestolactone (6) shows the lowest inhibitory activity against BChE (IC50 = 283.95 μM). Several structural features of testosterone derivatives were found to play an important role in BChE inhibition. With the exception of compound 2, oxidation of 17-OH to the corresponding keto-group decreased the activity, introduction of a hydroxy group at C-11 also decreased activity. While dehydrogenation at C-1/C-2 did not show a consistent effect, since the activities of testosterone (1) (IC50 = 53.50 μM) and 11α-hydroxytestosterone (10) (IC50 = 89.03 μM) were less than the corresponding dehydrogenated compounds with IC50 = 20.87 (compound 8) and 61.50 μM (compound 11), respectively. Androst-4-en-3,17-dione (2) and 11α-hydroxyandrost-4-en-3,17-dione (5) were more active than the corresponding dehydrogenated derivatives; 7 and 9, respectively. Lactonization of ring D appeared to decrease the activity as in compounds 3 and 6.
Observing that androst-4-en-3,17-dione (2) is a more potent BChE inhibitor than galanthamine (12), a known inhibitor of cholinesterase, more studies were carried out to investigate the type and the kinetics of the inhibition of BChE by androst-4-en-3,7-dione (2). Such studies can give a better understanding of the mechanism of inhibition and the site of interaction between the inhibitor and the enzyme. Information about the type of inhibition was predicted graphically by measuring the rate of hydrolysis of certain concentrations of the substrate butyrylthiocholine chloride at various inhibitor concentrations, then plotting the Lineweaver- Burk plot (). The maximum rates (Vmax), the Michaelis-Menten constant (Km) and the equilibrium constant of enzyme-inhibitor complex dissociation (Ki) were calculated from the intercepts and slopes of the lines obtained in . Lineweaver- Burk plot shows a typical non-competitive inhibition [Citation20]. Km value was found to be 0.26 μM while Ki was 13.5 μM. The maximum velocity continually decreases as the concentration of the inhibitor increases.
Figure 1. Steady state inhibition of BChE by androst-4-en-3,17-dione (2), the Lineweaver– Burk plot of reciprocal of initial velocities versus reciprocal of four fixed BChE concentrations in absence (▪) and presence of 5.0 μM (Δ), 10.0 μM (•), and 20.0 μM (▴) androst-4-en-3,17-dione (2).
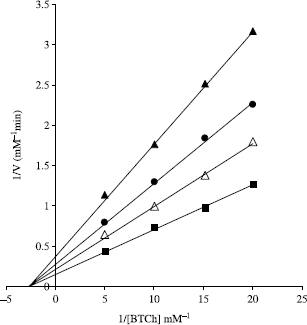
These findings suggest that compound 2 has no effect on the substrate binding sites of the enzyme and vice versa, but it binds after or before binding of the substrate, resulting in the formation of a catalytically inactive enzyme-substrate-inhibitor complex. In addition, compound 2, as a non-competitive inhibitor, may interact with the enzyme at positions other than the catalytic active site.
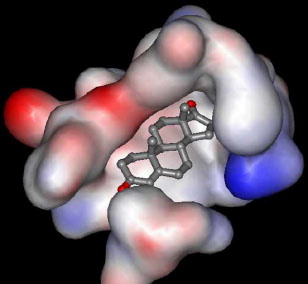
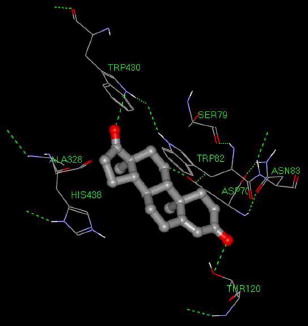
Molecular docking provided further evidence to support the non-competitive type inhibition. Using Auto Dock 3.0, the docking protocol was repeated several times and the best docking solution (the one with the lowest energy) was considered. The results, summarized in , indicated that BChE can accommodate compound 2 at the peripheral anionic site (PAS), located at the mouth of the catalytic site gorge (), allowing the interaction of compound 2 with Asp70, and Tyr332. Such orientation permits the formation of two hydrogen bonds between the carbonyl groups at C-3 and C-17 of compound 2 and Thr120 and Trp430 of BChE which seems to play an important role in the inhibitory effect of compound 2. In addition, hydrophobic-hydrophobic interactions may form between compound 2 and Ser79, Trp430, Ala328, Met437, Tyr440, and with the amino acid Trp82 which is located at the cationic π site of the enzyme. A week hydrophobic-hydrophobic interaction may also form between compound 2 and His438 at the catalytic triad (). No interaction was observed with the amino acid residues of the oxyanion hole; Gly116, and Gly117, or with Ser198, and Glu325 at the catalytic triad of esteratic site. These results indicate that, beside the above mentioned hydrogen bonds, the interactions between the amino acids of the enzyme and the inhibitor are of a hydrophobic type. This is consistent with the decrease in the activity of the compounds as their polarity increases, as indicated by the IC50 results in .
Conclusion
Testosterone (1) and some of its metabolites can inhibit BChE selectively. The most potent metabolite is adrost-4-en-3,17-dione (2) which shows a lower IC50 (1.55 μM)than galanthamine (8.5 μM). Kinetic studies revealed that compound 2 inhibits BChE in a non-competitive manner. Molecular modeling docking results were in good agreement with the inhibition kinetics results, and gave more structural insight to the experimental findings.
Acknowledgements
We are grateful to the Deanship of Scientific Research at the University of Jordan (Jordan) for financial support. We also acknowledge the Higher Education Commission (Pakistan) for financial support to A. Al-Aboudi under the short-term Foreign Faculty program.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- MM Cherrier, S Asthana, S Plymate, L Baker, AM Matsumoto, E Peskind, MA Raskind, K Brodkin, W Bremner, A Petrova, S LaTendresse, and S Craft. (2001). Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 57:80–88.
- JS Janowsky, SK Oviatt, and ES Orwoll. (1994). Testosterone influences spatial cognition in older men. Behav Neurosci 108:325–332.
- JS Janowsky, B Chavez, and E Orwoll. (2000). Sex steroids modify working memory. J Cognitive Neurosci 12:407–414.
- JE Morley, F Kaiser, WJ Raum, HM Perry, JF Flood, J Jensen, AJ Silver, and E Roberts. (1997). Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: Progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci USA 94:7537–7542.
- E Barrett-Connor, D Goodman-Gruen, and B Patay. (1999). Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab 84:3681–3685.
- E Hogervorst, J Lehmann, J McBroom, and D Smith. (2002). Apolipoprotein Eε4 and testosterone interact in the risk of Alzheimer's disease in men. Int J Geriatr Psychiatry 10:938–940.
- S Moffat. (2005). Effects of testosterone on cognitive and brain aging in elderly men. Ann NY Acad Sci 1055:80–92.
- M Cherrier, A Matsumoto, J Amory, S Asthana, W Bremner, E Peskind, M Raskind, and S Craft. (2005). Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 64:2063–2068.
- DA Gruenewald, and AM Matsumoto. (2003). Testosterone supplementation therapy for older men: Potential benefits and risks. J Am Geriat Soc 51:101–115.
- E Hogervorst, S Bandelow, and SD Moffat. (2005). Increasing testosterone levels and effects on cognitive functions in elderly men and women: A review. Curr Drug Targets CNS Neurol Disorder 4:531–540.
- GK Gouras, H Xu, RS Gross, JP Greenfield, B Hai, R Wang, and P Greengard. (2000). Testosterone reduces neuronal secretion of Alzheimer's β-amyloid peptides. Proc Natl Acad Sci USA 97:1202–1205.
- RS Tan, and SJ Pu. (2003). A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer's disease. The Aging Male 6:13–17.
- EK Perry. (1986). The cholinergic hypothesis ten years on. Br Med Bull 42:63–69.
- NH Greig, T Utsuki, DK Ingram, Y Wang, G Pepeu, C Scali, QS Yu, J Mamczarz, HW Holloway, T Giordano, D Chen, K Furukawa, K Sambamurti, A Brossi, and DK Lahiri. (2005). Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc Natl Acad Sci USA 102:17213–17218.
- E Giacobini. (2001). Selective inhibitors of butyrylcholinesterase: A valid alternative for therapy of Alzheimer's disease?. Drugs Aging 18:891–898.
- GL Ellman, KD Courtney, V Andres, and RM Featherstone. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95.
- A Al-Aboudi, M Yasin, G Musharraf, MI Choudhary, and A Ur-Rahman. (2008). Nat Prod Res (accepted for publication)
- P Fernandes, A Cruz, B Angelova, HM Pinheiro, and JS Cabral. (2003). Microbial conversion of steroid compounds: Recent developments. Enz Microb Technol 32:688–705.
- A Saxena, AM Redman, X Jiang, O Lockridge, and BP Doctor. (1997). Differences in active site gorge dimensions of cholinesterases revealed by binding of inhibitors to human butyrylcholinesterase. Biochemistry 36:14642–14651.
- IH Segel. Enzyme Kinetics Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. New York: Wiley; (1975) p 101–112.