Abstract
Beauvericin is a secondary metabolite natural product from microorganisms and has been shown to have a new potential antifungal activity. In this study, the metabolism and inhibition of beauvericin in human liver microsomes (HLM) and rat liver microsomes (RLM) were investigated. The apparent Km and Vmax of beauvericin in HLM were determined by substrate depletion approach and its inhibitory effects on cytochromes P450 (CYP) activities were evaluated using probe substrates, with IC50 and the (Ki) values were 1.2 μM (0.5 μM) and 1.3 μM (1.9 μM), respectively for CYP3A4/5 (midazolam) and CYP2C19 (mephenytoin). Similarly, beauvericin was also a potent inhibitor for CYP3A1/2 (IC50: 1.3 μM) in RLM. Furthermore, the pharmacokinetics of beauvericin in the rat were studied after p.o administration alone and co-administration with ketoconazole, which indicated a pharmacodynamic function may play a role in the synergistic effect on antifungal activity.
Introduction
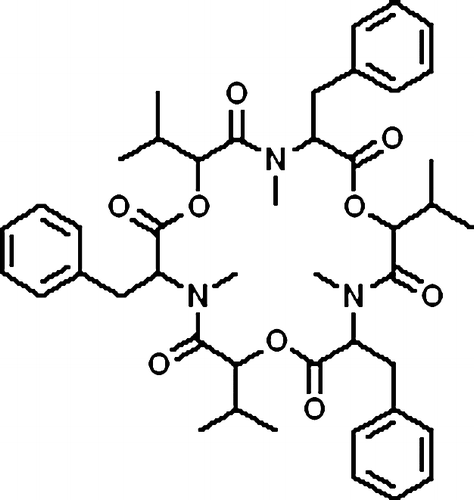
Beauvericin, a cyclic lactone trimer of the amide of N-methyl L-phenylalanine and D-L-hydroxyisovaleric acid (), was first isolated from Beauveria bassiana on the basis of assays of toxicity to brineshrimp and similar to the enniatins, which are produced by a number of Fusarium species [Citation1]. Recently, beauvericin has been reported to display a synergistic fungicidal effect combined with a low dosage of ketoconazole, and thereby a prospective strategy for antifungal therapy could be developed [Citation2]. It is obvious risk that fungal infections is greatly increased in patients who are severely immunocompromised due to cancer chemotherapy, organ or bone marrow transplantation, or through human immunodeficiency viral infections Citation2Citation3Citation4Citation5. Additionally, new antifungal medicines are needed regarding there are more and more drug-resistant fungal infections Citation2Citation6Citation7Citation8. Therefore, it is an unmet need to develop new chemicals or therapeutic application for antifungal.
A new chemical entity for clinical application should satisfy certain pharmacokinetic property, mainly revealed as the drug absorption, distribution, metabolism, and excretion (ADME) properties Citation9Citation10Citation11. Additionally, the risk of drug interaction potentials for new drug candidate should be well evaluated before the expensive clinical trials. In the present study, the main ADME properties and pharmacokinetic property in rats for beauvericin were conducted, including the estimation of the apparent Km and Vmax and the inhibitory potentials of beauvericin in drug metabolizing enzymes, cytochrome P450, using specific substrates in HLM and RLM. The resulting pharmacokinetic properties in rats are also discussed.
Materials and methods
Chemicals
Midazolam, 1′-hydroxymidazolam, mephenytoin, 4′-hydroxymephenytoin, phenytoin, dextromethorphan, dextrorphan, propranolol, α-hydroxytriazolam, henacetin, acetaminophen, salbutamol, diclofenac, 4′-hydroxydiclofenac, flufenamic acid were purchased from Sigma–Aldrich (St. Louis, MO, USA). HLM was obtained from BD Gentest (Woburn, MA). Acetonitrile, methnol (HPLC grade) was acquired from Burdick and Jackson (Muskeson, MI, USA). Formic acid (HPLC grade) were purchased from Dima technology INC., USA. Beauvericin and ketoconazole were kindly provided by Dr. Zhang Lixin (Institute of Microbiology, Chinese Academy of Sciences). Deionized water was prepared using a Mill-Q-System from Millipore Corp. (Milford, MA, USA). All other reagents and chemicals were of analytical or high-performance liquid chromatography (HPLC) grade.
Lc-ms/ms Conditions
Determination of all analytes was carried out using an LC/MS/MS system, which consisted of an HPLC apparatus (Shimadzu, Japan) including a SCL-10ADvp controller, two LC-10ADvp pumps and a DGU-14A solvent degasser, and a PE/SCIEX Applied Biosystems API3000 triple-quadrupole mass spectrometer (Foster City, CA, USA) equipped with electrospray ionization source. A MPS3C autosampler (GERSTEL Germany) was used for sample delivery. The HPLC columns used were a Gemini C18 110A column (2.0 × 100 mm, 5.0 μm) equipped with an ODS guard column (4 mm × 2.0 mm i.d) from Phenomenex (Torrance, CA,USA). Mobile phase of 0.1% formic acid in CH3OH/H2O(10/90) (A) and 0.1%formic acid in CH3OH/H2O(90/10) (B) were used for the chromatography in all experiments, run gradiently. Three HPLC gradient programs were designed to analyze these chemicals.
HPLC gradient program I, used for CYP2C9 and 2C19 assays, was as follows: 0 to 0.2 min, 25% B; 0.2 to 1.1 min, gradient to 90% B; 1.1to 2.0 min, 90% B; and 2.0 to 2.5 min, gradient to 25% B;
HPLC gradient program II, used for CYP3A4/5 and 2D6 assays, was as follows: 0 to 0.6 min, 10% B; 0.6 to 1.3 min, gradient to 95% B; 1.3to 2.5 min, 95% B; and 2.5 to 2.7 min, gradient to 10% B;
HPLC gradient program III, used for CYP1A2 assays and beauvericin analysis, was as follows: 0 to 0.4 min, 10% B; 0.4 to 1.5 min, gradient to 95% B; 1.5 to 3.0 min, 95% B; and 3.0 to 3.2 min, gradient to 10% B.
The run time was 3.8 min, column temperature was ambient, and the flow rate was 0.2 mL/min throughout the analyses.
The analytes were all detected by monitoring the precursor → product ion transition at unit resolution using multiple reaction monitoring (MRM) scan mode. Both analytes and their own ISs responded best to the positive ionization mode, with the protonated molecular ions [M + H]+ as the major species. Product ion spectra and fragmentation pathways of Beauvericin was shown in . The monitoring ion was set as m/z 784.1 / 262.3 for Beauvericin. For the five metabolites and ISs used as probes of cytochrome P450 activity, the MRM acquisitions were performed using the transition and collision energy described in previous paper [Citation12]. Mass spectrometric conditions were optimized to obtain maximum sensitivity. The following ESI conditions were applied: drying gas (nitrogen) heated at 350°C and used at a flow rate of 6 L/min; nebulizer gas (nitrogen) at a pressure of 3.5 MPa and heated nebulizer parameters were set as follows (arbitrary units): nebulizer, 12; curtain: 8; capillary voltage was set at 4500 V. Data was acquired using the Analyst 1.4 software.
Preparation of RLM
RLM was prepared as previously described [Citation13]. RLM prepared by several times was pooled together and the final concentration of proteins was measured to be 19 mg/mL by the method of Lowry et al. [Citation14].
Substrate depletion experiment of beauvericin
Beauvericin (40, 100, 400, and 1000 nM, respectively) was incubated with HLM (0.2 mg/mL) in a total incubation volume of 1.5 mL. Aliquots (0.2 mL) were removed at 0, 2.5, 5, 8, 15, 22 and 30 min and terminated by addition to 0.4 mL of acetonitrile on ice containing 300 ng of phenytoin as internal standard. The resulting solution was vortex-mixed, and the supernatant was separated by centrifugation at 10,000g for 5 min at 4°C. Aliquots (20 μL) of the supernatant were injected into an LC/MS/MS system.
Inhibition effect and kinetic analysis of beauvericin in HLM and RLM
In vitro incubations
All incubations were conducted by shaking reaction mixtures under air in a heated water bath at 37°C. Incubations were carried out in 100 mM KH2PO4, pH 7.4. Substrate, inhibitor, and microsomes were premixed at a certain concentration and pre-warmed for 5 min at 37°C, and incubations were commenced by addition of NADPH (1.0 mM). The final volume of the incubation mixture was 400 μL and the final concentration of organic solvent did not exceed 0.3%. After incubation at 37°C for a specific period of time, the reaction was terminated by the addition of ice-cold acetonitrile (800 μL) in which a specific amount of internal standard was contained. The resulting solution was vortex-mixed, and the supernatant was separated by centrifugation at 10,000g for 5 min at 4°C. Aliquots (20 μL) of the supernatant were injected into an LC/MS/MS system. In substrate saturation experiments in which product formation was measured, incubation times were set that had provided a linear reaction velocity in preliminary experiments. In substrate consumption experiments, this was intentionally not the case. All incubations were performed in duplicate, and the mean values were used for analysis.
Inhibition effects of beauvericin on the P450 Enzymes
The effect of various concentrations of beauvericin (0.05, 0.1, 0.5, 1, 10, 50 and 100 μM) on P450 activities was examined in HLM using probe substrates for corresponding specific isoforms of cytochrome P450. Five major human drug-metabolizing enzymes, CYP1A2, 2C9, 2C19, 2D6, and 3A4/5 were investigated [Citation9]. The following activities were measured for each type of P450 enzymes: phenacetin O-deethylation for CYP1A2, mephenytoin 4′-hydroxylation for CYP2C19, dextromethorphan O-demethylation for CYP2D6, diclofenac 4′-hydroxylation for CYP2C9, and midazolam 1′-hydroxylation for CYP3A4/5. The concentrations of phenacetin, mephenytoin, dextromethorphan, diclofenac, and midazolam were set at 45, 55, 10, 10 and 5 μM respectively, all were at approximately their respective Km values (CDER, FDA, http://www.fda.gov/cder/drug/drugInteractions/default.htm). Microsome concentrations and incubation times were all set at 0.2 mg protein/mL and 6 min respectively for each reaction, except for the isoforms of CYP2C19 in which the mephenytoin 4′-hydroxylation assay was carried in 0.3 mg protein/mL and 40 min reaction. The incubation was carried out in the same way with the method described above.
The inhibition effect of beauvericin on P450 activities in RLM was performed in a similar way with the above method for HLM. Dextromethorphan, diclofenac and midazolam were selected to be specific substrates for CYP2D2, 2C6, and 3A1/2 respectively [Citation15]. Each incubation was performed with 0.3 mg/mL RLM.
Pharmacokinetic experiments in rats
Male Sprague–Dawley rats (8 weeks old, weighing 200–240 g) were purchased from the Laboratory Animal Services Center of Southern Medical University in China. Animals were held in wire cages under conditions of constant temperature (22–25°C), lighting (12-hr dark-light cycle) and humidity (55 ± 5%) with a commercial food diet and water freely available, and were acclimated in the facility for 1 week prior to testing. The animal protocols for experiments were reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences.
Rats were orally dosed with 0.5, 1, and 2 mg/kg beauvericin, and co-administrated with 0.5 mg/kg ketoconazole at the dose of 0.5 mg/kg of beauvericin, respectively. Beauvericin and ketoconazole were formulated in 0.5% methylcellulose suspension before gavage. Access to water was maintained during the experiment, but animals were fasted beginning the night before the experiment. Blood samples (0.4 mL) were collected 0.133, 0.833, 1.5, 3.5, 5, 8, 12, 16 and 24 h after the dosing and immediately centrifuged at 2000 × g for 10 min. The plasma was then collected and stored at − 80°C until analysis. For analysis, 90 μL of plasma was mixed with 10 μL of the internal standard (IS) solution (4.0 ng/mL of phenytoin) in a 1.5 mL polypropylene test tube, and then 200 μL of methnol/acetonitrile (50/50, v/v) solution was added to precipitate proteins. The mixture was vortexed for 1 min, followed by centrifugation for 10 min at 16,000g. A 20 μL of the supernatant was injected into the LC/MS-MS apparatus for determination of the contents interested.
Data analysis
In substrate depletion experiment, the percentage remaining versus time at each substrate concentration was fitted to first order decay functions to determine initial substrate depletion rate constants (kdep). If substrate decline demonstrated nonlinearity on log percentage remaining versus time curves, only those initial timepoints wherein log-linearity was observed were used to determine depletion rate constants.
Km values from substrate consumption experiments were determined by plotting the kdep versus the substrate concentration on a linear-log plot with Origin software, version 7.5 (MicroCal Software, Inc., Northampton, MA), using the following Equation (1):
in which [S] is the substrate concentration, kdep([S] = 0) represents the theoretical maximum consumption rate constant at an infinitesimally low-substrate concentration, and Km is the Michaelis constant [Citation16].
And the following Equation (2) was used to calculate the Vmax values [Citation17]:
in which Vmax is the maximum velocity of enzyme and Km is the Michaelis constant calculated by Equation (1). Intrinsic clearance of the in vitro incubation was calculated as Clint = Vmax/Km.
IC50 values (inhibitor concentration that decreased enzyme activity by 50%) were determined by nonlinear regression of sigmoidal dose-response curves using Grafit, version 5.0. The determination of apparent Ki value (the equilibrium dissociation constant for the enzyme-inhibitor complex) was conducted from secondary reciprocal plots of apparent Km/Vmax (obtained from the slope of the Lineweaver-Burk plots) versus inhibitor concentration, the Ki value was equal to x-intercept of the secondary reciprocal plots [Citation18,Citation19]. Lineweaver-Burk plots and Dixon plots of the enzyme kinetic data were generated to determine the mode of inhibition [Citation20].
The pharmacokinetic parameters were calculated using the Drug and Statistics version 2.0 software package (Anhui Provincial Center for Drug Clinical Evaluation, China).
Data were presented as the mean ± S.D. and Statistical analyses were performed by one-way analysis of variance (ANOVA), and P values of < 0.05 were considered to be statistically significant.
Results
The apparent Km and Vmax of beauvericin in HLM determined by substrate depletion approach
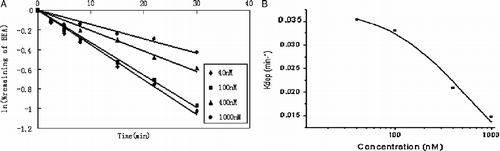
To estimate the enzyme kinetic parameters of beauvericin in HLM using substrate depletion approach, each kdep value at various concentrations were determined. Substrate concentrations range from 40 to 1000 nM and the depletion profiles are shown in (A). The apparent Km and Vmax values for beauvericin metabolism were, therefore, estimated to be 0.6 ± 0.1 μM and 21 ± 3 nmol/min/mg-protein, respectively, according to the Equations (1) and (2) ((B)). The intrinsic clearance value (Clint) was determined to be 38 ± 8 mL/min/mg-protein.
Figure 2. (A) Disappearance of beauvericin in HLM at various initial concentrations. Beauvericin at concentrations of 40, 100, 400, and 1000 nM was incubated with 0.2 mg/mL HLM. Each point represents the logarithmic value of the remaining percentage of substrate to the initial concentration in HLM. (B) Plots of in vitro depletion rate constants versus substrate concentration for HLM-catalyzed beauvericin metabolism. Each point represents the depletion rate constants of corresponding substrate concentration. The line represents the curve predicted from Equation (1).
Inhibitory effects of beauvricin on cytochrome P450 in HLM and RLM
The inhibitory effects of beauvericin on the activities of five common major human drug-metabolizing enzymes, cytochrome P450 and three rat cytochrome P450 were evaluated, as assessed by isoform-specific probe reactions. The selective substrate concentrations were prepared near to its respective Km value and beauvericin concentration range is from 0.05 to 100 μM. Both inhibitory results in HLM and RLM are summarized in and . Beauvericin occurred to be a strong inhibitor for midazolam 1′-hydroxylase (CYP3A4/5) and mephenytoin 4′-hydroxylase (CYP2C19) activities in HLM, with IC50 values of 1.2 ± 0.1 μM and 1.3 ± 0.4 μM, respectively. In contrast, Beauvericin displayed no significant inhibitory effects on the activities catalyzed by CYP1A2, 2C9, or 2D6 (IC50>100 μM). Additionally, beauvericin was found to be a potent inhibitor to CYP3A1/2 with the IC50 value of 1.3 ± 0.6 μM and not a inhibitor at all for CYP2C6 or 2D2 in RLM (IC50>100 μM) as shown in .
Table I. Inhibition effect of Beauvericin on cytochrome P450 isozyme-specific activities in HLM and RLM.
Figure 3. Inhibitory effects of beauvericin on CYP-catalyzed reactions in HLM (A) and RLM (B). Beauvericin were incubated under conditions described in Methods. The activity of each isoform was measured by isoform-specific substrate reaction probes at approximately their respective Km value. Each data point represents the mean of duplicate experiments.
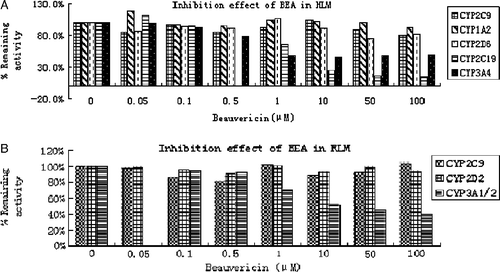
Figure 4. Inhibitory effect of beauvericin on CYP3A4/5 and 2C19 in HLM, and CYP3A1/2 in RLM for the value of IC50, using the software of Grafit, version 5.0. (A) Inhibition of CYP3A4/5 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (B) Inhibition of CYP2C19 (with mephenytoin [55 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (C)) Inhibition of CYP3A1/2 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in RLM (see Materials and Methods for details). Data are averages for duplicate incubations.
![Figure 4. Inhibitory effect of beauvericin on CYP3A4/5 and 2C19 in HLM, and CYP3A1/2 in RLM for the value of IC50, using the software of Grafit, version 5.0. (A) Inhibition of CYP3A4/5 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (B) Inhibition of CYP2C19 (with mephenytoin [55 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (C)) Inhibition of CYP3A1/2 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in RLM (see Materials and Methods for details). Data are averages for duplicate incubations.](/cms/asset/a0effdea-6863-4e38-b0ad-98e4842ab96b/ienz_a_336371_f0005_b.gif)
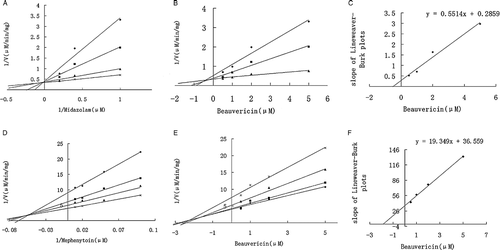
To better understand the inhibition mechanism by beauvericin, detail inhibition kinetics was conducted in HLM and revealed by Lineweaver-Burk plots, Dixon plots, and secondary reciprocal plots. Midazolam (1–5 μM) and mephenytoin (10–100 μM) were used as selective substrate of CYP3A4/5 and CYP2C19 respectively, and beauvericin (0.5–5 μM) was added to the reaction system as inhibitor. A competitive inhibition kinetics was indicated by methods of Lineweaver-Burk plot, Dixon plots, and secondary reciprocal plots for beauvericin inhibiting CYP3A4/5 activity, while a mixed type kinetics was suggested by these methods for beauvericin inhibiting CYP2C19 activity ((A)–(F)). The apparent Ki values were calculated to be 0.5 μM and 1.9 μM for beauvericin inhibiting CYP3A4/5 and 2C19 activities, respectively, using Non-linear regression analysis.
Figure 5. Lineweaver-Burk plots, Dixon plots, and secondary reciprocal plots of CYP3A4/5 catalyzed midazolam 1′-hydroxylation and CYP2C19-catalyzed mephenytoin 4′-hydroxylation by beauvericin (1–5 μM) in HLM. The symbols indicate mean of the transformed data for two independent experiments, and the lines were generated by linear regression analysis. Lineweaver-Burk plots of midazolam (A, 1–5 μM) and mephenytoin (D, 10–100 μM) in the presence of 0.5( × ), 1 (▴), 2 (▪), and 5 (♦) μM beauvericin. Dixon plots of midazolam (B) with 1 (♦), 2.5 (▪), and 5 (▴)μM and mephenytoin (E) with 10 ( × ), 20 (▴), 50 (▪), and 100 (♦) μM in the presence of beauvericin (0.5, 1, 2, and 5 μM). Secondary plots of the slopes taken from Lineweaver-Burk plots versus the beauvericin concentration for midazolam (C) and mephenytoin (F). Each data point represents the average of duplicate measurements.
Pharmacokinetics result of beauvericin and ketoconazole in rats
A sensitive liquid chromatography-electrospray ionization mass spectrometric (LC-MS/MS) method was established to analyze the content in rat plasma. The lower limit of detection of the method reached to 0.5 ng/mL for both beauvericin (the transition 784.1 → 262.3) and ketoconazole (the transition 531.2 → 489.4). The standard curves exhibited excellent linearity over a range of 0.5-180 ng/mL (r2>0.992) for beauvericin and 0.5-250 ng/mL (r2>0.994) for ketoconazole. The typical chromatograms of beauvericin in rat plasma are shown in . The mean plasma concentration-time profiles of beauvericin after p.o. administration at the doses of 0.5, 1, and 2 mg/kg and co-administration beauvericin and ketoconazole at the doses of 0.5 mg/kg for each are shown in . The pharmacokinetic parameters of these four representative doses are calculated using DAS Software and the results are shown in . The results indicated that pharmacokinetic exposures for beauvericin and ketoconazole were not significantly different with the comparison of administration with each alone and with the co-administration of both.
Figure 6. Typical chromatograms of beauvericin and phenytoin (internal standard, IS) in rat plasma. Plasma sample spiked with beauvericin (A) and IS (B); Blank plasma sample (C).
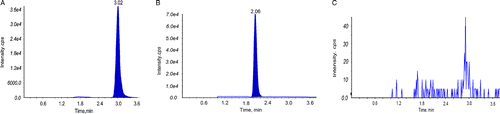
Figure 7. (A) Profiles of mean plasma concentration of beauvericin versus time after p.o. administration at the dose of 0.5, 1, 2 mg/kg. (B) Comparative profiles of mean plasma concentration of beauvericin after 0.5 mg/kg dose of beauvericin alone and co-administration with ketoconazole both at the dose of 0.5 mg/kg to rats. (C) Comparative profiles of mean plasma concentration of ketoconazole after 0.5 mg/kg dose of ketoconazole alone and co-administration with beauvericin both at the dose of 0.5 mg/kg to rats.
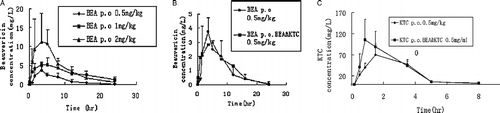
Table II-A. Pharmacokinetic parameters of beauvericin after oral administration to rats (n = 5; mean±S.D.).
Table II-B. Pharmacokinetic parameters of ketoconazole after oral administration to rats (n = 5; mean±S.D.).
Discussion
Biotransformation of beauvericin in HLM displayed NADPH dependency, indicating cytochrome P450 response mainly for its intrinsic clearance. Since the standards of metabolites are not readily available, the common enzyme kinetic approach monitoring the formation of metabolite(s) is not appropriate. Therefore, the substrate depletion approach is invaluable to use for determination of the apparent Km and Vmax of beauvericin in HLM Citation21Citation22Citation23. The main disadvantage for this approach is that the multiple pathways may be involved in the substrate depletion and thus the resulting apparent Km and Vmax would be represented in a combinating contribution of multiple cytochrome P450 isoforms [Citation23].
The apparent Km, Vmax, and Clint of beauvericin in HLM measured by the substrate depletion approach were determined to be 0.6 ± 0.1 μM, 21 ± 6 μM/min/mg protein, and 29 ± 14 mL/min/mg protein, respectively, revealing high affinity of beauvericin to cytochrome P450s. These results are well correlated with the inhibition measurements of beauvericin inhibiting activities catalyzed by CYP3A4/5 and 2C19 in HLM (Ki: 0.5 μM and 1.9 μM, ), especially in which the competitive inhibition kinetics was determined in CYP3A4/5-mediated midazolam 1′-hydroxylation reaction. Similarly, beauvericin was found to be a potent inhibitor to CYP3A1/2 (IC50: 1.3 μM) in RLM. Since the selective substrate for rat CYP2C has not been identified yet based on our knowledge, the inhibition of beauvericin in RLM for CYP2C was not evaluated in the present study.
The pharmacokinetic properties of beauvericin in rats were studied at the doses of 0.5, 1, 2 mg/kg respectively. As expected, the exposures of beauvericin in rats increased as the administration dosage increased ((A)). Ketoconazole is a well known potent CYP3A inhibitor, giving a Ki of 0.14 μM in RLM (Salem Omran Ali Abdalla, dissertation, Department of Clinical Pharmacology, Georg-August University, Gottingen. Germany, June 2007). Ketoconazole also displayed a short half-life of 1.38 h in rats [Citation24]. In our present study, the AUC0-t for beauvericin administered alone (0.5 mg/kg) and administrated simultaneously with ketoconazole (both 0.5 mg/kg) were calculated to be 27.9 h·mg·L− 1 and 31.1 h·mg·L− 1, respectively, indicating no significant difference in the exposures of beauvericin in rats. Additionally with the comparison of these two administrations, Cmax of 3.4 versus 3.8 mg·L− 1 and Tmax of 4.1 h versus 3.4 h did not display significantly different. Similarily, the exposures of ketoconazole were not significantly different in its administration alone and simultaneously co-administration with beauvericin. It is worthwhile to mention that the antifungal activity by a combination administration of beauvericin with ketoconazole displays more than 100-fold higher than that by a single application of either alone [Citation2]. Our finding indicates that the synergetic effect on antifungal activities obtained with co-administration of beauvericin and ketoconazole was not caused by their pharmacokinetic interactions.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notes
†Tel: 86 20 32290426. [email protected]
‡Email: [email protected]
References
- RD Plattner, PE Nelson. Production of beauvericin by a strain of Fusarium proliferatum isolated from Corn Fodder for Swine. Appl Environ Microbiol 1994;60:3894–3896.
- LX Zhang, KZ Yan, Y Zhang, R Huang, J Bian, CS Zheng, HX Sun, ZH Chen, N Sun, R An, FG Min, WB Zhao, Y Zhuo, JL You, YJ Song, ZY Yu, ZH Liu, KQ Yang, H Gao, HQ Dai, XL Zhang, J Wang, CZ Fu, G Pei, JT Liu, S Zhang, G Michael, YY Jiang, J Kuai, GC Zhou, XP Chen. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. PNAS 2007;104:4606–4611.
- JL Goodman, DJ Winston, RA Greenfield, PH Chandrasekar, B Fox, H Kaizer, RK Shadduck, TC Shea, P Stiff, DJ Friedman. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992;326:845–851.
- MT Larocco, SJ Burgert. Infection in the bone marrow transplant recipient and role of the microbiology laboratory in clinical transplantation. Clin Microbiol Rev 1997;10:277–297.
- V Sanchez, JA Vasquez, D Barth-Jones, L Dembry, JD Sobel, MJ Zervus. Nosocomial acquisition of Candida parapsilosis: An epidemiologic study. Am J Med 1993;94:577–582.
- JH Rex, MG Rinaldi, MA Pfaller. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother 1995;39:1–8.
- MA Ghannoum, LB Rice. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999;12:501–517.
- AM Sugar, SG Alsip, JN Galgiani, JR Graybill, WE Dismukes, GA Cloud, PC Craven, DA Stevens. Pharmacology and toxicity of high-dose ketoconazole. Antimicrob Agents Chemother 1997;31:1874–1878.
- TD Bjornsson, JT Callaghan, HJ Einolf, V Fischer, L Gan, S Grimm, J Kao, PS King, G Miwa, L Ni, G Kumar, J Mcleod, SR Obach, S Roberts, A Roe, A Shah, F Snikeris, JT Sullivan, D Tweedie, JM Vega, J Walsh, SA Wrighton. The conduct of in vitro and in vivo drug-drug interaction studies: A pharmaceutical research and manufacturers of America (PhRMA) perspective. Drug Metab Dispos 2003;31:815–832.
- CL Crespi, DM Stresser. Fluorometric screening for metabolism-based drug-drug interactions. J Pharmacol Toxicol Meth 2000;44:325–331.
- ZL Xia, JY Ying, R Sheng, S Zeng, YZ Hu, TW Yao. In vitro metabolism of BYZX in human liver microsomes and the structural elucidation of metabolite by liquid chromatography–mass spectrometry method. J Chromatogr B 2007;857:266–274.
- M Yao, MS Zhu, WS Michael, HJ Zhang, WG Humphreys, AD Rodrigues, R Dai. Development and full validation of six inhibition assays for five major cytochrome P450 enzymes in human liver microsomes using an automated 96-well microplate incubation format and LC–MS/MS analysis. J Pharm Biomed Anal 2007;44:211–223.
- DF Zhong, SQ Zhang, L Sun, XY Zhao. Metabolism of roxithromycin in phenobarbital-treated rat liver microsomes. Acta Pharmacol Sin 2002;23:455–460.
- OH Lowry, NJ Rosebrough, AL Farr, RJ Randall. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275.
- K Kobayashi, K Urashima, N Shimada, K Chiba. Substrate specificity for rat cytochrome P450 (CYP) isoforms: Screening with cDNA-expressed systems of the rat. Biochem Pharmacol 2002;63:889–896.
- SR Obach, AE Reed-Hagen. Measurement of Michaelis Constants for cytochrome P450-mediated biotransformation reactions using a substrate depletion approach. Drug Metab Dispos 2002;30:831–837.
- A Nath, WM Atkins. A theoretical validation of the substrate depletion approach to determining kinetic parameters. Drug Metab Dispos 2006;34:1433–1435.
- IH Segal. Enzyme kinetics. New York: Wiley-Interscience; 1975. p 957.
- TK Chang, J Chen, WB Lee. Differential inhibition and inactivation of human CYP1 enzymes by trans-resveratrol: Evidence for mechanism-based inactivation of CYP1A2. J Pharmacol Exp Ther 2001;299:874–882.
- D Zhang, M Zhu, WG Humphreys. Drug metabolism in drug design and developmen. New Jersey: Wiley; 2007. p 513–543.
- D Rodriques. Drug-drug interactions. New York: Marcel Dekker, Inc; 2002. p 217–293.
- JS Lee, RS Obach, MB Fisher. Drug metabolizing enyzmes: Cytochrome P450 and other enzymes in drug discovery and development. New York: Fontis Media, Lausanne, Switzerland and Marcel Dekker; 2003. p 211–254.
- HM Jones, JB Houston. Substrate depletion approach for determining in vitro metabolic clearence: Time dependences in hepatocyte and microsomal incubations. Drug Metab Dispos 2004;32:973–982.
- T Kotegawa, BE Laurijssens, ALB Durol, DJ Greenblatt. Pharmacokinetics and electroencephalographic effects of ketoconazole in the rat. Biopharm Drug Dispos 1999;20:49–52.

![Figure 1. Full-scan product ion spectra of [M + H]+ and fragmentation pathways for beauvericin.](/cms/asset/d3bcf73c-0f76-46a4-a156-bef9692b2518/ienz_a_336371_f0002_b.gif)