Abstract
Macrocyclic inhibitors for the serine protease plasmin were synthesized and evaluated. The inhibitors were constructed starting from a cyclohexanone core. This core was linked to either the C- or N-terminus of a peptide so that the inhibitors were designed to interact with the non-primed or primed binding sites of the protease. Macrocycles were prepared by connecting the side chain of Tyr or Trp, via a short linker, to one end of the peptide. The activities of the macrocyclic inhibitors, while modest, were up to 10-fold more potent than a related non-cyclic analog.
Introduction
Plasmin is a serine protease that plays important roles in the proteolytic modification of the extracellular matrix (ECM). These remodeling events are key steps involved in the cancer-related processes of angiogenesis and metastases Citation1,Citation2. Plasmin degrades a variety of ECM components, and also activates other important proteases such as the matrix metalloproteases (MMPs) 1, 3 and 9 [Citation3]. The pivotal regulatory role of plasmin in the ECM remodeling process makes it a potential therapeutic target for the treatment of cancer.
Plasmin is also a key player in the dissolution of fibrin clots because it is the major enzyme responsible for cleaving fibrin. Plasminogen, the inactive precursor to plasmin, initially binds to fibrin via its lysine binding site. Plasminogen is then converted to active plasmin by several proteases including tissue plasminogen activator, urokinase, factor XIIa and kallikrein. The activated plasmin subsequently cleaves the fibrin mesh into smaller fragments. Several fibrinolysis inhibitors have been used clinically to reduce bleeding during surgery [Citation4]. Aprotinin is a protein-based inhibitor that targets both plasmin and kallikrein, and is produced by Bayer under the name Trasylol. It is also known as bovine pancreatic trypsin inhibitor (BPTI). Aprotinin was recently withdrawn from the market because of concerns over side effects and increased risk of mortality. By contrast, small molecule antifibrinolytic drugs such as ϵ-aminocaproic acid and trans-4-(aminomethyl)cyclohexanecarboxylic acid (tranexamic acid) continue to be safe alternatives to aprotinin. However, these two small molecules target the lysine binding site of plasminogen, and do not influence the catalytic activity of activated plasmin. These observations highlight the need for development of new fibrinolysis inhibitors with a mechanism of action that targets the active site of plasmin and modifies its catalytic activity.
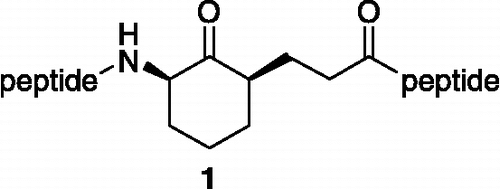
Over the last several years we have designed and synthesized a series of plasmin inhibitors 1 () [Citation5]. These inhibitors were constructed around a cyclic ketone core, and were designed to react with the active site serine residue to give a reversibly formed hemiacetal linkage [Citation5b]. The inhibitors also incorporated two peptide side chains, the identity of which were derived from the substrate specificity of plasmin. Several analogs of compound 1 showed potency in the low micromolar range and selectivities of greater than 100-fold for plasmin over other related serine proteases [Citation5f]. However, peptidic inhibitors are often associated with undesirable pharmacokinetic properties including poor oral bioavailability and cell membrane permeability.
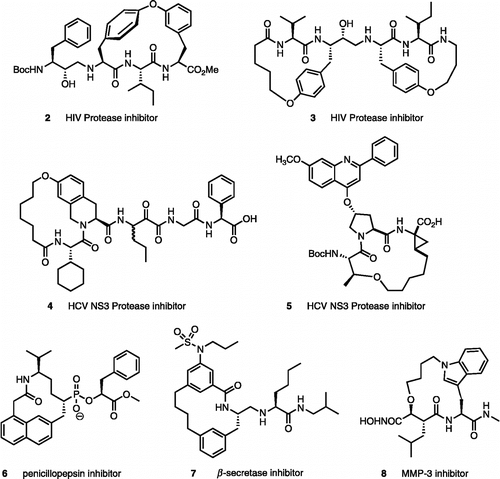
Macrocyclization of peptides has been a widely adopted strategy for designing peptidomimetic protease inhibitors with improved pharmacokinetic properties [Citation6]. For example, compounds 2 [Citation7] and 3 [Citation8] are potent and selective inhibitors of the human immunodeficiency virus type 1 (HIV-1) protease (). Compounds 4 [Citation9] and 5 [Citation10] are novel inhibitors of the hepatitis C virus (HCV) NS3 protease. Other macrocyclic peptidomimetics, such as compounds 6 [Citation11], 7 [Citation12] and 8 [Citation13] inhibit the aspartic proteases β-secretase and penicillopepsin, and the metalloprotease MMP-3.
In addition to their desirable pharmacokinetic characters, macrocyclic compounds provide several other advantages over peptides. First, the macrocycle often preorganizes the molecule into an extended conformation, which can be an ideal conformation for binding to the target enzyme [Citation6]. Second, the macrocycle decreases the conformational entropic penalty for binding to the enzyme when compared to more flexible non-cyclic analogs [Citation14]. Consequently, macrocyclic inhibitors often display enhanced activities.
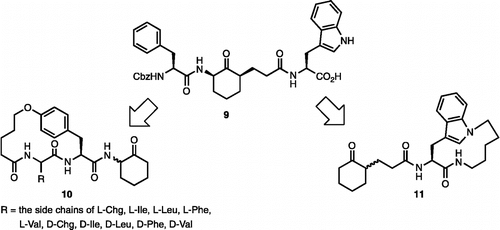
In previous studies we found that plasmin prefers to bind compounds with aromatic amino acids (e.g. Phe and Trp) at the P2 position, and the large aromatic amino acid Trp at the P2′ position () [Citation5f]. We also found that plasmin prefers hydrophobic amino acids at both the P3 and P3′ positions. Based on these results, we hypothesized that an intramolecular linkage between the P2 aromatic group and the N-terminus of P3, or between the P2′ Trp side chain and its C-terminus could provide favorable conformational constraints. These approaches, as shown in , lead to the design of the macrocyclic inhibitors 10 and 11.
Materials and methods
All experiments were conducted using anhydrous conditions under an atmosphere of nitrogen, except where stated, with oven-dried apparatus and employing standard techniques for handling air-sensitive materials. All solvents were distilled and stored under argon before use. All reagents were used as received. Aqueous solutions of sodium bicarbonate, sodium carbonate and sodium chloride (brine) were saturated. Analytical thin layer chromatography (TLC) plates were visualized by ultraviolet irradiation, ninhydrin or phosphomolybdic acid (PMA) staining solutions. Flash column chromatography was carried out under a positive pressure of nitrogen. 1H NMR spectra were recorded on 300 MHz or 400 MHz spectrometers. Data are presented as follows: chemical shift (in ppm on the δ scale relative to δ = 0.00 ppm for TMS), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad), coupling constant (J/Hz), which were taken directly from the spectra and are uncorrected, and integration. 13C NMR spectra were recorded at 75 or 100 MHz, and all chemical shift values are reported in ppm on the δ scale, with an internal reference of δ 77.0 or 49.0 for CDCl3 or CD3OD, respectively. High-resolution mass spectra were measured using electron impact (EI) or fast atom bombardment (FAB) ionization.
Inhibition studies
Inhibitors 10a-j and 11 were assayed against plasmin using H-D-Val-Ile-Lys-pNA (pNA = p-nitroanilide) as the substrate [Citation5f]. Initial rates were measured using UV spectroscopy to monitor formation of p-nitroaniline (405 nm). The assay mixtures contained 50 mM sodium phosphate buffer at pH 7.4, and 10% DMSO to ensure solubility of the inhibitors. Under these conditions, the Km value for the substrate was measured to be 170 μM.
Chemistry
(S)-benzyl 2-((S)-2-(tert-butoxycarbonylamino)-2-cyclohexylethanamido)-3-(4-hydroxyphenyl)propanoate (Boc-chg-tyr-obn) (12A)
Boc-Chg-OH (1.5 mmol) was dissolved in DMF (10 mL). To this solution was added H-Tyr-OBn (1.5 mmol), HBTU (758 mg, 2.0 mmol), and DIEA (530 μL, 390 mg, 3.0 mmol). The reaction was stirred at room temperature for 2 h, and then partitioned between EtOAc (250 mL) and 1 N HCl (200 mL). The organic layer was washed with 1 N HCl (200 mL), saturated NaHCO3 (200 mL) and brine (150 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation. The crude material was purified by flash chromatography (EtOAc: hexanes) to yield dipeptide 12a (710 mg, 1.40 mmol, 93%): 1H NMR (400 MHz, CDCl3) δ 0.80-1.02 (m, 2H), 1.08-1.22 (m, 3H), 1.47 (s, 9H), 1.55-1.82 (m, 6H), 2.95-3.10 (d, J = 4.8 Hz, 2H), 3.80-4.00 (t, J = 8.0 Hz, 1H), 4.85-5.00 (dt, J = 3.6, 8.0 Hz, 1H), 5.05-5.25 (d, J = 12.0 Hz, 1H), 5.15-5.25 (d, J = 12.0 Hz, 1H), 5.25-5.35 (d, J = 9.2 Hz, 1H), 6.60-6.70 (d, J = 8.0 Hz, 2H), 6.80-6.90 (m, 3H), 5.30-5.45 (m, 5H), 7.66 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 14.1, 25.8, 26.1, 28.4, 29.5, 37.1, 42.2, 53.4, 59.5, 67.3, 80.2, 115.6, 126.5, 128.6, 128.7, 130.4, 135.0, 155.6, 156.2, 171.2, 171.6; HRMS-FAB (M + Na+) calcd for C29H38NaN2O6 533.2628, found 533.2638.
(S)-benzyl 2-((R)-2-(tert-butoxycarbonylamino)-2-cyclohexylethanamido)-3-(4-hydroxyphenyl)propanoate (Boc-d-chg-tyr-obn) (12B)
Compound 12b was synthesized using a procedure analogous to compound 12a (665 mg, 1.30 mmol, 86%): 1H NMR (400 MHz, CDCl3) δ 1.05-1.12 (m, 1H), 1.13-1.25 (m, 2H), 1.46 (s, 9H), 1.55-1.65 (m, 3H), 1.65-1.75 (m, 3H), 1.80-1.90 (m, 2H), 2.90-3.10 (d, J = 6.8 Hz, 2H), 3.90-4.10 (t, J = 8.0 Hz, 1H), 4.85-4.95 (dd, J = 3.6, 8.0 Hz, 1H), 5.05-5.25 (m, 3H), 6.25-6.45 (br s, 1H), 6.55-6.61 (d, J = 7.8 Hz, 1H), 6.65-6.71 (d, J = 8.4 Hz, 2H), 6.85-6.95 (d, J = 8.0 Hz, 2H), 7.30-7.50 (m, 5H), 7.66 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 14.2, 25.9, 26.0, 27.6, 28.3, 29.7, 37.2, 40.4, 53.2, 59.3, 67.4, 80.1, 115.6, 128.6, 128.7, 130.3, 135.0, 155.9, 156.2, 171.4, 171.5; HRMS-FAB (M + Na+) calcd for C29H38NaN2O6 533.2628, found 533.2643.
(S)-benzyl 2-((2S,3s)-2-(tert-butoxycarbonylamino)-3-methylpentanamido)-3-(4-hydroxyphenyl)propanoate (Boc-ile-tyr-obn) (12C)
Compound 12c was synthesized using a procedure analogous to compound 12a (670 mg, 1.30 mmol, 86%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.90 (m, 6H), 1.00-1.15 (m, 1H), 1.48 (s, 9H), 1.70-1.85 (m, 1H), 2.97-3.12 (d, J = 4.8 Hz, 2H), 3.85-4.00 (t, J = 8.0 Hz, 1H), 4.85-5.00 (td, J = 5.4, 8.0 Hz, 1H), 5.05-5.25 (m, 3H), 6.50-6.70 (d, J = 8.0 Hz, 2H), 6.75-6.90 (d, J = 8.0 Hz, 2H), 7.00-7.20 (br s, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 11.2, 15.4, 24.7, 28.4, 53.3, 59.2, 76.7, 80.3, 115.5, 126.6, 128.6, 128.7, 130.4, 135.0, 155.4, 156.0, 171.1, 171.5; HRMS-FAB (M + Na+) calcd for C27H36NaN2O6 507.2471, found 507.2485.
(S)-benzyl 2-((2R,3r)-2-(tert-butoxycarbonylamino)-3-methylpentanamido)-3-(4-hydroxyphenyl)propanoate (Boc-d-ile-tyr-obn) (12D)
Compound 12d was synthesized using a procedure analogous to compound 12a (700 mg, 1.37 mmol, 91%): 1H NMR (400 MHz, CD3OD) δ 0.70-0.90 (m, 6H), 0.93-1.10 (m, 1H), 1.30-1.50 (m, 11H), 1.60-1.75 (m, 1H), 2.85-2.95 (dd, J = 10.0, 12.0 Hz, 1H), 3.05-3.15 (dd, J = 8.0, 12.0 Hz, 1H), 3.90-4.00 (d, J = 7.6 Hz, 1H), 4.60-4.75 (m, 1H), 5.10-5.20 (dd, J = 10.0, 12.0 Hz, 2H), 6.65-6.75 (d, J = 8.0 Hz, 2H), 6.95-7.05 (d, J = 8.0 Hz, 2H), 7.25-7.45 (m, 5H); 13C NMR (100 MHz, CD3OD) δ 10.3, 14.4, 24.0, 27.3, 36.1, 37.2, 54.1, 59.1, 66.6, 79.2, 115.0, 127.1, 127.9, 128.2, 129.8, 135.7, 156.1, 156.4, 171.4, 172.9; HRMS-FAB (M + Na+) calcd for C27H36NaN2O6 507.2471, found 507.2468.
(S)-benzyl 2-((S)-2-(tert-butoxycarbonylamino)-4-methylpentanamido)-3-(4-hydroxyphenyl)propanoate (Boc-leu-tyr-obn) (12E)
Compound 12e was synthesized using a procedure analogous to compound 12a (665 mg, 1.3 mmol, 86%): 1H NMR (400 MHz, CDCl3) δ 0.80-1.00 (d, J = 2.0 Hz, 6H), 1.47 (s, 9H), 1.50-1.70 (m, 2H), 2.97-3.12 (d, J = 4.8 Hz, 2H), 4.10-4.30 (m, 1H), 4.85-5.00 (td, J = 5.4, 8.0 Hz, 1H), 5.05-5.25 (m, 3H), 6.50-6.70 (d, J = 8.0 Hz, 2H), 6.75-6.90 (d, J = 8.0 Hz, 2H), 6.90-7.00 (d, J = 7.6 Hz, 1H), 7.30-7.50 (m, 5H), 7.50-7.90 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 22.0, 22.8, 24.7, 28.4, 37.0, 41.2, 53.0, 53.5, 67.3, 80.4, 115.5, 126.4, 128.6, 128.8, 130.4, 135.0, 155.5, 156.0, 171.2, 172.5; HRMS-FAB (M + Na+) calcd for C27H36NaN2O6 507.2471, found 507.2485.
(S)-benzyl 2-((R)-2-(tert-butoxycarbonylamino)-4-methylpentanamido)-3-(4-hydroxyphenyl)propanoate (Boc-d-leu-tyr-obn) (12F)
Compound 12f was synthesized using a procedure analogous to compound 12a (690 mg, 1.37 mmol, 91%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.90 (d, J = 6.4 Hz, 6H), 1.40-1.50 (m, 10H), 1.55-1.70 (m, 2H), 2.97-3.12 (m, 2H), 4.10-4.30 (m, 1H), 4.85-5.00 (td, J = 5.4, 8.0 Hz, 1H), 4.95-5.05 (d, J = 7.6 Hz, 1H), 5.05-5.15 (d, J = 12.4 Hz, 1H), 5.15-5.30 (d, J = 12.0 Hz, 1H), 6.60-6.70 (d, J = 7.6 Hz, 2H), 6.80-6.95 (m, 3H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 21.8, 22.9, 24.7, 28.3, 37.0, 41.1, 53.0, 53.3, 67.4, 80.4, 115.7, 126.7, 128.6, 128.7, 130.3, 135.0, 155.6, 155.8, 171.6, 172.7; HRMS-FAB (M + Na+) calcd for C27H36NaN2O6 507.2471, found 507.2490.
(S)-benzyl 2-((S)-2-(tert-butoxycarbonylamino)-3-phenylpropanamido)-3-(4-hydroxyphenyl)propanoate (Boc-phe-tyr-obn) (12G)
Compound 12g was synthesized using a procedure analogous to compound 12a (770 mg, 1.50 mmol, 99%): 1H NMR (400 MHz, CDCl3) δ 1.41 (s, 9H), 2.90-3.12 (m, 4H), 4.40-4.50 (m, 1H), 4.70-4.90 (dd, J = 6.0, 12.8 Hz, 1H), 5.00-5.20 (d, J = 1.6 Hz, 1H), 6.50-6.60 (d, J = 7.6 Hz, 1H), 6.60-6.70 (d, J = 8.0 Hz, 2H), 6.70-6.80 (d, J = 8.4 Hz, 2H), 7.01 (s, 1H), 7.10-7.20 (m, 2H), 7.20-7.30 (m, 3H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 28.3, 37.1, 38.3, 53.4, 55.7, 67.3, 80.5, 95.7, 115.6, 126.6, 127.0, 128.6, 128.7, 129.3, 130.4, 135.0, 136.4, 155.5, 171.0, 171.3; HRMS-FAB (M + Na+) calcd for C30H34NaN2O6 541.2315, found 541.2325.
(S)-benzyl 2-((R)-2-(tert-butoxycarbonylamino)-3-phenylpropanamido)-3-(4-hydroxyphenyl)propanoate (Boc-d-phe-tyr-obn) (12H)
Compound 12h was synthesized using a procedure analogous to compound 12a (725 mg, 1.40 mmol, 93%): 1H NMR (400 MHz, CDCl3) δ 1.40 (s, 9H), 2.80-2.90 (m, 1H), 2.90-3.05 (m, 2H), 3.05-3.20 (m, 1H), 4.30-4.50 (m, 1H), 4.75-4.90 (dd, J = 6.0, 12.8 Hz, 1H), 5.00-5.20 (m, 3H), 6.40-6.50 (s, 1H), 6.50-6.60 (d, J = 7.6 Hz, 2H), 6.60-6.75 (m, 4H), 7.10-7.20 (d, J = 7.2 Hz, 2H), 7.20-7.30 (m, 5H), 7.30-7.50 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 28.3, 37.0, 53.2, 67.4, 115.6, 126.6, 127.0, 128.5, 128.6, 128.7, 129.3, 130.3, 135.0, 136.5, 155.3, 171.2, 171.3; HRMS-FAB (M + Na+) calcd for C30H34NaN2O6 541.2315, found 541.2298.
(S)-benzyl 2-((S)-2-(tert-butoxycarbonylamino)-3-methylbutanamido)-3-(4-hydroxyphenyl)propanoate (Boc-val-tyr-obn) (12I)
Compound 12i was synthesized using a procedure analogous to compound 12a (635 mg, 1.35 mmol, 90%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.90 (d, J = 6.4 Hz, 3H), 0.90-1.00 (d, J = 6.8 Hz, 3H), 1.47 (s, 9H), 1.95-2.15 (m, 1H), 3.00-3.10 (d, J = 4.8 Hz, 2H), 3.80-3.95 (dd, J = 8.0, 8.4 Hz, 1H), 4.85-4.95 (dd, J = 5.6, 13.2 Hz, 1H), 5.05-5.25 (m, 3H), 6.45-6.55 (d, J = 7.6 Hz, 1H), 6.65-6.75 (d, J = 12.0 Hz, 2H), 6.75 (s, 1H), 6.75-6.90 (d, J = 8.0 Hz, 2H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 17.9, 19.2, 28.4, 31.0, 37.0, 53.3, 60.0, 67.3, 80.3, 115.6, 126.7, 128.6, 128.7, 130.4, 135.0, 155.3, 156.1, 171.2, 171.4; HRMS-FAB (M + Na+) calcd for C26H34NaN2O6 493.2315, found 493.2326.
(S)-benzyl 2-((R)-2-(tert-butoxycarbonylamino)-3-methylbutanamido)-3-(4-hydroxyphenyl)propanoate (Boc-d-val-tyr-obn) (12J)
Compound 12j was synthesized using a procedure analogous to compound 12a (635 mg, 1.35 mmol, 90%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.90 (d, J = 6.4 Hz, 3H), 0.90-1.00 (d, J = 6.8 Hz, 3H), 1.47 (s, 9H), 1.95-2.15 (m, 1H), 3.00-3.10 (d, J = 4.8 Hz, 2H), 3.80-3.95 (dd, J = 8.0, 8.4 Hz, 1H), 4.85-4.95 (dd, J = 5.6, 13.2 Hz, 1H), 5.05-5.25 (m, 3H), 6.45-6.55 (d, J = 7.6 Hz, 1H), 6.65-6.75 (d, J = 12.0 Hz, 2H), 6.75 (s, 1H), 6.75-6.90 (d, J = 8.0 Hz, 2H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 17.9, 19.2, 28.4, 31.0, 37.0, 53.3, 60.0, 67.3, 80.3, 115.6, 126.7, 128.6, 128.7, 130.4, 135.0, 155.3, 156.1, 171.2, 171.4; HRMS-FAB (M + Na+) calcd for C26H34NaN2O6 493.2315, found 493.2330.
(S)-Benzyl 2-((S)-2-cyclohexyl-2-(5-iodopentanamido)ethanamido)-3-(4-hydroxyphenyl)propanoate (13a)
To a solution of compound 12a (510 mg, 1.0 mmol) in CH2Cl2 (10 mL) was added TFA (5 mL). The reaction was stirred at room temperature for 30 min, and the solvent was removed by rotary evaporation to yield the free amine. To a solution of the resulting amine in THF (4 mL) were added H2O (4 mL), aqueous K2CO3 (20%, 1.25 mL), and 5-bromovaleryl chloride (146 μL, 220 mg, 1.1 mmol) as a solution in THF (1 mL). The reaction was stirred vigorously at room temperature for 8 min and then partitioned between EtOAc (300 mL) and 1 N HCl (250 mL). The organic layer was washed with saturated NaHCO3, brine (200 mL) and dried over MgSO4. The solvent was removed by rotary evaporation. The crude material was purified by flash chromatography (EtOAc:hexanes 1:2-1:1) to yield the corresponding primary bromide (535 mg, 0.95 mmol, 95%). This compound (280 mg, 0.5 mmol) was dissolved in acetone (20 mL) and NaI (150 mg, 1.0 mmol) was added. The reaction was stirred under reflux for 2 h and cooled to room temperature. The white precipitate was removed by filtration, and the filtrate was concentrated by rotary evaporation. The crude material was purified by passing it through a short silica plug (EtOAc:hexanes 1:1-2:1) to give the corresponding primary iodide 13a (310 mg, 0.5 mmol, 100%): 1H NMR (400 MHz, CD3OD) δ 0.85-1.10 (m, 3H), 1.10-1.30 (m, 3H), 1.50-1.90 (m, 11H), 2.10-2.30 (br s, 2H), 2.80-2.95 (m, 1H), 2.96-3.10 (m, 1H), 3.20-3.30 (m, 1H), 3.31-3.40 (s, 2H), 4.10-4.22 (m, 1H), 4.60-4.70 (m, 1H), 5.00-5.20 (s, 2H), 6.60-6.75 (d, J = 7.6, 2H), 6.90-7.05 (d, J = 7.6, 2H), 7.25-7.50 (m, 5H), 7.80-8.00 (m, 1H); 13C NMR (100 MHz, CD3OD) δ 4.7, 25.6, 25.9, 26.4, 28.5, 29.3, 32.8, 34.1, 36.3, 39.8, 54.1, 58.0, 66.6, 114.8, 127.1, 127.9, 128.1, 128.2, 130.0, 135.6, 156.0, 171.3, 172.1, 174.0; HRMS-FAB (M + Na+) calcd for C29H37INaN2O5 643.1645, found 643.1660.
(S)-Benzyl 2-((R)-2-cyclohexyl-2-(5-iodopentanamido)ethanamido)-3-(4-hydroxyphenyl)propanoate (13b)
The synthesis of compound 13b was analogous to the procedure used for compound 13a (280 mg, 0.45 mmol, 90% for two steps): 1H NMR (400 MHz, CD3OD) δ 0.70-1.00 (m, 2H), 1.00-1.25 (m, 3H), 1.26-1.40 (m, 1H), 1.45-1.75 (m, 7H), 1.76-2.00 (m, 2H), 2.15-2.30 (m, 2H), 2.75-2.95 (m, 1H), 3.10-3.40 (m, 3H), 4.10-4.30 (m, 1H), 4.60-4.75 (m, 1H), 5.00-5.20 (m, 2H), 6.50-6.75 (d, J = 7.6, 2H), 6.90-7.10 (d, J = 7.6, 2H), 7.25-7.50 (m, 5H); 13C NMR (75 MHz, CD3OD) δ 5.0, 26.0, 26.2, 26.8, 28.5, 29.6, 33.1, 34.5, 36.4, 40.2, 40.4, 54.3, 58.1, 66.9, 115.2, 115.3, 127.6, 128.2, 128.4, 128.5, 128.6, 130.1, 130.3, 136.2, 156.5, 171.7, 172.4, 174.3; HRMS-FAB (M + Na+) calcd for C29H37INaN2O5 643.1645, found 643.1652.
(S)-Benzyl 3-(4-hydroxyphenyl)-2-((S)-2-(5-iodopentanamido)-4-methylpentanamido)propanoate (13e)
The synthesis of compound 13e was analogous to the procedure used for compound 13a (280 mg, 0.47 mmol, 95% for two steps): 1H NMR (300 MHz, CD3OD) δ 0.80-0.95 (m, 6H), 1.30-1.50 (m, 3H), 1.55-1.90 (m, 4H), 2.10-2.30 (m, 2H), 2.80-3.00 (m, 1H), 3.05-3.20 (m, 1H), 4.35-4.50 (dd, J = 4.8, 7.5 Hz; 1H), 4.60-4.75 (dd, J = 5.7, 7.2 Hz; 1H), 5.00-5.20 (d, J = 4.2 Hz, 2H), 6.60-6.70 (d, J = 8.1 Hz, 2H), 6.90-7.00 (d, J = 8.1 Hz, 2H), 7.25-7.40 (m, 5H), 7.95-8.15 (m, 2H); 13C NMR (75 MHz, CD3OD) δ 5.3, 21.2, 22.5, 24.8, 26.8, 33.1, 34.6, 36.6, 41.0, 51.9, 54.4, 67.0, 115.4, 127.3, 128.3, 128.4, 128.6, 130.4, 136.0, 156.5, 171.7, 173.7, 174.4; HRMS-FAB (M + Na+) calcd for C27H35INaN2O5 617.1488, found 617.1492.
(S)-Benzyl 3-(4-hydroxyphenyl)-2-((S)-2-(5-iodopentanamido)-3-phenylpropanamido)propanoate (13g)
The synthesis of compound 13g was analogous to the procedure used for compound 13a (280 mg, 0.45 mmol, 90% for two steps): 1H NMR (400 MHz, CD3OD) δ 1.45-1.70 (m, 4H), 2.10-2.20 (m, 2H), 2.70-2.80 (dd, J = 4.0, 14.0 Hz, 1H), 2.85-2.95 (m, 1H), 3.00-3.20 (m, 4H), 4.60-4.75 (m, 2H), 5.05-5.15 (s, 2H), 6.60-6.70 (dd, J = 2.0, 6.8 Hz, 2H), 6.90-7.00 (dd, J = 2.0, 6.8 Hz, 2H), 7.15-7.27 (m, 5H), 7.27-7.45 (m, 5H); 13C NMR (100 MHz, CD3OD) δ 4.8, 26.2, 32.3, 34.1, 36.2, 37.4, 54.1, 54.2, 66.6, 114.9, 126.3, 127.0, 127.9, 128.0, 128.1, 128.2, 128.9, 135.6, 137.1, 156.1, 171.2, 172.2, 173.9; HRMS-ESI (M + Na+) calcd for C30H33INaN2O5 651.1332, found 651.1347.
(S)-Benzyl 3-(4-hydroxyphenyl)-2-((R)-2-(5-iodopentanamido)-3-phenylpropanamido)propanoate (13h)
The synthesis of compound 13h was analogous to the procedure used for compound 13a (270 mg, 0.44 mmol, 88% for two steps): 1H NMR (400 MHz, CD3OD) δ 1.45-1.60 (m, 4H), 2.05-2.20 (m, 2H), 2.65-2.75 (dd, J = 9.2, 13.6 Hz, 1H), 2.80-2.95 (dd, J = 9.2, 13.6 Hz, 1H), 2.92-3.05 (m, 2H), 3.06-3.20 (m, 2H), 4.50-4.80 (m, 2H), 5.00-5.20 (m, 2H), 6.60-6.70 (d, J = 8.4 Hz, 2H), 6.80-7.00 (d, J = 8.4 Hz, 2H), 7.05-7.15 (d, J = 7.2 Hz, 2H), 7.15-7.90 (m, 8H); 13C NMR (100 MHz, CD3OD) δ 4.9, 26.2, 32.3, 34.1, 36.2, 37.6, 54.0, 66.6, 115.0, 126.4, 126.9, 128.0, 128.1, 128.2, 128.9, 130.0, 135.7, 137.0, 156.1, 171.2, 172.0, 173.9; HRMS-ESI (M + Na+) calcd for C30H33INaN2O5 651.1332, found 651.1346.
(S)-Benzyl 3-(4-hydroxyphenyl)-2-((S)-2-(5-iodopentanamido)-3-methylbutanamido)propanoate (13i)
The synthesis of compound 13i was analogous to the procedure used for compound 13a (265 mg, 0.45 mmol, 91% for two steps): 1H NMR (400 MHz, CD3OD) δ 0.85-0.95 (d, J = 6.8 Hz, 6H), 1.65-1.75 (m, 1H), 1.76-1.90 (m, 1H), 1.95-2.05 (m, 1H), 2.20-2.30 (dt, J = 0.9, 6.8 Hz, 2H), 2.85-2.95 (dd, J = 8.0, 11.2 Hz, 1H), 2.95-3.05 (dd, J = 6.4, 14.0 Hz, 1H), 3.20-3.30 (m, 2H), 4.10-4.20 (d, J = 7.6 Hz, 1H), 4.60-4.70 (dd, J = 6.0, 7.6 Hz, 1H), 5.05-5.15 (d, J = 0.9 Hz, 2H), 6.60-6.70 (dd, J = 2.0, 6.4 Hz, 2H), 6.90-7.00 (dd, J = 2.0, 6.4 Hz, 2H), 7.25-7.30 (m, 2H), 7.30-7.40 (m, 3H); 13C NMR (100 MHz, CD3OD) δ 4.7, 17.4, 18.3, 26.4, 30.5, 32.7, 34.1, 36.3, 54.2, 58.6, 66.6, 114.9, 127.1, 128.0, 128.1, 128.2, 130.0, 135.6, 156.0, 171.3, 172.2, 174.1; HRMS-FAB (M + Na+) calcd for C26H33INaN2O5 603.1332, found 603.1351.
(S)-Benzyl 3-(4-hydroxyphenyl)-2-((R)-2-(5-iodopentanamido)-3-methylbutanamido)propanoate (13j)
The synthesis of compound 13j was analogous to the procedure used for compound 13a (260 mg, 0.45 mmol, 90% for two steps): 1H NMR (400 MHz, CD3OD) δ 0.70-0.85 (d, J = 6.8 Hz, 6H), 1.65-1.75 (m, 1H), 1.76-1.85 (m, 1H), 1.86-2.00 (m, 1H), 2.15-2.35 (m, 2H), 2.80-2.90 (dd, J = 4.8, 14.0 Hz, 1H), 3.00-3.10 (dd, J = 4.2, 13.6 Hz, 1H), 3.15-3.25 (t, J = 6.8 Hz, 2H), 3.30-3.50 (m, 1H), 4.20-4.30 (dd, J = 7.2, 8.4 Hz, 1H), 4.60-4.70 (m, 1H), 5.05-5.15 (d, J = 5.6 Hz, 2H), 6.60-6.70 (d, J = 8.4 Hz, 2H), 6.90-7.00 (d, J = 8.4 Hz, 2H), 7.25-7.40 (m, 5H), 7.85-7.95 (d, J = 8.8 Hz, 1H), 8.15-8.25 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 4.9, 17.0, 18.4, 26.5, 30.6, 32.7, 34.2, 36.2, 54.0, 54.1, 58.4, 58.5, 66.6, 115.0, 127.1, 127.9, 128.0, 128.2, 129.9, 135.7, 156.1, 171.31, 171.33, 172.3, 174.2; HRMS-FAB (M + Na+) calcd for C26H33INaN2O5 603.1332, found 603.1355.
Benzyl ester 14a
The iodide 13a (310 mg, 0.5 mmol) was dissolved in DMF (30 mL). To this solution was added K2CO3 (342 mg, 2.5 mmol). The reaction was stirred at room temperature for 10 h, and then diluted with EtOAc (200 mL). The organic layer was washed with 1 N HCl (3 × 250 mL), saturated NaHCO3 (200 mL), and brine (200 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation. The crude material was purified by flash chromatography (EtOAc:hexanes 1:1-2:1) to yield 14a (172 mg, 0.35 mmol, 70%): 1H NMR (400 MHz, CDCl3) δ 0.85-1.00 (m, 2H), 1.00-1.20 (m, 3H), 1.30-1.50 (m, 3H), 1.51-1.80 (m, 7H), 2.00-2.10 (m, 1H), 2.15-2.20 (m, 1H), 2.50-2.60 (t, J = 13.2 Hz, 1H), 3.40-3.50 (dd, J = 4.8, 13.2 Hz, 1H), 3.96-4.06 (t, J = 8.0 Hz, 1H), 4.10-4.30 (m, 2H), 5.00-5.10 (m, 1H), 5.15-5.20 (d, J = 12.4 Hz, 1H), 5.21-5.30 (d, J = 12.4 Hz, 1H), 5.70-5.80 (d, J = 8.8 Hz, 1H), 6.10-6.20 (d, J = 10.0 Hz, 1H), 6.70-6.80 (dd, J = 2.4, 8.0 Hz, 1H), 6.80-6.90 (ddd, J = 2.0, 8.4, 13.6 Hz, 2H), 7.15-7.25 (dd, J = 2.0, 8.4 Hz, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 21.7, 25.8, 25.9, 26.0, 28.8, 29.2, 36.1, 38.4, 41.2, 52.4, 57.6, 67.4, 67.8, 116.5, 118.9, 128.4, 128.5, 128.7, 128.8, 130.0, 131.2, 135.1, 155.6, 170.2, 171.3, 172.0; HRMS-FAB (M + Na+) calcd for C29H36NaN2O5 515.2522, found 515.2531.
Benzyl ester 14b
Compound 14b was synthesized using a procedure analogous to benzyl ester 14a (184 mg, 0.38 mmol, 75%): 1H NMR (400 MHz, CDCl3) δ 0.70-0.95 (m, 2H), 1.00-1.15 (m, 1H), 1.16-1.30 (m, 2H), 1.35-1.75 (m, 11H), 1.80-2.00 (m, 3H), 2.05-2.20 (m, 1H), 3.15-3.25 (d, J = 5.6 Hz, 2H), 4.10-4.25 (m, 2H), 4.30-4.45 (m, 1H), 4.55-4.65 (dd, J = 6.8, 13.6 Hz, 1H), 5.05-5.15 (d, J = 8.8 Hz, 1H), 5.15-5.25 (d, J = 12.0 Hz, 1H), 5.26-5.35 (d, J = 12.4 Hz, 1H), 5.80-5.90 (d, J = 6.8 Hz, 1H), 6.88 (s, 2H), 6.95-7.00 (d, J = 6.8 Hz, 1H), 7.01-7.10 (d, J = 8.0 Hz, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 20.8, 25.9, 26.1, 27.6, 27.9, 29.9, 34.0, 35.5, 38.8, 52.5, 58.4, 67.4, 68.5, 118.1, 118.4, 128.3, 128.6, 128.7, 130.2, 130.7, 158.0, 170.9, 171.5, 172.3; HRMS-FAB (M + Na+) calcd for C29H36NaN2O5 515.2522, found 515.2536.
Benzyl ester 14c
Compound 14c was synthesized using a procedure analogous to benzyl ester 14a (140 mg, 0.30 mmol, 60%): 1H NMR (300 MHz, CDCl3) δ 0.70-0.90 (m, 6H), 0.95-1.10 (m, 1H), 1.30-1.55 (m, 3H), 1.56-1.85 (m, 4H), 1.95-2.10 (m, 1H), 2.12-2.30 (m, 1H), 2.45-2.60 (m, 1H), 3.35-3.50 (dd, J = 5.6, 9.9 Hz, 1H), 3.95-4.05 (t, J = 8.4 Hz, 1H), 4.10-4.35 (m, 2H), 5.00-5.15 (m, 1H), 5.15-5.25 (d, J = 12.0 Hz, 1H), 5.26-5.35 (d, J = 12.4 Hz, 1H), 5.60-5.75 (d, J = 6.8 Hz, 1H), 5.80-5.90 (d, J = 8.4 Hz, 1H), 6.25-6.35 (m, 1H), 6.80-7.00 (m, 2H), 7.15-7.25 (m, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 11.3, 15.0, 21.6, 25.1, 26.0, 36.1, 38.1, 38.4, 52.4, 57.3, 67.5, 67.8, 115.4, 116.5, 118.9, 128.4, 128.5, 128.6, 128.7, 128.8, 130.0, 130.9, 131.2, 135.1, 155.7, 170.1, 171.3, 171.9; HRMS-FAB (M + Na+) calcd for C27H34NaN2O5 489.2365, found 489.2380.
Benzyl ester 14d
Compound 14d was synthesized using a procedure analogous to benzyl ester 14a (155 mg, 0.33 mmol, 65%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.90 (m, 6H), 0.90-1.00 (m, 1H), 1.30-1.55 (m, 3H), 1.56-1.75 (m, 1H), 1.80-2.00 (m, 3H), 2.05-2.20 (m, 1H), 3.15-3.25 (d, J = 8.8 Hz, 2H), 4.15-4.25 (m, 2H), 4.30-4.45 (m, 1H), 4.50-4.70 (dd, J = 8.0, 12.4 Hz, 1H), 5.10-5.25 (m, 2H), 5.26-5.35 (d, J = 12.4 Hz, 1H), 5.85-5.95 (d, J = 8.0 Hz, 1H), 6.80-6.90 (m, 2H), 6.95-7.00 (m, 1H), 7.00-7.10 (m, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 11.4, 15.7, 20.7, 24.5, 27.6, 34.0, 35.5, 35.7, 52.5, 58.4, 67.4, 68.5, 118.2, 118.4, 128.3, 128.6, 128.7, 130.2, 130.7, 135.3, 158.0, 170.9, 171.5, 172.4; HRMS-FAB (M + Na+) calcd for C27H34NaN2O5 489.2365, found 489.2375.
Benzyl ester 14e
Compound 14e was synthesized using a procedure analogous to benzyl ester 14a (155 mg, 0.33 mmol, 65%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.90 (d, J = 6.3 Hz, 6H), 1.30-1.55 (m, 5H), 1.60-1.85 (m, 2H), 1.90-2.10 (m, 2H), 2.10-2.25 (m, 1H), 2.50-2.65 (t, J = 9.3 Hz, 1H), 3.35-3.50 (dd, J = 4.8, 13.5 Hz, 1H), 4.10-4.35 (m, 3H), 4.90-5.10 (m, 1H), 5.15-5.22 (d, J = 12.3 Hz, 1H), 5.23-5.35 (d, J = 12.3 Hz, 1H), 5.55-5.65 (d, J = 8.7 Hz, 1H), 6.00-6.10 (d, J = 9.6 Hz, 1H), 6.70-7.00 (m, 3H), 7.15-7.25 (d, J = 8.4 Hz, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 21.9, 22.8, 23.0, 25.0, 26.5, 36.3, 38.5, 42.8, 51.5, 52.9, 67.8, 68.3, 117.4, 119.1, 128.8, 129.0, 129.1, 130.3, 131.6, 135.5, 156.2, 171.6, 171.8, 172.1; HRMS-FAB (M + Na+) calcd for C27H34NaN2O5 489.2365, found 489.2381.
Benzyl ester 14f
Compound 14f was synthesized using a procedure analogous to benzyl ester 14a (128 mg, 0.28 mmol, 55%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.95 (m, 6H), 1.30-1.55 (m, 4H), 1.65-1.75 (m, 2H), 1.80-1.95 (m, 2H), 2.00-2.20 (m, 1H), 3.15-3.25 (d, J = 6.4 Hz, 2H), 4.15-4.25 (m, 1H), 4.30-4.40 (m, 2H), 4.50-4.60 (dd, J = 4.4, 5.2 Hz, 1H), 5.10-5.20 (m, 2H), 5.21-5.30 (d, J = 12.0 Hz, 1H), 5.95-6.05 (d, J = 7.2 Hz, 1H), 6.80-6.9 (m, 2H), 6.95-7.05 (dd, J = 2.0, 8.4 Hz, 1H), 7.05-7.10 (dd, J = 1.6, 8.4 Hz, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 20.6, 21.7, 22.9, 24.8, 27.6, 33.9, 35.5, 40.0, 51.8, 52.8, 67.4, 68.5, 118.3, 118.6, 128.4, 128.6, 128.7, 130.2, 130.7, 135.4, 157.9, 171.4, 171.8, 172.4; HRMS-FAB (M + Na+) calcd for C27H34NaN2O5 489.2365, found 489.2375.
Benzyl ester 14g
Compound 14g was synthesized using a procedure analogous to benzyl ester 14a (150 mg, 0.30 mmol, 60%): 1H NMR (400 MHz, CDCl3) δ 1.30-1.55 (m, 1H), 1.60-1.80 (m, 2H), 1.81-2.00 (m, 1H), 2.00-2.25 (m, 2H), 2.40-2.60 (m, 1H), 2.74-3.00 (m, 2H), 3.00-3.15 (m, 1H), 3.25-3.40 (dd, J = 5.2, 14.0 Hz, 1H), 4.10-4.30 (m, 1H), 4.30-4.40 (m, 1H), 5.20-5.35 (m, 1H), 6.55-6.75 (m, 1H), 6.76-6.90 (m, 2H), 7.05-7.25 (m, 6H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 21.7, 26.0, 36.0, 38.3, 39.2, 52.5, 54.3, 67.3, 67.6, 115.6, 116.2, 118.6, 127.0, 128.2, 128.4, 128.5, 128.6, 128.7, 128.8, 129.3, 129.4, 130.4, 131.1, 135.2, 136.2, 155.8, 169.9, 170.9, 172.0; HRMS-FAB (M + Na+) calcd for C30H32NaN2O5 523.2209, found 523.2220.
Benzyl ester 14h
Compound 14h was synthesized using a procedure analogous to benzyl ester 14a (125 mg, 0.25 mmol, 50%): 1H NMR (400 MHz, CDCl3) δ 1.20-1.50 (m, 3H), 1.50-1.65 (m, 1H), 1.70-1.90 (m, 3H), 1.90-2.00 (m, 1H), 2.90-3.00 (m, 1H), 3.00-3.30 (m, 3H), 4.10-4.20 (m, 1H), 4.20-4.30 (m, 1H), 5.00-5.30 (m, 3H), 5.80-5.90 (d, J = 8.8 Hz, 1H), 6.70-6.80 (m, 1H), 6.80-6.85 (m, 1H), 6.95-7.00 (m, 1H), 7.00-7.05 (m, 1H), 7.10-7.15 (m, 2H), 7.20-7.25 (m, 4H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 20.5, 27.6, 34.1, 35.5, 36.4, 52.6, 54.0, 67.4, 68.4, 118.6, 118.7, 126.9, 128.1, 128.6, 128.7, 129.3, 129.9, 131.0, 135.3, 136.6, 157.9, 170.9, 171.4, 172.5; HRMS-FAB (M + Na+) calcd for C30H32NaN2O5 523.2209, found 523.2222.
Benzyl ester 14i
Compound 14i was synthesized using a procedure analogous to benzyl ester 14a (158 mg, 0.35 mmol, 70%): 1H NMR (400 MHz, CDCl3) δ 0.80-0.90 (d, J = 6.8 Hz, 6H), 1.80-2.00 (m, 2H), 1.70-1.82 (m, 2H), 1.83-1.90 (m, 1H), 2.00-2.10 (m, 1H), 2.15-2.25 (m, 1H), 2.50-2.60 (t, J = 12.8 Hz, 1H), 3.40-3.50 (dd, J = 4.8, 13.6 Hz, 1H), 3.95-4.05 (dd J = 7.6, 8.4 Hz, 1H), 4.10-4.30 (m, 2H), 5.00-5.10 (m, 1H), 5.15-5.25 (d, J = 12.0 Hz, 1H), 5.25-5.35 (d, J = 12.0 Hz, 1H), 5.70-5.80 (d, J = 8.8 Hz, 1H), 6.10-6.15 (d, J = 10.0 Hz, 1H), 6.75-6.82 (dd, J = 2.4, 8.4 Hz, 1H), 6.85-6.95 (m, 2H), 7.17-7.25 (dd, J = 2.4, 8.8 Hz, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 18.5, 18.8, 21.7, 26.4, 31.8, 36.2, 52.5, 58.1, 67.5, 116.6, 118.9, 128.6, 128.7, 128.8, 130.0, 131.2, 155.7, 170.2, 171.4, 172.0; HRMS-FAB (M + Na+) calcd for C26H32NaN2O5 475.2209, found 475.2221.
Benzyl ester 14j
Compound 14j was synthesized using a procedure analogous to benzyl ester 14a (158 mg, 0.35 mmol, 70%): 1H NMR (400 MHz, CDCl3) δ 0.75-0.85 (d, J = 6.8 Hz, 3H), 0.85-0.95 (d, J = 6.8 Hz, 3H), 1.40-1.60 (m, 2H), 1.60-1.70 (m, 1H), 1.80-2.12 (m, 3H), 2.10-2.20 (m, 1H), 2.22-2.32 (m, 1H), 3.15-3.30 (dd, J = 1.6, 7.6 Hz, 1H), 4.10-4.15 (dd, J = 6.4, 8.4 Hz, 1H), 4.15-4.25 (m, 1H), 4.30-4.40 (m, 1H), 4.50-4.60 (dd, J = 6.8, 13.6 Hz, 1H), 5.10-5.20 (d J = 12.0 Hz, 1H), 5.20-5.30 (m, 2H), 5.95-6.05 (d, J = 7.2 Hz, 1H), 6.80-6.90 (m, 2H), 6.92-7.00 (dd, J = 3.2, 11.6 Hz, 1H), 7.00-7.05 (dd, J = 3.2, 11.6 Hz, 1H), 7.30-7.50 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 17.6, 19.4, 20.7, 27.6, 29.2, 33.9, 35.5, 52.5, 59.1, 67.4, 68.5, 118.2, 118.4, 128.3, 128.6, 128.7, 130.3, 130.7, 135.3, 158.0, 171.1, 171.5, 172.5; HRMS-FAB (M + Na+) calcd for C26H32NaN2O5 475.2209, found 475.2225.
Amide 17a
A solution benzyl ester 14a (150 mg, 0.30 mmol) in MeOH (20 mL) was hydrogenated using 1 atm of H2 gas over 10% Pd(OH)2/C (20 mg) at room temperature for 4 h. The catalyst was removed by filtration and the solvent was removed by rotary evaporation to give the corresponding carboxylic acid 15a. The carboxylic acid was dissolved in CH2Cl2 (10 mL). To this solution were added HBTU (340 mg, 0.90 mmol), DIEA (210 μL, 156 mg, 1.20 mmol) and primary amine 16 (46 mg, 0.45 mmol) as a solution in DMF (100 μL). The reaction was stirred at room temperature for 24 h, then partitioned between CH2Cl2 (100 mL) and 1 N HCl (100 mL). The organic layer was washed with saturated NaHCO3 (75 mL) and brine (75 mL), dried over MgSO4 and concentrated. The crude material was purified by flash chromatography (gradient of 100% EtOAc to 10% MeOH/CH2Cl2) to yield the corresponding amide 17a (118 mg, 0.21 mmol, 70%) as a mixture of two diastereomers: 1H NMR (400 MHz, CDCl3) δ 0.85-1.05 (m, 3H), 1.06-1.20 (m, 4H), 1.30-1.55 (m, 9H), 1.56-1.75 (m, 9H), 1.76-1.85 (m, 3H), 1.86-2.00 (m, 2H), 2.00-2.15 (m, 2H), 2.15-2.25 (m, 1H), 2.65-2.80 (m, 2H), 3.25-3.40 (m, 1H), 3.75-3.90 (m, 3H), 3.91-4.10 (m, 5H), 4.11-4.30 (m, 3H), 4.80-5.00 (m, 1H), 5.70-5.90 (m, 1H), 6.40-6.60 (m, 2H), 6.75-6.85 (m, 1H), 6.85-6.90 (m, 1H), 6.90-7.00 (m, 1H), 7.20-7.26 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 25.5, 25.7, 25.8, 25.9, 26.1, 27.7, 28.8, 28.9, 29.0, 29.2, 29.3, 35.9, 36.0, 38.6, 38.8, 41.2, 41.3, 53.7, 53.9, 57.5, 57.7, 59.2, 59.3, 59.4, 67.8, 67.9, 97.5, 97.6, 116.4, 116.5, 116.6, 118.6, 118.8, 128.5, 129.3, 129.4, 130.0, 131.2, 131.3, 131.4, 155.5, 155.6, 170.2, 170.3, 170.4, 170.5, 170.6, 171.8, 171.9, 172.2; HRMS-FAB (M + Na+) calcd for C31H45NaN3O6 578.3206, found 578.3218.
Amide 17b
Compound 17b was synthesized using a procedure analogous to amide 17a (100 mg, 0.18 mmol, 60%): 1H NMR (400 MHz, CDCl3) δ 0.80-1.00 (m, 2H), 1.05-1.30 (m, 5H), 1.35-1.55 (m, 7H), 1.56-1.75 (m, 8H), 1.85-2.05 (m, 5H), 2.10-2.20 (m, 1H), 2.60-2.80 (m, 1H), 3.20-3.40 (m, 2H), 3.80-4.13 (m, 6H), 4.15-4.32 (m, 2H), 4.35-4.65 (m, 2H), 5.15-5.40 (m, 1H), 6.00-6.30 (m, 1H), 6.85-7.00 (m, 3H), 7.00-7.10 (m, 1H), 7.11-7.25 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 21.2, 21.3, 21.4, 22.1; 24.1, 25.5, 25.6, 26.1, 27.0, 27.4, 28.1, 28.3, 28.8, 29.0, 29.7, 29.8, 33.7, 33.8, 34.1, 35.1, 35.6, 35.8, 36.1, 38.8, 38.9, 39.3, 41.1, 53.5, 54.3, 55.3, 58.2, 58.6, 58.8, 59.3, 59.5, 62.0, 68.3, 68.7, 97.4, 97.7, 117.7, 117.8, 118.2, 118.3, 128.8, 130.2, 130.7, 130.8, 131.3, 157.4, 157.7, 157.9, 171.0, 171.2, 172.3, 172.5; HRMS-FAB (M + Na+) calcd for C31H45NaN3O6 578.3206, found 578.3225.
Amide 17c
Compound 17c was synthesized using a procedure analogous to amide 17a (121 mg, 0.23 mmol, 75%): 1H NMR (400 MHz, CDCl3) δ 0.70-0.90 (m, 6H), 0.91-1.10 (m, 1H), 1.15-1.55 (m, 9H), 1.55-1.70 (m, 3H), 1.71-1.83 (m, 3H), 1.84-2.00 (m, 2H), 2.05-2.15 (m, 1H), 2.16-2.30 (m, 1H), 2.65-2.80 (m, 1H), 3.20-3.40 (m, 1H), 3.70-4.13 (m, 5H), 4.14-4.30 (m, 3H), 4.80-5.00 (m, 1H), 5.90-6.10 (m, 1H), 6.50-6.70 (m, 1H), 6.75-6.90 (m, 2H), 6.91-7.05 (m, 1H), 7.15-7.35 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 11.3, 15.0, 15.1, 20.9, 21.6, 21.7, 23.7, 25.0, 25.1, 25.4, 25.7, 26.2, 27.3, 29.0, 29.1, 31.5, 36.0, 36.5, 38.2, 38.3, 38.4, 38.6, 38.8, 52.4, 52.6, 53.8, 54.0, 57.1, 57.2, 59.1, 59.2, 59.3, 68.0, 68.1, 97.5, 97.6, 116.7, 116.8, 118.6, 118.8, 120.1, 125.6, 129.5, 129.6, 129.7, 129.8, 129.9, 131.4, 131.5, 155.5, 155.6, 170.4, 170.5, 170.7, 170.8, 171.8, 171.9, 172.3, 174.0; HRMS-ESI (M + Na+) calcd for C29H43NaN3O6 552.3050, found 552.3060.
Amide 17d
Compound 17d was synthesized using a procedure analogous to amide 17a (121 mg, 0.23 mmol, 75%): 1H NMR (400 MHz, CDCl3) δ 0.75-0.90 (m, 6H), 0.91-1.10 (m, 1H), 1.15-1.30 (m, 1H), 1.31-1.58 (m, 7H), 1.59-1.71 (m, 2H), 1.72-1.85 (m, 2H), 1.86-2.10 (m, 4H), 2.10-2.30 (m, 1H), 2.60-2.80 (m, 1H), 3.20-3.30 (m, 1H), 3.80-4.30 (m, 6H), 4.35-4.70 (m, 2H), 5.10-5.40 (m, 1H), 6.10-6.40 (m, 1H), 6.70-7.05 (m, 3H), 7.06-7.25 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 11.2, 11.4, 15.4, 15.7, 21.0, 21.7, 23.9, 24.0, 24.7, 25.5, 25.6, 26.0, 27.3, 28.9, 29.0, 34.0, 34.1, 35.9, 36.3, 38.6, 53.4, 55.1, 58.6, 58.7, 59.2, 59.3, 59.4, 68.3, 68.7, 97.5, 97.7, 117.5, 117.6, 117.8, 118.1, 128.6, 128.7, 129.0, 129.3, 130.1, 130.7, 130.9, 131.3, 157.4, 157.6, 157.9, 171.0, 171.1, 171.2, 171.3, 172.1, 172.3; HRMS-FAB (M + Na+) calcd for C29H43NaN3O6 552.3050, found 552.3060.
Amide 17e
Compound 17e was synthesized using a procedure analogous to amide 17a (120 mg, 0.23 mmol, 75%): 1H NMR (400 MHz, CDCl3) δ 075-0.95 (m, 6H), 1.20-1.60 (m, 10H), 1.61-1.85 (m, 4H), 1.86-2.15 (m, 3H), 2.16-2.30 (m, 1H), 2.65-2.85 (m, 2H), 3.20-3.40 (m, 1H), 3.70-4.10 (m, 4H), 4.11-4.30 (m, 2H), 4.31-4.50 (m, 1H), 4.60-4.90 (m, 1H), 5.60-5.90 (m, 1H), 6.40-6.60 (m, 1H), 6.75-6.90 (m, 2H), 6.95-7.05 (m, 1H), 7.15-7.26 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 22.4, 22.8, 22.9, 24.6, 24.7, 25.5, 25.7, 26.1, 29.0, 29.1, 36.0, 36.1, 38.6, 39.1, 42.9, 43.0, 51.1, 51.2, 53.7, 53.9, 59.1, 59.2, 59.3, 59.4, 67.9, 68.0, 97.5, 97.7, 116.8, 118.5, 118.6, 129.3, 130.0, 130.1, 131.5, 131.6, 155.7, 155.8, 170.4, 170.6, 171.4, 171.5, 171.6, 171.7; HRMS-FAB (M + Na+) calcd for C29H43NaN3O6 552.3050, found 552.3061.
Amide 17f
Compound 17f was synthesized using a procedure analogous to amide 17a (105 mg, 0.20 mmol, 65%): 1H NMR (400 MHz, CDCl3) δ 0.75-0.95 (m, 6H), 1.15-1.60 (m, 9H), 1.61-1.83 (m, 5H), 1.84-2.05 (m, 3H), 2.05-2.30 (m, 2H), 2.60-2.80 (m, 1H), 3.20-3.30 (m, 1H), 3.75-4.10 (m, 4H), 4.15-4.30 (m, 1H), 4.31-4.70 (m, 3H), 5.20-5.70 (m, 1H), 6.20-6.40 (m, 1H), 6.85-7.05 (m, 2H), 7.06-7.26 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 21.7, 21.8, 21.9, 22.9, 23.0, 24.1, 24.8, 24.9, 25.4, 25.7, 27.1, 27.5, 28.1, 28.8, 29.0, 34.1, 34.2, 36.1, 36.7, 40.6, 40.7, 52.2, 52.3, 53.5, 54.6, 59.3, 59.4, 68.3, 68.6, 97.4, 97.7, 117.6, 117.8, 118.1, 118.2, 128.9, 129.2, 130.4, 130.9, 131.2, 157.5, 157.7, 171.1, 171.7, 171.8, 172.1, 172.2; HRMS-ESI (M + Na+) calcd for C29H43NaN3O6 552.3050, found 552.3048.
Amide 17g
Compound 17g was synthesized using a procedure analogous to amide 17a (95 mg, 0.17 mmol, 65%): 1H NMR (400 MHz, CDCl3) δ 1.30-1.50 (m, 7H), 1.60-1.80 (m, 3H), 1.80-1.95 (m, 3H), 2.00-2.20 (m, 3H), 2.70-2.85 (m, 2H), 2.86-2.95 (m, 2H), 3.10-3.30 (m, 1H), 3.80-4.00 (m, 4H), 4.01-4.10 (m, 1H), 4.11-4.20 (m, 1H), 4.21-4.30 (m, 1H), 4.40-4.50 (m, 1H), 4.70-4.80 (m, 1H), 5.10-5.30 (m, 1H), 6.00-6.20 (m, 1H), 6.30-6.40 (m, 1H), 6.70-6.90 (m, 2H), 6.91-7.00 (m, 1H), 7.00-7.18 (m, 3H), 7.19-7.26 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 21.6, 21.7, 25.5, 25.7, 26.2, 26.3, 29.1, 35.9, 38.6, 39.0, 39.1, 53.6, 53.7, 54.0, 54.2, 59.2, 59.3, 59.4, 67.6, 67.7, 97.5, 97.6, 116.4, 118.3, 118.4, 126.9, 127.0, 128.5, 129.0, 129.3, 130.5, 136.1, 136.3, 156.0, 170.0, 170.1, 171.8, 171.9; HRMS-FAB (M + Na+) calcd for C32H41NaN3O6 586.2893, found 586.2875.
Amide 17h
Compound 17h was synthesized using a procedure analogous to amide 17a (100 mg, 0.18 mmol, 60%): 1H NMR (400 MHz, CDCl3) δ 1.20-1.40 (m, 4H), 1.41-1.55 (m, 3H), 1.56-1.70 (m, 3H), 1.75-1.90 (m, 3H), 1.90-2.10 (m, 4H), 2.60-2.80 (m, 1H), 2.90-3.00 (m, 1H), 3.05-3.25 (m, 2H), 3.26-3.40 (m, 1H), 3.70-4.10 (m, 5H), 4.12-4.25 (m, 1H), 4.28-4.40 (m, 1H), 4.40-4.55 (m, 1H), 4.55-4.65 (m, 1H), 5.20-5.60 (m, 1H), 6.00-6.20 (m, 1H), 6.50-7.00 (m, 3H), 7.00-7.30 (m, 8H); 13C NMR (100 MHz, CDCl3) δ 20.6, 20.7, 21.7, 25.5, 25.7, 27.3, 27.7, 34.1, 35.8, 36.5, 36.7, 53.2, 54.9, 55.0, 59.3, 59.4, 68.6, 97.6, 97.8, 117.8, 118.2, 118.7, 126.8, 126.9, 128.6, 128.7, 129.1, 129.2, 130.6, 130.7, 131.1, 136.8, 137.0, 157.9, 170.8, 170.9, 171.0, 172.4, 172.5; HRMS-FAB (M + Na+) calcd for C32H41NaN3O6 586.2893, found 586.2882.
Amide 17i
Compound 17i was synthesized using a procedure analogous to amide 17a (123 mg, 0.24 mmol, 80%): 1H NMR (400 MHz, CDCl3) δ 0.75-0.90 (m, 6H), 1.15-1.55 (m, 8H), 1.56-2.02 (m, 8H), 2.03-2.35 (m, 3H), 2.60-2.80 (m, 2H), 3.20-3.40 (m, 1H), 3.70-4.10 (m, 5H), 4.11-4.30 (m, 3H), 4.30-4.50 (m, 1H), 5.80-6.00 (m, 1H), 6.50-6.90 (m, 4H), 6.91-7.01 (m, 1H), 7.15-7.30 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 18.9, 19.0, 19.2, 19.3, 22.0, 22.1, 24.1, 25.8, 26.0, 26.5, 28.0, 29.4, 29.5, 32.3, 36.5, 38.9, 39.4, 54.2, 54.4, 58.3, 58.4, 59.5, 59.6, 59.7, 68.3, 68.5, 97.9, 98.0, 117.0, 117.1, 119.1, 129.8, 129.9, 130.4, 130.5, 131.8, 131.9, 156.0, 170.8, 170.9, 171.1, 172.3, 172.4; HRMS-FAB (M + Na+) calcd for C28H41NaN3O6 538.2893, found 538.2881.
Amide 17j
Compound 17j was synthesized using a procedure analogous to amide 17a (92 mg, 0.18 mmol, 60%): 1H NMR (400 MHz, CDCl3) δ 0.70-1.00 (m, 6H), 1.15-1.60 (m, 7H), 1.61-1.83 (m, 3H), 1.84-2.50 (m, 6H), 2.60-2.80 (m, 1H), 3.20-3.40 (m, 2H), 3.70-4.15 (m, 5H), 4.17-4.30 (m, 1H), 4.31-4.40 (m, 1H), 4.41-4.80 (m, 1H), 5.30-5.70 (m, 1H), 6.40-6.60 (m, 1H), 6.90-7.00 (m, 2H), 7.00-7.21 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 18.1, 18.3, 19.2, 19.5, 21.0, 21.7, 25.4, 25.6, 27.1, 27.3, 29.0, 29.9, 30.0, 33.9, 34.1, 35.8, 36.6, 54.9, 59.3, 59.4, 59.6, 59.9, 68.3, 68.6, 97.4, 97.7, 117.6, 117.7, 117.8, 117.9, 129.0, 129.3, 129.9, 130.5, 131.2, 131.5, 157.5, 157.7, 171.2, 171.3, 171.4, 171.5, 172.2, 172.3; HRMS-FAB (M + Na+) calcd for C28H41NaN3O6 538.2893, found 538.2899.
Inhibitor 10a
To compound 17a (110 mg, 0.2 mmol), an aqueous TFA solution (10 mL of a 33% solution) was added at 0°C. The reaction was warmed to room temperature, stirred for an additional 12h, and then concentrated by rotary evaporation. The resulting residue was diluted with EtOAc (50 mL) and washed with saturated aqueous Na2CO3 (50 mL) and brine (50 mL). It was then dried over MgSO4, and the solvent was removed by rotary evaporation. The crude oil was purified by flash chromatography (EtOAc:hexanes 2:1) to yield inhibitor 10a as a mixture of two diastereomers (50 mg, 0.1 mmol, 50%): 1H NMR (400 MHz, CDCl3) δ 0.80-1.00 (m, 2H), 1.10-1.25 (m, 3H), 1.30-1.55 (m, 3H), 1.56-1.71 (m, 7H), 1.72-1.85 (m, 7H), 2.00-2.30 (m, 3H), 2.35-2.55 (m, 1H), 2.56-2.75 (m, 3H), 3.25-3.40 (m, 1H), 4.00-4.10 (m, 1H), 4.11-4.30 (m, 2H), 4.40-4.60 (m, 1H), 4.80-5.00 (m, 1H), 5.60-5.85 (m, 1H), 6.20-6.40 (m, 1H), 6.75-6.90 (m, 2H), 6.90-7.00 (m, 1H), 7.01-7.11 (m, 1H), 7.15-7.25 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 22.1, 22.2, 24.1, 26.8, 26.9, 28.8, 29.5, 29.6, 29.7, 35.5, 35.6, 36.1, 37.6, 38.8, 38.9, 39.1, 41.3, 41.4, 41.5, 53.2, 54.4, 54.5, 58.2, 58.5, 64.4, 68.2, 117.8, 117.9, 119.0, 129.3, 129.4, 130.0, 130.1, 131.8, 155.9, 171.1, 171.2, 171.4, 171.5, 174.0, 207.4, 207.6; HRMS-ESI (M + H+) calcd for C28H40N3O5 498.2968, found 498.2970.
Inhibitor 10b
Compound 10b was synthesized using a procedure analogous to inhibitor 10a (50 mg, 0.10 mmol, 50%): 1H NMR (400 MHz, CDCl3) δ 0.80-1.00 (m, 2H), 1.01-1.30 (m, 3H), 1.45-1.55 (m, 2H), 1.56-1.82 (m, 8H), 1.83-2.00 (m, 3H), 2.10-2.25 (m, 2H), 2.35-2.50 (m, 1H), 2.51-2.75 (m, 2H), 3.10-3.40 (m, 2H), 4.00-4.15 (m, 1H), 4.16-4.32 (m, 1H), 4.31-4.45 (m, 1H), 4.46-4.60 (m, 2H), 5.40-5.65 (m, 1H), 6.20-6.60 (m, 2H), 6.85-7.00 (m, 2H), 7.01-7.11 (m, 2H), 7.30-7.50 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 21.2, 24.7, 24.8, 26.0, 26.4, 27.6, 28.4, 28.9, 30.2, 34.0, 35.3, 36.0, 39.2, 39.3, 41.4, 41.5, 55.0, 55.2, 58.7, 58.8, 59.4, 59.5, 64.4, 68.6, 118.2, 118.3, 118.5, 118.6, 128.9, 130.3, 130.4, 131.5, 131.7, 158.2, 158.3, 172.0, 173.6, 207.4, 207.5; HRMS-ESI (M + H+) calcd for C28H40N3O5 498.2968, found 498.2977.
Inhibitor 10c
Compound 10c was synthesized using a procedure analogous to inhibitor 10a (56 mg, 0.12 mmol, 60%): 1H NMR (400 MHz, CDCl3) δ 0.70-0.90 (m, 6H), 0.91-1.10 (m, 1H), 1.25-1.75 (m, 7H), 1.76-1.95 (m, 4H), 2.05-2.25 (m, 2H), 2.25-2.40 (m, 1H), 2.41-2.65 (m, 3H), 2.66-2.81 (m, 1H), 3.20-3.40 (m, 1H), 4.00-4.30 (m, 3H), 4.40-4.70 (m, 1H), 4.85-5.10 (m, 1H), 6.70-6.90 (m, 2H), 6.90-7.00 (m, 1H), 7.10-7.46 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 11.6, 11.7, 15.5, 21.9, 24.4, 25.4, 26.5, 28.3, 31.3, 35.3, 35.6, 36.1, 38.3, 38.5, 38.7, 39.1, 41.5, 41.6, 54.3, 57.7, 58.3, 58.4, 68.4, 118.0, 118.2, 119.1, 120.5, 120.6, 129.6, 130.0, 130.1, 130.2, 130.3, 132.0, 155.8, 171.3, 171.5, 173.7, 207.4, 207.5; HRMS-ESI (M + H+) calcd for C26H38N3O5 472.2811, found 472.2825.
Inhibitor 10d
Compound 10d was synthesized using a procedure analogous to inhibitor 10a (52 mg, 0.11 mmol, 55%): 1H NMR (400 MHz, CDCl3) δ 0.70-0.90 (m, 6H), 0.91-1.11 (m, 1H), 1.30-1.60 (m, 4H), 1.61-1.75 (m, 2H), 1.76-2.00 (m, 4H), 2.01-2.10 (m, 1H), 2.11-2.31 (m, 2H), 2.35-2.75 (m, 2H), 3.20-3.40 (m, 2H), 4.10-4.30 (m, 2H), 4.30-4.45 (m, 1H), 4.45-4.65 (m, 2H), 5.50-5.80 (m, 1H), 6.40-6.70 (m, 1H), 6.90-7.00 (m, 2H), 7.00-7.15 (m, 2H), 7.40-7.60 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 11.2, 11.3, 11.9, 21.0, 21.1, 24.4, 25.2, 27.5, 27.6, 28.3, 33.9, 34.0, 35.2, 35.9, 36.2, 36.3, 41.4, 41.5, 55.1, 55.2, 58.7, 58.8, 59.2, 59.3, 64.4, 68.5, 68.6, 118.0, 118.1, 118.5, 118.6, 128.9, 129.0, 129.1, 130.2, 130.3, 131.6, 131.7, 158.2, 158.3, 172.1, 172.2, 172.4, 172.5, 173.6, 207.4, 207.5; HRMS-ESI (M + H+) calcd for C26H38N3O5 472.2811, found 472.2820.
Inhibitor 10e
Compound 10e was synthesized using a procedure analogous to inhibitor 10a (45 mg, 0.10 mmol, 50%): 1H NMR (400 MHz, CDCl3) δ 0.70-0.90 (m, 6H), 1.20-1.30 (m, 1H), 1.30-1.60 (m, 7H), 1.75-2.00 (m, 3H), 2.00-2.15 (m, 1H), 2.16-2.25 (m, 1H), 2.30-2.40 (m, 1H), 2.50-2.65 (m, 2H), 2.66-2.80 (m, 1H), 3.20-3.40 (m, 1H), 4.10-4.30 (m, 2H), 4.40-4.70 (m, 3H), 4.90-5.10 (m, 1H), 6.65-6.81 (m, 2H), 6.86-6.90 (m, 1H), 6.90-7.00 (m, 1H), 7.10-7.20 (m, 1H), 7.27-7.35 (m, 1H), 7.40-7.60 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 22.4, 22.9, 24.2, 24.3, 25.0, 25.2, 26.2, 27.5, 28.3, 28.4, 35.5, 35.9, 39.0, 39.1, 41.3, 41.4, 42.7, 42.8, 51.8, 51.9, 54.8, 58.6, 58.7, 64.4, 65.4, 68.2, 113.4, 118.5, 118.6, 118.9, 129.0, 129.1, 129.9, 130.0, 131.9, 155.9, 159.7, 160.3, 171.2, 171.4, 172.8, 172.9, 174.6, 174.7, 207.5, 207.8; HRMS-ESI (M + H+) calcd for C26H38N3O5 472.2811, found 472.2816.
Inhibitor 10f
Compound 10f was synthesized using a procedure analogous to inhibitor 10a (61 mg, 0.13 mmol, 65%): 1H NMR (400 MHz, CDCl3) δ 0.70-0.90 (m, 6H), 1.30-1.75 (m, 9H), 1.75-2.00 (m, 3H), 2.00-2.25 (m, 3H), 2.30-2.70 (m, 3H), 3.00-3.20 (m, 1H), 3.20-3.40 (m, 1H), 4.10-4.40 (m, 3H), 4.45-4.75 (m, 3H), 5.70-5.90 (m, 1H), 6.50-6.80 (m, 1H), 6.90-7.25 (m, 4H), 7.60-7.70 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 21.8, 21.9, 23.3, 23.4, 24.4, 25.7, 25.8, 27.7, 27.8, 28.2, 34.0, 35.0, 35.1, 36.3, 36.8, 40.2, 40.3, 41.3, 41.4, 52.8, 52.9, 54.8, 54.9, 58.7, 64.4, 68.4, 118.0, 118.1, 118.7, 118.8, 128.6, 128.7, 130.4, 131.7, 131.8, 158.4, 158.5, 159.4, 160.0, 172.5, 173.0, 173.1, 174.0, 174.1, 207.4, 207.5; HRMS-ESI (M + H+) calcd for C26H38N3O5 472.2811, found 472.2814.
Inhibitor 10g
Compound 10g was synthesized using a procedure analogous to inhibitor 10a (60 mg, 0.12 mmol, 60%): 1H NMR (400 MHz, CDCl3) δ 1.30-1.58 (m, 3H), 1.60-1.80 (m, 3H), 1.81-2.00 (m, 2H), 2.10-2.35 (m, 3H), 2.40-2.70 (m, 4H), 2.80-3.00 (m, 2H), 3.10-3.30 (m, 1H), 4.10-4.30 (m, 2H), 4.40-4.60 (m, 3H), 4.70-4.90 (m, 1H), 6.40-6.60 (m, 1H), 6.70-6.85 (m, 2H), 6.86-7.00 (m, 2H), 7.00-7.26 (m, 7H); 13C NMR (100 MHz, CDCl3) δ 21.9, 24.4, 26.1, 26.3, 28.2, 28.3, 35.2, 35.5, 36.0, 38.8, 38.9, 39.6, 39.7, 41.4, 54.5, 54.8, 54.9, 58.5, 58.6, 67.8, 68.0, 117.2, 117.5, 119.0, 127.5, 128.7, 128.8, 128.9, 129.5, 129.6, 130.3, 131.8, 135.9, 136.0, 156.0, 156.1, 170.8, 170.9, 171.1, 174.2, 207.3, 207.4; HRMS-ESI (M + H+) calcd for C29H36N3O5 506.2655, found 506.2624.
Inhibitor 10h
Compound 10h was synthesized using a procedure analogous to inhibitor 10a (51 mg, 0.10 mmol, 50%): 1H NMR (400 MHz, CDCl3) δ 1.30-1.50 (m, 2H), 1.50-1.70 (m, 4H), 1.75-1.90 (m, 2H), 1.90-2.00 (m, 3H), 2.10-2.25 (m, 1H), 2.40-2.50 (m, 1H), 2.50-2.65 (m, 2H), 3.00-3.10 (m, 2H), 3.15-3.25 (m, 1H), 3.35-3.45 (m, 1H), 4.20-4.30 (m, 1H), 4.30-4.40 (m, 1H), 4.40-4.55 (m, 3H), 5.25-5.35 (m, 1H), 5.95-6.05 (m, 1H), 6.90-7.00 (m, 2H), 7.00-7.10 (m, 2H), 7.11-7.20 (m, 2H), 7.20-3.28 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 21.1, 21.3, 24.2, 27.8, 27.9, 28.2, 28.3, 34.1, 34.2, 35.0, 35.2, 35.8, 36.1, 36.5, 36.6, 41.4, 54.8, 55.3, 55.6, 58.6, 58.7, 68.5, 68.6, 118.6, 118.7, 118.9, 127.3, 128.6, 128.7, 129.0, 129.2, 129.5, 129.6, 129.9, 130.7, 131.4, 131.5, 136.9, 137.0, 158.3, 158.5, 171.6, 171.7, 171.9, 173.8, 173.9, 207.5, 207.6; HRMS-ESI (M + H+) calcd for C29H36N3O5 506.2655, found 506.2670.
Inhibitor 10i
Compound 10i was synthesized using a procedure analogous to inhibitor 10a (50 mg, 0.11 mmol, 55%): 1H NMR (400 MHz, CDCl3) δ 0.70-0.90 (m, 6H), 1.25-1.40 (m, 1H), 1.45-1.65 (m, 3H), 1.70-2.00 (m, 5H), 2.10-2.25 (m, 2H), 2.35-2.80 (m, 5H), 3.20-3.40 (m, 1H), 4.00-4.25 (m, 2H), 4.26-4.35 (m, 1H), 4.40-4.55 (m, 1H), 4.55-4.70 (m, 1H), 4.90-5.11 (m, 1H), 6.70-7.00 (m, 4H), 7.15-7.25 (m, 1H), 7.30-7.70 (m, 1H), 7.70-7.90 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 19.1, 19.2, 19.3, 21.9, 24.3, 26.3, 27.5, 28.3, 32.0, 32.1, 35.3, 35.5, 35.9, 39.0, 39.1, 41.4, 41.5, 54.8, 58.6, 58.7, 58.9, 64.4, 68.3, 118.4, 118.5, 119.0, 129.2, 129.3, 130.0, 130.1, 131.9, 155.8, 171.4, 171.5, 171.7, 171.8, 175.0, 175.1, 207.5, 207.6; HRMS-ESI (M + H+) calcd for C35H36N3O5 458.2655, found 458.2664.
Inhibitor 10j
Compound 10j was synthesized using a procedure analogous to inhibitor 10a (55 mg, 0.12 mmol, 60%): 1H NMR (400 MHz, CDCl3) δ 0.60-1.00 (m, 6H), 1.40-1.60 (m, 3H), 1.60-1.75 (m, 2H), 1.75-2.05 (m, 4H), 2.10-2.30 (m, 3H), 2.40-2.70 (m, 3H), 3.10-3.20 (m, 1H), 3.20-3.40 (m, 1H), 4.00-4.10 (m, 1H), 4.15-4.40 (m, 2H), 4.50-4.70 (m, 2H), 5.80-6.00 (m, 1H), 6.70-7.00 (m, 3H), 7.00-7.25 (m, 2H), 7.60-7.80 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 18.4, 18.5, 19.5, 21.2, 24.4, 27.5, 27.6, 28.2, 30.1, 33.9, 34.0, 35.0, 35.1 36.1, 36.4, 41.3, 41.4, 55.1, 58.8, 60.6, 64.5, 68.4, 117.2, 117.8. 117.9, 118.5, 128.8, 130.0, 131.8, 131.9, 158.3, 158.4, 159.5, 160.1, 172.5, 172.6, 172.7, 174.2, 207.2, 207.3; HRMS-ESI (M + H+) calcd for C35H36N3O5 458.2655, found 458.2660.
7-(But-3-enyl)-1,5-dioxaspiro[5.5]undecane (21)
To a solution of diisopropylamine (8.42 mL, 6.06 g, 60.0 mmol) in THF (60 mL), n-butyllithium (23.5 mL, 58.8 mmol, 2.5 M in hexanes) was added at − 78°C under an atmosphere of nitrogen. The temperature of the solution was slowly increased to 0°C and maintained at that temperature for an additional 10 min. To this solution ketoester 18 (5.0 g, 29.4 mmol) was slowly added. After 15 min, 4-bromo-but-1-ene (3.80 mL, 5.34 g, 44.0 mmol) was added dropwise. The reaction was stirred at room temperature for 30 h, and then quenched with water. The THF was removed by rotary evaporation, and the mixture was partitioned between EtOAc (500 mL) and 1 N HCl (250 mL). The organic layer was washed with 1 N HCl (250 mL), saturated NaHCO3 (250 mL), and brine (250 mL). It was then dried over MgSO4, and the resulting solution was concentrated by rotary evaporation. The crude oil was purified by flash chromatography (EtOAc:hexanes 1:18) to yield 19 as a mixture of ketone and enol tautomers (5.16 g, 22.9 mmol, 78%). To a solution of compound 19 (6.0 g, 19.7 mmol) in MeOH (50 mL), 2 N aqueous NaOH (50 mL) was added. The reaction was heated at reflux for 24 h, and then cooled to room temperature. The MeOH was removed by rotary evaporation. The resulting aqueous solution was extracted with EtOAc (3 × 50 mL), the organic layers were combined and the solvent was removed by rotary evaporation to yield the corresponding alkene 20 (2.75 g, 90%). A solution of compound 20 (3.0 g, 20 mmol) in THF (10 mL) was cooled in an ice bath. To this solution, 1,3-propanediol (30 mL, 31.7 g, 417 mmol) and TMSCl (5.0 mL, 4.3 g, 40 mmol) were added. The reaction was stirred at room temperature for 48 h, and then partitioned between EtOAc (500 mL) and saturated NaHCO3 (400 mL). The organic layer was washed with saturated NaHCO3 (400 mL) and brine (400 mL). It was then dried over MgSO4, and the resulting solution was concentrated by rotary evaporation. The crude oil was purified by flash chromatography (EtOAc:hexanes 1:18) to yield 21 (4.0 g, 19.0 mmol, 95%): 1H NMR (400 MHz, CDCl3) δ 1.15-1.30 (m, 4H), 1.30-1.40 (m, 1H), 1.40-1.50 (m, 1H), 1.50-1.65 (m, 3H), 1.66-1.75 (m, 1H), 1.80-2.00 (m, 3H), 2.05-2.20 (m, 1H), 2.35-2.55 (m, 1H), 3.70-3.83 (m, 2H), 3.85-3.95 (td, J = 3.2, 11.2 Hz, 1H), 3.96-4.05 (td, J = 3.2, 11.2 Hz, 1H), 4.87-4.93 (dd, J = 1.2, 10.4 Hz, 1H), 4.95-5.05 (dd, J = 1.6, 17.2 Hz, 1H), 5.70-5.90 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 22.3, 25.7, 26.7, 26.9, 28.2, 32.1, 58.8, 58.9, 99.1, 114.0, 139.5; HRMS-EI (M + H+) calcd for C13H23O2 210.1620, found 210.1615.
3-(1,5-Dioxaspiro[5.5]undecan-7-yl)propanoic acid (22)
Compound 21 (250 mg, 1.0 mmol) was dissolved in a 2:1 mixture of acetone and water (60 mL). To this solution NaIO4 (1.1 mg, 5.4 mmol), KMnO4 (120 mg, 750 μmol), and NaHCO3 (100 mg, 1.0 mmol) were added. The reaction was stirred at room temperature for 4 h, and then the acetone was removed by rotary evaporation. The remaining material was partitioned between EtOAc (100 mL) and 1 N HCl (75 mL). The organic layer was washed with 1 N HCl (3 × 75 mL), brine (75 mL), and dried over MgSO4. The solvent was removed by rotary evaporation to give the carboxylic acid 22 (205 mg, 0.9 mmol, 90%): 1H NMR (400 MHz, CDCl3) δ 1.15-1.45 (m, 5H), 1.46-1.75 (m, 5H), 1.85-2.05 (m, 1H), 2.10-2.30 (m, 1H), 2.30-2.70 (m, 3H), 3.70-3.85 (m, 2H), 3.85-3.98 (m, 1H), 4.00-4.10 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 22.1, 23.4, 25.6, 27.5, 28.0, 32.8, 58.8, 58.9, 99.1, 180.3; HRMS-FAB (M + Na+) calcd for C12H20NaO4 251.1259, found 251.1269.
(S)-tert-Butyl 1-(5-hydroxypentylamino)-3-(1H-indol-3-yl)-1-oxopropan-2-ylcarbamate (24)
Boc-Trp-OH (456 mg, 1.5 mmol) was dissolved in DMF (10 mL). To this solution were added 5-aminohexan-1-ol (206 mg, 2.0 mmol), HBTU (758 mg, 2.0 mmol), and DIEA (530 μL, 390 mg, 3.0 mmol). The reaction was stirred at room temperature for 2 h, and then partitioned between EtOAc (250 mL) and 1 N HCl (200 mL). The organic layer was washed with 1 N HCl (200 mL), saturated NaHCO3 (200 mL) and brine (150 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation. The crude material was purified by flash chromatography (EtOAc: hexanes) to yield alcohol 24 (560 mg, 1.43 mmol, 95%): 1H NMR (400 MHz, CDCl3) δ 0.90-1.10 (m, 2H), 1.15-1.31 (m, 4H), 1.35-1.51 (m, 11H), 2.50-2.60 (s, 1H), 2.90-3.20 (m, 3H), 3.21-3.40 (m, 1H), 3.50-3.60 (m, 2H), 4.30-4.50 (br s, 1H), 5.20-5.45 (br s, 1H), 6.95-7.05 (s, 1H), 7.05-7.25 (m, 2H), 7.30-7.40 (d, J = 11.2 Hz, 1H), 7.60-7.70 (d, J = 11.2 Hz, 1H), 8.80-9.00 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 25.6, 26.6, 28.7, 29.1, 29.4, 32.6, 39.6, 62.8, 111.8, 119.1, 119.9, 122.4, 123.8, 127.7, 136.7, 156.0, 172.3; HRMS-FAB (M + Na+) calcd for C21H31NaN3O4 412.2212, found 412.2220.
(S)-5-(2-(tert-butoxycarbonylamino)-3-(1H-indol-3-yl)propanamido)pentyl 4-methylbenzenesulfonate (25)
Alcohol 24 (400 mg, 1.0 mmol) was dissolved in CH2Cl2 (10 mL). To this solution were added TsCl (285 mg, 1.5 mmol) and pyridine (530 μL, 390 mg, 3.0 mmol). The reaction was stirred at room temperature for 2 h, and then partitioned between EtOAc (250 mL) and 1 N HCl (200 mL). The organic layer was washed with 1 N HCl (200 mL), saturated NaHCO3 (200 mL) and brine (150 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation. The crude material was purified by flash chromatography (EtOAc: hexanes) to yield compound 25 (560 mg, 1.43 mmol, 97%): 1H NMR (300 MHz, CDCl3) δ 0.90-1.15 (m, 4H), 1.40-1.60 (m, 11H), 2.40-2.50 (m, 3H), 2.90-3.25 (m, 3H), 3.25-3.40 (m, 1H), 3.30-3.50 (m, 2H), 4.30-4.50 (br s, 1H), 5.20-5.40 (br s, 1H), 5.70-5.80 (br s, 1H), 6.90-7.23 (m, 3H), 7.30-7.45 (m, 3H), 7.55-7.70 (d, J = 9.6 Hz, 1H), 7.75-7.85 (d, J = 10.4 Hz, 2H), 8.80-9.00 (br s, 1H); 13C NMR (75 MHz, CDCl3) δ 21.6, 22.6, 28.3, 28.4, 39.1, 70.6, 110.3, 111.5, 118.8, 119.5, 122.0, 123.4, 127.4, 127.8, 130.0, 132.8, 136.4, 145.0, 155.5, 171.7; HRMS-FAB (M + Na+) calcd for C29H39NaN3O6S 580.2457, found 580.2437.
Macrocycle 26
Compound 25 (290 mg, 0.5 mmol) was dissolved in THF (10 mL). To this solution was added NaH (150 mg, 2.5 mmol). The reaction was stirred at room temperature for 48 hr, and then partitioned between EtOAc (250 mL) and 1 N HCl (200 mL). The organic layer was washed with 1 N HCl (200 mL), saturated NaHCO3 (200 mL) and brine (150 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation. The crude material was purified by flash chromatography (EtOAc: hexanes) to yield macrocyclic compound 26 (56 mg, 0.15 mmol, 30%): 1H NMR (400 MHz, CDCl3) δ 0.05-0.30 (br s, 1H), 0.40-0.60 (br s, 1H), 1.35-1.60 (m, 12H), 1.70-1.85 (br s, 1H), 1.86-2.00 (br s, 1H), 2.70-2.90 (br s, 1H), 2.95-3.10 (m, 1H), 3.15-3.50 (m, 2H), 4.10-4.30 (m, 3H), 5.10-5.40 (m, 1H), 6.90-7.00 (s, 1H), 7.15-7.30 (m, 2H), 7.31-7.40 (m, 1H), 7.60-7.80 (d, J = 12.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 19.9, 25.8, 25.9, 28.7, 29.0, 38.4, 44.2, 56.7, 80.3, 108.4, 110.9, 119.0, 119.8, 121.9, 128.3, 131.0, 136.9, 155.4, 172.1; HRMS-FAB (M + Na+) calcd for C21H29NaN3O3 394.2107, found 394.2115.
Amide 28
To a solution of compound 26 (190 mg, 0.5 mmol) in CH2Cl2 (10 mL) was added TFA (5 mL). The reaction was allowed to stir at room temperature for 30 min. The solvent was removed to yield the crude amine 27. The resulting compound 27 was dissolved in DMF (10 mL). To this solution were added HBTU (379 mg, 1.0 mmol) and DIEA (265 μL, 195 mg, 1.5 mmol). The reaction was stirred at room temperature for 2 h, and then partitioned between EtOAc (150 mL) and 1 N HCl (100 mL). The organic layer was washed with 1 N HCl (100 mL), saturated NaHCO3 (100 mL) and brine (100 mL). The organic layer was dried over MgSO4 and concentrated by rotary evaporation. The crude material was purified by flash chromatography (EtOAc: hexanes) to yield amide 28 as a mixture of two diastereomers (170 mg, 0.35 mmol, 70%): 1H NMR (400 MHz, CDCl3) δ -0.10-0.25 (m, 1H), 0.40-0.80 (m, 1H), 1.00-1.20 (m, 1H), 1.30-1.90 (m, 10H), 2.15-2.50 (m, 4H), 2.51-2.75 (m, 2H), 2.76-2.85 (m, 2H), 2.90-3.10 (m, 1H), 3.25-3.55 (m, 3H), 3.65-4.00 (m, 3H), 4.20-4.40 (m, 1H), 4.40-4.70 (m, 1H), 5.00-5.60 (m, 1H), 5.80-6.80 (m, 1H), 6.80-7.00 (m, 1H), 7.05-7.25 (m, 2H), 7.30-7.40 (m, 1H), 7.60-7.70 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 14.4, 20.0, 20.2, 23.0, 25.3, 25.6, 25.8, 27.9, 28.3, 28.6, 29.6, 34.0, 34.1, 34.2, 35.4, 38.1, 38.4, 42.0, 43.1, 43.6, 44.1, 49.8, 50.0, 55.0, 55.2, 58.6, 58.9, 62.0, 99.4, 107.8, 108.1, 110.6, 110.9, 111.2, 118.3, 118.8, 119.7, 119.9, 121,4, 121.7, 127.9, 128.5, 130.6, 131.2, 136.4, 136.7, 170.0, 171.7, 171.8, 172.7, 173.5, 213.4; HRMS-FAB (M + Na+) calcd for C28H39NaN3O4 504.2838, found 504.2852.
Inhibitor 11
To compound 28 (250 mg, 0.5 mmol), an aqueous TFA solution (10 mL of a 33% solution) was added at 0°C. The reaction was warmed to room temperature, stirred for an additional 12h, and then concentrated by rotary evaporation. The resulting residue was diluted with EtOAc (50 mL) and washed with saturated aqueous Na2CO3 (50 mL) and brine (50 mL). It was then dried over MgSO4, and the solvent was removed by rotary evaporation. The crude oil was purified by flash chromatography (EtOAc:hexanes 2:1) to yield inhibitor 11 as a mixture of two diastereomers (97 mg, 224 μmol, 55%): 1H NMR (300 MHz, CDCl3) δ -0.10-0.10 (br s, 1H), 0.60-0.90 (br s, 1H), 1.20-1.80 (m, 9H), 1.81-2.00 (m, 1H), 2.00-2.20 (m, 5H), 2.21-2.50 (m, 5H), 2.55-2.80 (m, 1H), 2.95-3.10 (m, 1H), 3.25-3.40 (m, 1H), 3.41-3.60 (m, 1H), 4.00-4.20 (m, 1H), 4.21-4.40 (m, 1H), 4.40-4.60 (m, 1H), 5.50-5.70 (m, 1H), 6.40-6.60 (m, 1H), 6.89 (s, 1H), 7.10-7.30 (m, 2H), 7.31-7.45 (m, 1H), 7.60-7.80 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 21.0, 26.0, 26.2, 28.4, 29.0, 34.3, 34.4, 34.6, 34.7, 38.5, 42.4, 42.5, 44.4, 50.2, 50.4, 55.3, 108.5, 111.0, 119.1, 120.0, 122.0, 128.3, 131.0, 137.0, 172.1, 172.9, 213.6, 213.7; HRMS-FAB (M + Na+) calcd for C25H33N3NaO3 446.2420, found 446.2410.
Results and discussion
Chemistry
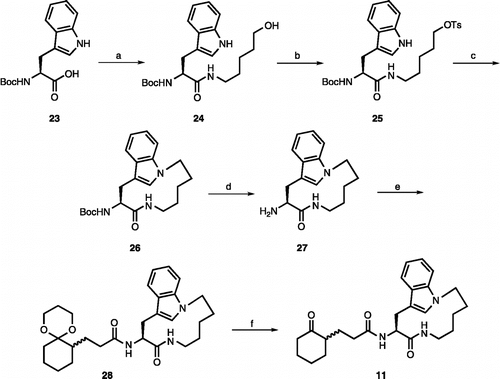
The synthesis of inhibitors 10a-j is detailed in . Boc-protected amino acids were coupled with H-Tyr-OBn using HBTU to give dipeptides 12a-j. The Boc protecting groups were removed using 1:2 TFA/CH2Cl2 to generate the corresponding primary amines. Acylation of these amines with 5-bromopentanoyl chloride under the Schotten-Baumann conditions provided the primary bromides in excellent yield. These were treated with NaI in acetone to generate compounds 13a-j. Ring closure of compounds 13a-j in the presence of K2CO3 gave macrocycles 14a-j. The benzyl esters of compounds 14a-j were removed by catalytic hydrogenation, and the resulting carboxylic acids 15a-j were coupled with racemic primary amine 16 [Citation5e] in the presence of HBTU to yield amides 17a-j. Finally, the acetal protecting group was removed using aqueous TFA to give inhibitors 10a-j as mixtures of two diastereomers.
Scheme 1. Reagents and conditions: (a) Boc-aa-OH, HBTU, DIEA, rt, 2 h (86-99%); (b) TFA/CH2Cl2 (1:2), rt, 30 min; (c) 20% K2CO3, 5-bromopentanoyl chloride, rt, 8 min; (d) NaI, acetone, reflux, 2 h (88-100%); (e) K2CO3, rt, 10 h (50-75%); (f) H2, Pd(OH)2/C, rt, 4 h; (g) 16, HBTU, DIEA, rt, 24 h (60-80%); (h) TFA/H2O (1:2), rt, 12 h (50-65%). Compounds 13c, 13d and 13f were not isolated in pure form, but instead the crude materials were used directly in the next reaction. Compounds 17a-j and 10a-j are 1:1 mixtures of two diastereomers where the stereochemistry of the R substituent is defined, but the stereocenter on the cyclohexane ring is not.
The synthesis inhibitor 11 required carboxylic acid 22, which was prepared starting from ketoester 18 (). Compound 18 was treated with two equivalents of LDA, and the resulting dianion was reacted with 4-bromo-1-butene to generate alkene 19. Hydrolysis of the ester using NaOH/MeOH, followed by spontaneous decarboxylation gave compound 20. The ketone was converted to acetal 21 using 1,3-propanediol in the presence of TMSCl. Finally, oxidative cleavage of the alkene in 21 using NaIO4 and KMnO4 provided acid 22.
Scheme 2. (a) LDA (2 equiv), 0°C, then 1-bromo-4-butene, rt, 30 h (78%); (b) 2 N NaOH:MeOH (1:1), reflux, 24 h (90%); (c) 1,3-propanediol, TMSCl, 0°C to rt, 48 h (95%); (d) NaIO4, KMnO4, NaHCO3, acetone/water (2:1), rt, 4 h (90%).
Inhibitor 11 was prepared as shown in . The coupling reaction between Boc-Trp-OH 23 and 5-amino-1-hexanol using HBTU gave alcohol 24. The hydroxy group was converted to the corresponding tosylate, and the resulting compound 25 was subjected to a series of bases to determine optimal cyclization conditions. We found that NaH gave a modest but acceptable yield of the desired macrocycle 26. The Boc protecting group in 26 was removed with TFA to generate amine 27, which was then coupled with acid 22 to give compound 28. Finally, the acetal was hydrolyzed using aqueous TFA to yield inhibitor 11.
Inhibition of plasmin
The assay results for inhibitors 10a-j, evaluated as mixtures of the two diastereomers, against plasmin are shown in . None of these compounds showed >20% inhibition at a concentration of 250 μM. We measured the IC50 values of three inhibitors with the highest activities, compounds 10c, 10d and 10h (), and compared these values with the activity of a closely related non-cyclic inhibitor, compound 29.
Figure 4. Assay results for inhibitors 10a-j (250 μM) against plasmin. The R group in each inhibitor is defined by the side chain of the amino acid shown on the x-axis of the plot. The data are an average of three independent measurements.
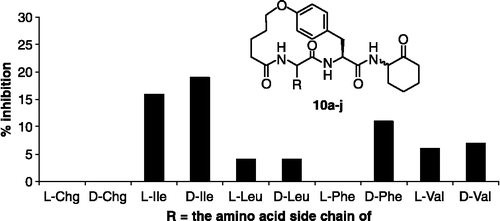
Table I. IC50 values of inhibitors 10c, 10d and 10h against plasmin.
Inhibitor 10d, with R = the side chain of D-Ile, is the best inhibitor with an IC50 value of 450 μM. This compound is closest in structure with its linear analog, compound 29. Inhibitor 10d is greater than 10-fold more potent than the linear analog 29, suggesting that the combination of macrocyclization and acylation of the N-terminus significantly improves its interactions with the active site of plasmin. The enhancement in activity caused by macrocyclization is further accentuated by the fact that 29 contains a sulfur atom, while 10d contains a methylene group at the analogous position. A sulfur atom at this position has been shown to enhance the electrophilicity of the ketone, and improve inhibition activity by approximately three-fold [Citation5a].
Inhibitor 10c, with R = the side chain of L-Ile, is the second most potent compound with an IC50 value of 550 μM. The observed preference of the enzyme for a D-Ile residue at the P3 position is consistent with results reported by Okada and coworkers [Citation15]. Additionally, inhibitor 10h, where R = the side chain of D-Phe, has an IC50 value of 930 μM, which is still >5 fold more potent than 29. The other seven inhibitors, which showed < 10% inhibition at a concentration of 250 μM, were poor inhibitors of plasmin. This result suggests that, within the context of the macrocyclic scaffold 10, plasmin prefers Ile at P3 over other similar amino acids including Leu, Val, and cyclohexylglycine.
We also examined the activity of compound 11 against plasmin, but it showed no detectable inhibition at concentrations up to 500 μM. Two factors may contribute to the low activity of this compound. First, the linker between the indole nitrogen atom and the C-terminus of the inhibitor may be too long so that it does not significantly limit the conformational freedom of the molecule. Second, the inhibitor incorporates only a single amino acid unit, Trp, at the P2′ position. The corresponding S2′ subsite on plasmin is known to have a relatively minor influence on binding interactions with peptide substrates. Thus, there is likely limited affinity between the S2′ subsite and the peptide portion of inhibitor 11.
In summary, we have designed and synthesized several macrocyclic inhibitors of the serine protease plasmin. While the inhibitors showed only modest activities, we did observe greater than 10-fold improvement in activity for several of the compounds (10c and d) when compared to the related linear analog compound 29.
Acknowledgements
This research was supported by the US National Institutes of Health NIGMS (Grant R01 GM057327 to CTS).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- MD Sternlicht, Z Werb. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516.
- H Nagase, JF WoessnerJr. Matrix metalloproteinases. J Biol Chem 1999;274:21491–21494.
- P Mignatti, DB Rifkin. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme Protein 1996;49:117–137. (b) Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. Acta Pathol Mic Sc 1999;107:38–44
- PM Mannucci. Hemostatic drugs. N Engl J Med 1998;339:245–253.
- JL Conroy, TC Sanders, CT Seto. Using the electrostatic field effect to design a new class of inhibitors for cysteine proteases. J Am Chem Soc 1997;119:4285–4291. (b) Conroy JL, Seto CT. 13C NMR studies demonstrate that tetrahydropyranone-based inhibitors bind to cysteine proteases by reversible formation of a hemithioketal adduct. J Org Chem 1998;63:2367–2370. (c) Abato P, Yuen CM, Cubanski JY, Seto CT. Inhibitors of plasmin that extend into both the S and S′ binding sites: cooperative interactions between S1 and S2. J Org Chem 2002;67:1184–1191. (d) Abato P, Conroy JL, Seto CT. A combinatorial library of serine and cysteine protease inhibitors that interact with both the S and S′ binding sites. J Med Chem 1999;42:4001–4009. (e) Xue F, Seto CT. A comparison of cyclohexanone and tetrahydro-4H-thiopyran-4-one 1,1-dioxide as pharmacophores for the design of peptide-based inhibitors of the serine protease plasmin. J Org Chem 2005:70:8309–8321. (f) Xue F, Seto CT. Selective inhibitors of the serine protease plasmin: probing the S3 and S3′ subsites using a combinatorial library. J Med Chem 2005;68:6908–6917. (g) Xue F, Seto CT. Structure-activity studies of cyclic ketone inhibitors of the serine protease plasmin: Design, synthesis and biological activity. Bioorg Med Chem 2006;14:8467–8487. (h) Sanders TC, Seto CT. 4-Heterocyclohexanone-based inhibitors of the serine protease plasmin. J Med Chem 1999;42:2969–2976
- WA Loughlin, JDA Tyndall, MP Glenn, DP Fairlie. Beta-strand mimetics. Chem Rev 2004;104:6085–6117. (b) Tyndall JDA, Nall T, Fairlie DP. Proteases universally recognize beta strands in their active sites. Chem Rev 2005;105:973–999. (c) Tyndall JD, Fairlie DP. Macrocycles mimic the extended peptide conformation recognized by aspartic, serine, cysteine and metallo proteases. Curr Med Chem 2001;8:893–907
- JW Janetka, P Raman, K Satyshur, GR Flentke, DH Rich. Novel cyclic biphenyl ether peptide β-strand mimetics and HIV-protease inhibitors. J Am Chem Soc 1997;119:441–442.
- RC Reid, DR March, MJ Dooley, DA Bergman, G Abbenante, DP Fairlie. A novel bicyclic enzyme inhibitor as a consensus peptido-mimetic for the receptor-bound conformations of twelve peptidic inhibitors of HIV-1 protease. J Am Chem Soc 1996;118:8511–8517.
- KX Chen, FG Njoroge, J Pichardo, A Prongay, N Butkiewicz, N Yao, V Madison, V Girijavallabhan. Potent 7-hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid-based macrocyclic inhibitors of hepatitis C virus NS3 protease. J Med Chem 2006;49:567–574.
- YS Tsantrizos, J-M Ferland, A McClory, M Poirier, V Farina, NK Yee, X Wang, N Haddad, X Wei, J Xu, L Zhang. Olefin ring-closing metathesis as a powerful tool in drug discovery and development - potent macrocyclic inhibitors of the hepatitis C virus NS3 protease. J Organomet Chem 2006;691:5163–5171.
- JH Meyer, PA Bartlett. Macrocyclic inhibitors of penicillopepsin 1. Design, synthesis, and evaluation of an inhibitor bridged between P1 and P3. J Am Chem Soc 1998;120:4600–4609.
- SJ Stachel, CA Coburn, S Sankaranarayanan, EA Price, BL Pietrak, Q Huang, J Lineberger, AS Espeseth, X Jin, J Elis, MK Holloway, S Munshi, T Allison, D Hazuda, AJ Simon, SL Graham, JP Vacca. Macrocyclic inhibitors of β-secretase: functional activity in an animal model. J Med Chem 2006;49:6147–6150.
- CB Xue, ME Voss, DJ Nelson, JJ Duan, RJ Cherney, IC Jacobson, X He, J Roderick, L Chen, RL Corbett, L Wang, DT Meyer, K Kennedy, WF DeGradodagger, KD Hardman, CA Teleha, BD Jaffee, RQ Liu, RA Copeland, MB Covington, DD Christ, JM Trzaskos, RC Newton, RL Magolda, RR Wexler, CP Decicco. Design, synthesis, and structure-activity relationships of macrocyclic hydroxamic acids that inhibit tumor necrosis factor α release in vitro and in vivo. J Med Chem 2001;44:2636–2660.
- S Thaisrivongs, DT Pals, SR Turner, LT Kroll. Conformationally constrained renin inhibitory peptides: γ-lactam-bridged dipeptide isostere as conformational restriction. J Med Chem 1988;31:1369–1376.
- Y Okada, Y Tsuda, N Teno, K Wanaka, M Bohgaki, A Hijikata-Okunomiya, T Naito, S Okamoto. Synthesis of active center-directed peptide inhibitors of plasmin. Chem Pharm Bull 1988;36:1289–1297, (b) S Okamoto, S Sato, Y Tanaka, U Okamoto. An active stereoisomer (trans form) of AMCHA and its antifibrinolytic (antiplasminic) action in vitro and in vivo. Keio J Med 1964;13:177–185.