Abstract
Chalcones (1,3-diaryl-2-propen-1-ones) are α, β-unsaturated ketones with cytotoxic and anticancer properties. Several reports have shown that compounds with cytotoxic properties may also interfere with DNA topoisomerase functions. Five derivatives of 4′-hydroxychalcones were examined for cytotoxicity against transformed human T (Jurkat) cells as well as plasmid supercoil relaxation experiments using mammalian DNA topoisomerase I. The compounds were 3-phenyl-1-(4′-hydroxyphenyl)-2-propen-1-one (I), 3-(p-methylphenyl)-1-(4′-hydroxyphenyl)-2-propen-1-one (II), 3-(p-methoxyphenyl)-1-(4′-hydroxyphenyl)-2-propen-1-one (III), 3-(p-chlorophenyl)-1-(4′-hydroxyphenyl)-2-propen-1-one (IV), and 3-(2- thienyl)-1-(4′-hydroxyphenyl)-2-propen-1-one (V). The order of the cytotoxicity of the compounds was; IV > III > II > I > V. Compound IV, had the highest Hammett and log P values (0.23 and 4.21, respectively) and exerted both highest cytotoxicity and strongest DNA topoisomerase I inhibition. Compounds I and II gave moderate interference with the DNA topoisomerase I while III & V did not interfere with the enzyme.
Introduction
Chalcones are alpha, beta-unsaturated ketones, reported to have cytotoxic and anticancer properties against a number of human cells, including the HeLa cervical carcinoma, the PANC-1 pancreatic cancer and the GOTO neuroblastoma [Citation1–4]. Many reports in recent years have shown that numerous naturally occurring and synthetic compounds with cytotoxic properties also interfere with normal DNA topoisomerase functions [Citation5,Citation6]. DNA topoisomerases are essential enzymes that regulate conformational changes in DNA topology by catalyzing concerted breakage and rejoining of DNA strands during many genetic processes, including DNA replication, transcription, recombination and transposition [Citation7]. Intermediates in the strand passage reaction involve either single- or double-stranded breaks, defining type I and type II enzymes, respectively [Citation7,Citation8]. Type I topoisomerases (Topo I) make a single-stranded break in a DNA duplex, mediate passage of the intact strand through the break, and then reseal it. Type II topoisomerases (Topo II), on the other hand, create transient breaks in both strands of a duplex, pass an intact DNA segment through the break and then reseal the cleavage site [Citation7,Citation8]. Over the past years, DNA topoisomerases have been recognized as an effective approach for the development of chemotherapeutics [Citation5,Citation6,Citation9–12].
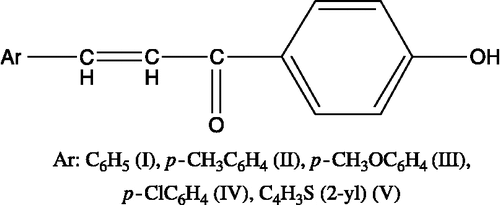
In this study, we synthesized 4′-hydroxychalcone derivatives to characterize their biological activities. Because of the known importance of the relationship between the biological activity and the chemical structure, we synthesized a number of 4′-hydroxychalcone derivatives, namely, 1-(4′-hydroxyphenyl)-3-phenyl-2-propen-1-one (I), 1-(4′-hydroxyphenyl)-3-(4-methylphenyl)-2-propen-1-one (II), 1-(4′-hydroxyphenyl)-3-(4- methtoxyphenyl)-2-propen-1-one (III), 1-(4′-hydroxyphenyl)-3-(4-chlorophenyl)-2-propen-1-one (IV) and 1-(4′-hydroxyphenyl)-3-(thiophene-2-yl)-2-propen-1-one (V) () and characterized to screen for their cytotoxic potentials via MTT test using transformed human T (Jurkat) cells. We, then, extended our characterization studies to cover plasmid supercoil relaxation assays using topo I to identify if the cytotoxicity obtained with a given derivative also matches with topo I inhibition.
Materials and methods
Synthesis of 4′-hydroxychalcone derivatives ()
4′-Hydroxychalcone derivatives were synthesized as described in literature [Citation4,Citation13]. Calculation of the partition coefficient was carried out using Chem. Officeultra 7.0 software ().
Table I. Cytotoxicity of the compounds against Jurkat cells, Log P and Hammett values of the substituents on the p- position of the phenyl ring.
Cytotoxicity of the compounds against Jurkat cells
Jurkat cells were transformed human T lymphocytes (American Type Culture Collection, TIB-152, Jurkat, clone E6-1, T cell leukemia, Human, Rockville, Maryland). They were maintained in RPMI-1640, 10% fetal bovine serum with penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37°C under a humidified atmosphere of 95% air and 5% CO2 [Citation14]. The cells were collected by centrifugation when they were near to confluence. Viable cell counts were performed in a hemocytometer using trypan blue (0.4%) dye exclusion method [Citation15]. They were, then, seeded on to 24 well plates (∼100,000 cells/mL). The test compound (15 mg) was dissolved in 0.5 mL DMSO and added into the cell culture medium (RPMI-1640) of 1200 μL the following day. Then, three fold (1 + 2 new medium) dilution series (6 steps) were prepared in duplicate each containing 250 μL of medium. After 48 h of the addition of the test compound to the wells, MTT was added (25 μL for 0.5 mL medium; 1:20 volume/volume), and the cells were incubated for 3 h at 37°C under a humidified atmosphere of 95% air and 5% CO2. Following the addition of MTT lysis buffer (0.5 mL for 0.5 mL medium; 1:1 volume: volume), the plates were incubated overnight at 37°C in a conventional incubator. One mL of medium was transferred to eppendorf tubes and centrifuged at 10000 g for 5 min at room temperature and two samples (250 μL) from each eppendorf tube were transferred to 96 well plates. The absorbance values were read at 570 nm by plate reader (Tecan Spectra Fluor, Salzburg, Austria) using the software called Biolise (Biolise; version 2.0 rev 15) in duplicate. The viability of the cells containing only the vehicle (DMSO) was accepted as 100%. The medium containing only reagents used in the MTT test without cells was used as negative control (0% viability). The value of the negative control was substracted from all absorbance values before drawing a dose-response curve. IC50 value of each compound was calculated by using the suitable segment of this curve, i.e., the part where dose-response curve seemed linear and had an r value of over 0.8. If the compound was found to be more cytotoxic than the initial concentrations used, test was repeated by lower doses to find out the IC50 value of the compound. The cytotoxicity test was carried out as two independent experiments in duplicate.
Plasmid supercoil relaxation assays
Plasmid supercoil relaxation assays were carried out as described [Citation16]. Briefly, 20 mL of reaction mixture contained one unit of calf thymus topoisomerase I, 0.5 mg of supercoiled (sc) pBR322 (TAKARA, Otsu-Shiga, Japan), in the presence or absence of the test compounds (10 μg/μL to 0.05 μg/μL) in 35 mM Tris-HCl, (pH 8.0), 72 mM KCl, 5 mM MgCl2, 5 mM DTT, 5 mM spermidine, and 0.1% bovine serum albumin. The relaxation products were analyzed on 1% agarose gels in TBE buffer (45 mM Tris borate and 1 mM EDTA, pH 8.0) in a horizontal electrophoresis apparatus (5 V/cm) (Thermo EC250) and photographed under UV light after staining in ethidium bromide (EtdBr) solution (0.5 μg/mL). The relationship between the binding of EtdBr and the amount of fluorescence given by sc and relaxed DNA (rlx DNA) under UV light was carried out as described [Citation17]. DNA bands were quantified from gel photographs using BioRad Multianalyst (ver. 1.1). One unit of the enzyme activity was defined as the activity removing the supercoils from 500 ng of sc plasmid substrate at 37°C in 30 min. All reactions were carried out in DNase-free 1.5 mL microcentrifuge tubes.
Results and discussion
In this study a number of 4′-hydroxychalcone derivatives, I-V () were synthesized to identify their biological activities [Citation4,Citation13,Citation18]. The compounds were then subjected to cytotoxicity test using transformed human T (Jurkat) cells. The results of the MTT cytotoxicity test are summarized in . As seen in , the order of the cytotoxicity of the compounds was: IV > III > II > I > V.
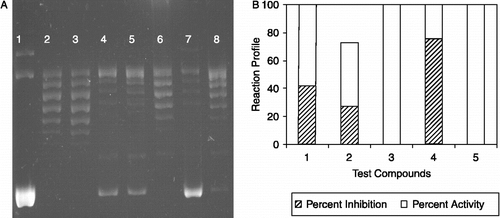
Many reports in recent years have shown that numerous naturally occurring and synthetic compounds with cytotoxic potential exert, in part, their functions through the inhibition of DNA topoisomerase enzymes [Citation5,Citation6,Citation10,Citation11]. The ability of specific agents to interfere with the catalytic cycle of topo I and stabilize the covalent complex can be biochemically addressed in sc plasmid DNA relaxation or in lineaer DNA cleavage assays [Citation8]. We employed plasmid supercoil relaxation assays to identify if the cytoxic measurements correlate with topoisomerase-interfering properties of the compounds I to V. Our assay employs sc plasmid DNA and relies on the ability of topoisomerase I to relax sc DNA, which can be separated as discrete bands using gel electrophoresis [Citation8,Citation12]. An inhibition of relaxation activity due to the presence of a particular inhibitor is monitored in the form of an accumulated faster-migrating sc DNA. We carried out a series of assays starting from high concentration of compounds, I to V (10 μg/μL) and they gave inhibitions to varying degrees (data not shown). When the tests were repeated with lower concentrations (1 μg/μL to 0.05 μg/μL), a more differentiable result was obtained using the five compounds (see for a representative assay result obtained with 1 μg/μL test compounds). As seen in , the sc DNA, pBR322, (, lane 1) was fully relaxed by the enzyme (, lane 2) and the organic solvent, DMSO, did not exert any detectable inhibition on the topo I activity in the identical experimental conditions (, lane 3). Relaxation of pBR322 was partially inhibited upon incubation with the compounds I and II (, lanes 4 and 5, respectively). The compound III was not effective on the supercoil relaxation activity of the enzyme (, lane 6) while the inclusion of the compound IV resulted in a highly considerable inhibition in the topo I reaction (, lane 7). Faster-migrating sc band was neglectable when compound V was used (, lanes 8). Densitometric quantification of the remaining sc plasmid vs rlx DNA band intensities is shown in . Residual rlx DNA band, seen in the first lane, was taken into consideration in the quantification of percent inhibition. The plot was drawn to show the average percent activity/inhibition during the reaction profile for the individual compounds. Starting from a normalized 100% topo I activity in the absence of test compound, the average percent inhibition for the compounds I and II were calculated as an average of 42% and 26%, respectively, while compound IV inhibited the reaction by 76% when added to reaction mixtures at 1 μg/μL concentration (). There was no faster migrating sc DNA band for the compound III that could be detected with the assay method we employed and the sc band was neglectable for the compound V (0.02%). We also used a serial dilution of a stock solution of 10 μg/μL Camptothecin (CPT), a well-known topoisomerase I poision, in DMSO for comparisons which gave rise to a concentration-dependent inhibition showing that the detected sc DNA bands were because of the inclusion of the test compounds in reactions [12 and data not shown].
Figure 2. The effect of 4′-hydroxychalcone derivatives on mammalian DNA topoisomerase activity. A. A representative agarose gel photograph of supercoil relaxation with 1 unit of DNA topoisomerase I in the presence of varying concentrations of 4′-hydroxychalcone derivatives (see “Materials and Methods” for the details). Lane 1, plasmid substrate, pBR322 with no enzyme; lane 2, pBR322 with 1 u of DNA topoisomerase I; lane 3, same as lane 2 in the presence of DMSO, lanes 4 to 8, pBR322 with 1 u DNA topoisomerase I in the presence of 1 μg/μL of test cpds from I to V.B. Quantitative assessment of the inhibitions obtained with the compounds. DNA bands were quantified from gel photographs and plotted with the relationship between the binding of EtdBr and the amount of fluorescence given by sc and rlx DNA under UV light.
In summary, the compound IV, the most cytotoxic compound in our test, also had the highest inhibition on topoisomerase I. The compounds I and II had comparable results by partially inhibiting topo I while compounds III and V did not effect the enzyme's relaxation ability. The orders of Log P and cytotoxicity for the compounds were IV>II>I>III>V, and IV>III>II>I>V, respectively. Compound IV, which is the most lipophilic compound had the highest Log P value. As seen, only three compounds with relatively high Log P values inhibited the topoisomerase I enzyme. On the other hand, the order of Hammett values () for the para position of the phenyl ring was obtained as IV>I>II>III while the inhibition percentages of topoisomerase I was 76, 42, and 28% for the compounds IV, I, and II, respectively. Therefore Hammett values of the compounds might correlate with topoisomerase inhibition. The resonance energies of the phenyl ring in compound I and thiophene ring in compound V were 36 kcal/mol and 29 kcal/mol, respectively. Considering our results for the Compounds I and V in cytotoxicity and topoisomerase assays, our study suggests that the cytotoxicity and topoisomerase interference properties of the compounds may be due to change of phenyl to thiophene.
Conclusions
Taken together, this is the first report on the effects of 4′-hydroxy chalcones on mammalian DNA topoisomerase I. Exploring the detailed mechanism of interaction of the compounds I, II and IV with topo I is currently in progress.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notes
* Present Address: Department of Biochemistry, Faculty of Science, Ege University, Izmir, Turkey
References
- DN Dhar. The chemistry of chalcones and related compounds. New York: Wiley-Intersciences; 1981.
- JA Benvenuto, TH Connor, DK Monteith, JL Laidlaw, SC Adams, TS Matney, JC Theiss. Degradation and inactivation of antitumor drugs. J Pharm Sci 1993;82:988–991.
- Y Satomi. Inhibitory effects of 3′-methyl-3-hydroxy-chalcone on proliferation of human malignant tumor cells and on skin carcinogenesis. Int J Cancer 1993;55:506–514.
- JR Dimmock, SK Raghavan, BM Logan. Anti-leukemic evaluation of some mannich-bases derived from 2-arylidene-1,3-diketones. Eur J Med Chem 1983;18:248–254.
- JS Kim, C Yu, A Liu, LF Liu, EJ La Voie. Terbenzimidazoles: Influence of 2″-, 4-, and 5-substituents on cytotoxicity and relative potency as topoisomerase I poisons. J Med Chem 1997;40:2818–2824.
- MPS Ishar, G Singh, S Singh, Sreenivasan, G Singh. Design, synthesis, and evluation of novel 6-chloro-/fluorochromone derivatives as potential topoisomerase inhibitor anticancer agents. Bioorg Med Chem Lett 2006;16:1366–1370.
- JC Wang. DNA topoisomeases. Ann Rev Biochem 1996;65:635–692.
- MA Bjornsti, N Osheroff. DNA topology and enzymes Humana Press. New Jersey: Totowa; 1999.
- Z Topcu. DNA topoisomerases as targets for anticancer drugs. J Clin Pharm Ther 2001;26:405–416.
- C Martín-Cordero, M López-Lázaro, M Gálvez, MJ Ayuso. Curcumin as a DNA Topoisomerase II Poison. J Enz Inhib Med Chem 2003;18:505–509.
- M Gálvez, C Martín-Cordero, MJ Ayuso. Iridoids as DNA topoisomerase I poisons. J Enz Inhib Med Chem 2005;20:389–392.
- AS Alpan, HS Gunes, Z Topcu. 1H-benzimidazole derivatives as mammalian DNA topoisomerase I inhibitors. Acta Biochem Pol 2007;54:561–565.
- HI Gul, KO Yerdelen, M Gul, U Das, B Pandit, PK Li, H Secen, F Sahin. Synthesis of 4′-hydroxy-3′-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch Pharm (Weinheim) 2007;340:195–201.
- MB Hansen, SE Nielsen, K Berg. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 1989;119:203–210.
- RI Freshny. Culture of Animal Cells; A Manual of Basic Technique. New York: John & Wiley Sons, Inc; 2005.
- Z Topcu, FJ Castora. Mammalian mitochondrial DNA topoisomerase I preferentially relaxes supercoils in plasmids containing specific mitochondrial DNA sequences. Biochem Biophys Acta 1995;1264:377–387.
- Z Topcu. Densitometric quantification of DNA topoisomers in ethidium bromide-stained agarose gels and chemiluminescence-detected X ray films. Acta Biochem Pol 2000;47:835–839.
- F Sahin, C Safak, O Yegen, AA Bilgin. Synthesis of some chalcone derivatives and their antifungal effects against Candida albicans. FABAD J 1984;9:124–132.